ISSN: 0973-7510
E-ISSN: 2581-690X
Human leukocyte antigens (HLA) are gene products found in the major histocompatibility complex, aiding in differentiation of “self” from “non-self” antigens, and is crucial for the communication between immune cells of the human body. HLA-B27, in specific, has a strong interrelation with different types of Spondyloarthritis (SpA). The aim of this study is to study the frequency of HLA-B27 in suspected Spondyloarthritis patients presenting with axial and peripheral joint involvement, who attended our tertiary care centre from August-2017 to January-2021. Patients fulfilling Assessment of Spondyloarthritis International Society (ASAS) criteria for Spondyloarthritis were included in the study, and were further classified into Spondyloarthritis sub-groups. Blood samples were collected for CRP and ESR tests along with HLA-B27 detection by RT-PCR method. Magnetic resonance imaging was done for sacroiliac joints in patients with lower back pain. Analysis of 289 samples of SpA patients revealed 156 (54%) to be HLA-B27 positive and 133 (46%) to be HLA-B27 negative. There were 98 patients (63%) with Ankylosing spondylitis, 33 (21%) had Reactive arthritis, 19 (12%) had Psoriatic arthritis, 6 (4%) had Undifferentiated Spondyloarthritis, and inflammatory bowel disease was diagnosed in 0% in HLA-B27 positive Spondyloarthritis patients. The frequency of HLA-B27 among the Spondyloarthritis (SpA) patients in our study was found to be 54% (156), more common clinical manifestation in men belonging to the age group of 16-25 years positive patients. Ankylosing spondylitis (AS) was found to be the most common sub-groups observed among the SpA patients.
Ankylosing spondylitis, HLA-B27, Psoriatic arthritis, Reactive arthritis, RT-PCR Spondyloarthritis
Spondyloarthritis (SpA) is a group of inflammatory arthritis, with common clinical manifestations, etiological factors and genetic characteristics, with a strong link to class I HLA antigens.1-3 This group of diseases is characterized by its axial involvements and absence of rheumatoid factor,4 frequently affecting sacroiliac (SI) joints of the spine that connects spine with the pelvis. SpA also has predispose to major inflammation in joints throughout the body. It causes discomfort, stiffness and twinging along the length of the spine, some of the patients having extra articular manifestations such in skin, eyes, lungs and heart.
The clinical manifestations sub-groups of SpA include inflammatory arthritis that have some overlapping features, such as Ankylosing spondylitis (AS), Psoriatic arthritis (PsA), Reactive arthritis (ReA), Juvenile onset Spondyloarthritis(JOSpA), Inflammatory bowel disease (IBD) and Undifferentiated Spondyloarthritis (uSpA) associated with spondylitis.
The disease begins in the second or third decade of life, and has a higher men preponderance compared to women (ranges from 2.6:1 – 5:1).5 The prevalence of SpA among first degree relatives of the affected patients is found to be 12%.6 Ankylosing spondylitis is the most common sub-group of SpA and has a strong association with HLA-B27 which has been reported in many studies for over 30 years, with several reports evidencing the pathogenic role of B27 gene in the development of AS.1,6,7
HLA-B27 detection, as a genetic marker, assists majorly in the disease diagnosis,8-10 which is one of the risk factors associated with SpA. In 1973, the association between HLA-B27 and ankylosing spondylitis was first reported11,12 and was later confirmed to be related with other Spondyloarthritis as well, by many other investigators. In addition to showing a striking association with AS and Spondyloarthritis, HLA-B27 also plays a crucial role in disease pathogenesis. The prevalence of HLA-B27 in AS ranges from 88 to 90% in patients compared to 4-8% among general population.13-17
The association of HLA-B27 varies from 19-94% in sero-negative spondyloarthritis (SSpA) patients and ranges from 1.4-8% in general population in India.18-20 A patient with clinical manifestations similar to rheumatoid arthritis who is tested negative for Rheumatoid Factor (RF) and Anti Cyclic Citrullinated Peptide (Anti-CCP), tested positive for HLA-B27 or scan proved on diagnosing sacroiliitis will be defined as Sero-negative Spondyloarthritis. The most common subtype associated with SpA is B*27:05 and B*27:04 among the South Indian population.4,21 Some of the clinical parameters that help to determine the disease’s severity comprises of the duration of disease, C-reactive protein (CRP) levels, Erythrocyte sedimentation rate (ESR), and age factors. This study aims to detect the frequency of HLA-B27 antigen among the Spondyloarthritis (sero-negative) patients.
This is a cross-sectional observational study. Patients who attended the rheumatology clinic in SRM Medical College Hospital and Research Centre, Kattankulathur, a tertiary care centre in South India, from August-2017 to January-2021, who fulfilled the ASAS criteria for Spondyloarthritis were included. The patients diagnosed with other auto immune diseases like rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus (SLE) were excluded from the study. Informed consent was obtained from all patients. Sample size was calculated based on the prevalence with sample calculation formula using 4pq/L2.
Blood samples were collected from patients in the inclusion criteria, as per sample collection protocols and were tested for ESR. Erythrocyte sedimentation ratio was markedly raised in the arthritis condition, because of inflammation and tissue destruction are any other infections. The ESR value was detected by westergren method, by its 1.6ml blood sample was collected in a 3.8% sodium citrate tube in the volume of 0.4ml mixed thoroughly, and fill the mixture in to the westergren tube up to the mark zero, the tube were stand vertically with the westergren stand in a room temperature without any disturbance. After the interval of 30 minutes, noted the reading of settling the red blood cell surface and final reading considering completion of 1 hour in the measurement unit of millimeters. The measurement of CRP provides information for the detection and evaluation of infection, tissue injury, inflammatory disorders and associated diseases, we use to detect CRP value by Standard F kit analyzed with SD Biosensor instrument, based on the principle of reflectometry and immunoassay technology. Human sample is react with monoclonal anti-CRP, latex in the tablet of the spoit (Red) and makes the complexes. When applying the sample mixture to the test device, the complexes move along the membrane and are captured by the monoclonal anti-CRP in the test line on the membrane as the result of the antigen-antibody reaction. The intensity of the reflected light is scanned and converted into an electric signal which is proportional to the intensity of the light produced on the membrane. The biosensor analyzer can analyze the C-reactive protein concentration of the clinical specimen based on preprogrammed algorithms and display the test result on the screen.
HLA-B27 (genetic marker) detection was using a Chip based Real-Time PCR (RT-PCR) method, based on the principle of TaqMan assay. DNA extraction from whole blood samples collected from patients in EDTA tubes was performed with Trueprep® AUTO/AUTO v2 Universal Cartridge based Sample Prep Device and Trueprep® AUTO/AUTO v2 Universal Cartridge based Sample Prepkit, which uses a proprietary matrix enclosed in a cartridge to purify nucleic acid from clinical samples. The Truenat™ HLA-B27 chip labelled with patient ID is placed on the chip tray, away from the analyzer, without touching the reaction well. The cap of the microtube containing freeze-dried PCR reagents is removed, and using the filter barrier tip, 6μL of purified DNA is transferred into the microtube. The tube was allowed to stand for 30-60s to get a clear solution. The clear solution (6 μL) was dispensed into the reaction well of Truenat™ HLA-B27 chip, and the test was initiated. At the end of the test run, a result was displayed on the Truelab® analyzer screen showing whether HLA-B27 was “DETECTED” or “NOT DETECTED”, along with the threshold cycle value(Ct value) of the internal positive control(IPC) and the validity of the test.
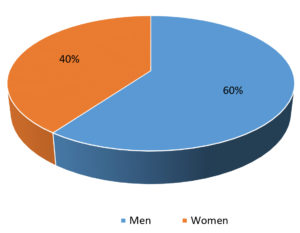
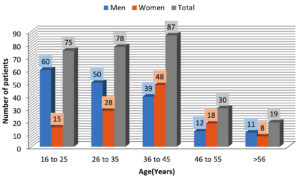
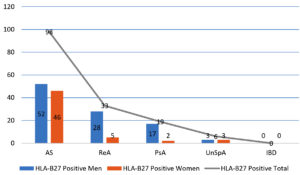
Out of the total 289 Spondyloarthritis patients, 172 (60%) cases were men and 117 (40%) were women (Fig. 1). Men: Women ratio was 1.5:1. For ease of data analysis patients were grouped into 5 age group ranges (Fig. 2). The maximum number of patients within the age group 16-25 years in men and 36-45 years in women. Among 289 SpA patients, 156 (54%) were found to be HLA-B27 positive, of which 100 (64%) patients were men and 56 (36%) were women (Fig. 3).
The most common sub-group identified among the patients was found to be ankylosing spondylitis (AS) 65.1% (188). The other sub-groups of SpA along with their prevalence are listed in (Table 1).
Table (1):
Spondyloarthritis (SpA), and their distribution by gender.
SpA group classification |
Total |
|---|---|
AS |
188(65.10%) |
ReA |
61(21.10%) |
PsA |
31(10.70%) |
uSpA |
6(2.10%) |
IBD |
3(1.0%) |
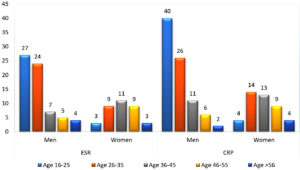
Frequency of clinical manifestations present in HLA-B27 positive persons by gender in (Table 2). HLA-B27 positive prevalence onset of clinical manifestations was found to be highest in the age group 16-25 years (34%), and least 7% among patients aged >56 years.
Table (2):
Frequency of HLA-B27 positivity in different sub-groups of Spondyloarthritis by gender.
SpA subtypes |
Men |
Women |
Total |
|---|---|---|---|
AS |
52 |
46 |
98(62.8%) |
ReA |
28 |
5 |
33(21.2%) |
PsA |
17 |
2 |
19(12.2%) |
IBD |
0 |
0 |
0 |
uSpA |
3 |
3 |
6(3.8%) |
Total |
100 |
56 |
156 |
MRI screening shows 163 sacroiliitis patients among 289 total study group, which were classified as bilateral sacroiliitis 139 (85.3%), left sacroiliitis 17 (10.4%), and right sacroiliitis 7 (4.3%).In this study HLA-B27 was found to be associated with knee pain 86 members followed neck pain 38 and lower back pain was seen in 37 patients.
Inflammatory markers were found to be elevated mostly in the age group of 16-25 years (Fig. 4). CRP and ESR values were found to be high in 82% (128/156) and 65% (102/156)respectively patients with HLA-B27 positive. Another observation was that 55.6% (74/133) ESR and CRP 69.9 % (93/133) of patients who were HLA-B27 negative showed elevated CRP and ESR values with the presence of sacroiliitis (Table 3).
Table (3):
Distribution of inflammatory markers abnormalities seen in patients by age.
| AGE GROUP | ESR+ | CRP+ | ESR+ & CRP+ |
|---|---|---|---|
| HLA-B27 positive cases | |||
| 16-25 | 30 | 44 | 30 |
| 26-35 | 33 | 40 | 32 |
| 36-45 | 18 | 24 | 16 |
| 46-55 | 12 | 13 | 14 |
| >56 | 9 | 7 | 7 |
| TOTAL | 102 | 128 | 99 |
| HLA-B27 negative cases | |||
| 16-25 | 18 | 24 | 16 |
| 26-35 | 13 | 25 | 12 |
| 36-45 | 28 | 30 | 24 |
| 46-55 | 10 | 11 | 8 |
| >56 | 5 | 3 | 3 |
| TOTAL | 74 | 93 | 63 |
Extra-articular manifestations in HLA-B27 positive patients, mostly included Uveitis 15 patients and Skin rashes 19 members. Clinical manifestations of HLA-B27 positive with their prevalence are present in (Table 4).
Table (4):
Distribution of articular and extra-articular manifestations inHLA-B27 positive Spondyloarthritis patients.
| Articular sites | |
|---|---|
| Pain Site | B27 Positive |
| Neck | 38 |
| Low back | 37 |
| Shoulder | 14 |
| Elbow | 19 |
| Hand | 12 |
| Write | 9 |
| Knee | 86 |
| Ankle | 20 |
| Feet | 18 |
| Hip | 7 |
| Upper back | 4 |
| Heel | 19 |
| Thigh | 11 |
| Sole | 18 |
| Chest | 11 |
| Extra-articular sites | |
| Uveitis | 15 |
| Skin rashes | 19 |
The strong genetic association of HLA-B27 with AS has been established long ago.21 HLA-B27 was found to be more commonly associated with AS (62.8%) followed by ReA (21.2%) and PsA (12.2%) in our study. Kamal et al.,2 observed that HLA-B27 was predominantly associated with AS (69%) similar to our study.
Spondyloarthritis is the second common inflammatory arthritis in India after rheumatoid arthritis.22,23 The specific genetic marker HLA-B27 was positive in 54% of Spondyloarthritis patients, with a men predominance. In a study conducted by Umamaheshwari et al, involving 50 patients, 26% of cases were found to be HLA-B27 positive,24 while in other studies by Mishra MN et al.,25 and Nessa et al.,26 the frequency of HLA-B27 antigen among SpA patients were 56% and 49.2% respectively with our study.
A higher percentage of men (64%) were found to be HLA-B27 positive among the SpA patients compared to women (36%) similar to the findings from other studies.24,26 Although Vaidya et al.,3 have reported that no difference was observed in the prevalence of HLA-B27 between men and women, they have observed a higher prevalence of the disease among the men population. Similarly, in our study, disease prevalence was higher in men (60%) compared to women (40%).
One of the studies from India3 showed that HLA-B27 is more prevalent in SpA patients in the second decade of life, which is similar to our study second to third decade of life. The most prevalent clinical manifestation was found to be AS 65.1%n and least in UuSpA was found to be 2.1% and 1% case of inflammatory bowel disease (IBD)was found in the study.
Kamal et al,2 had reported a higher prevalence of clinical manifestations such as Enteropathicarthropathy (22.4%), followed by reactive arthritis (19%) and psoriatic arthritis (18.3%) in their study. Vikram Haridas et al.21 reported the prevalence of AS in 74% when compared to 3% of healthy controls among the south Indian population.
In earlier days (Seager et al17), radiological findings did not provide conclusive results of sacroiliitis, but recent technological developments like MRI have aided in the diagnosis of sacroiliitis present in SpA patients.17 Similarly, MRI had shown changes (92% sensitivity) in HLA-B27 positive patients presenting with SpA in a study by Umamaheshwari et al.24
The major manifestation was found to be knee pain 86 patients, neck pain 38 and 37 patients met with lower back pain. 15 patients suffer from Uveitis and 19 Skin rashes were found to be the only extra-articular manifestations present. Umamaheshwari et al.,24 had observed joint involvement in most patients (20%), followed by extra-articular manifestations such as chronic UTI (14%), ocular, and skin involvement (8%), and CVS involvement (6%). Vaidya B et al.,3 in their study reported a higher incidence of large joint pains (70.5%) and inflammatory spinal pain (53.9%) as major manifestations in SpA patients. On early detection can prevent misdiagnosis of Spondyloarthritis as rheumatoid arthritis, which is the usual scenario where with further continuation of treatment on assuming it as RA it may lead to severe complications.27
This study highlights the prevalence of HLA-B27 among SpA patients, among which men in the age group of 16-25 years were found to be more prevalent. AS was the most common sub-group identified. Lower back pain was among the most reported complaints in articular pain sites while uveitis and conjunctivitis were the extra-articular manifestations presented. HLA-B27 detection can aid in suspecting SpA, diagnosing the advancement of the illness, and to identify patients with potentially severe outcome, which would be of paramount importance to clinicians, that prompting suitable treatment strategy. This study spots the light on the Indian population to use HLA-B27 as a genetic marker for early detection of expected Spondyloarthritis even before its appropriate clinical manifestation are elicited. HLA-B27 as a routine periodical investigation in yearly screening or being included in Master Health Checkup as early detection can prevent misdiagnosis of Spondyloarthritis as rheumatoid arthritis. Lifestyle modifications can prevent the progress of the Spondyloarthritis, if detected early.
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to Miss. Rachel S for support in writing the manuscript and acknowledge the continuous assistance provided by our lab technicians,
Mrs. Sowmyalaksmi N and Mr. Gopi throughout the study period.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, SRM Medical College Hospital and Research Centre, Tamil Nadu, India with wide Reference No: 1209/IEC/2017.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Zochling J, Smith EU. Seronegative spondyloarthritis. Best Pract Res Clin Rheumatol. 2010;24(6):747-756.
Crossref - Esalat-Manesh K, Taghadosi M, Arj A. A survey on the frequency of HLA-B27 in patients engaged with seronegative spondyloarthropathies in Kashan, Iran. Zahedan J Res Med Sci. 2015;17(6):e983.
Crossref - Vaidya B, Nakarmi S, Batajoo P. Clinical profile of Spondyloarthritis in females as compared to male patients with reference to HLAB27: a single center study from Nepal. Journal of Advances in Internal Medicine. 2013;2(1):3-5.
Crossref - Howe HS, Zhao L, Song YW, Springer L, Edmonds J, Gu J, Yu DT. Seronegative spondyloarthropathy-studies from the Asia Pacific region. Ann Acad Med Singap. 2007;36(2):135.
- Chatzikyriakidou A, Voulgari PV, Drosos AA. What is the role of HLA-B27 in spondyloarthropathies? Auto-Immun Rev. 2011;10(8):464-468.
Crossref - Prasad P, Niraj MK. Association of HLA-B27 with Ankylosing Spondylitis in Jharkhand. IOSR-JDMS. 2017;16(7):05-07.
Crossref - Chavan H, Samant R, Deshpande A, Mankeshwar R. Correlation of HLA B27 subtypes with clinical features of ankylosing spondylitis. Int J Rheum Dis. 2011;14(4):369-374.
Crossref - Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, B27, with ankylosing spondylitis. N Engl J Med. 1973;288(14):704-706.
Crossref - Brown MA, Pile KD, Kennedy LG, et al. A genome-wide screen for susceptibility loci in ankylosing spondylitis. Arthritis Rheum. 1998;41:588-595.
Crossref - Carter N, Williamson L, Kennedy LG, Brown MA, Wordsworth BP. Susceptibility to ankylosing spondylitis. Rheumatology (Oxford). 2000;39(4):445.
Crossref - Sonkar GK, Usha U. Role of HLA B27 in diagnosis of seronegative spondyloarthropathies. Indian J Pathol Microbiol. 2007;50(4):908-913.
- Sonkar GK, Usha U, Singh S. Is HLA-B27 a useful test in the diagnosis of juvenile spondyloarthropathies? Singapore Med J. 2008;49(10):795-799.
- Singh S, Sonkar GK, Singh U. Association of various inflammatory diseases with human leukocyte antigens B27, B7, Bw4 and Bw6 in patients with SSA. Rheumatol Int. 2009;29(9):1013-1016.
Crossref - Akgul O. Classification criteria for spondyloarthropathies. WJO.2011, 2(12):107.
- Chopra A. The COPCORD world of musculoskeletal pain and arthritis. Rheumatol. 2013;52(11):1925-1928.
Crossref - Chopra A. Disease burden of rheumatic diseases in India: COPCORD perspective. 2015.
Crossref - Seager K, Bashir HV, Geczy AF, Edmonds J, De Vere-Tyndall A. Evidence for a specific B27associated cell surface marker on lymphocytes of patients with Ankylosing spondylitis. Nature. 1979;277(5691):68-70.
Crossref - Shankarkumar U, Ghosh K, Colah RB, Gorakshakar AC, Gupte SC, Mohanty D. HLA antigen distribution in selected caste groups from Mumbai, Maharastra, India. Journal of Human Ecology. 2002;13(3):209-215.
Crossref - Dhurandhar PS, Shankarkumar U. HLA association in seronegativespondyloarthritis patients from Mumbai, India. Int J Hum Genet. 2007;7(3):235-239.
Crossref - Parasannanavar DJ, Rajadhyaksha A, Ghosh K. Application of a simple in-house PCR-SSP technique for HLA-B* 27 typing in spondyloarthritis patients. Arthritis. 2013;2013:504109.
Crossref - Haridas V, Shetty P, Kumar MN, et al. Human leukocyte Antigen-B* 27 allele subtype prevalence and disease association of ankylosing spondylitis among south indian population. Indian J Rheumatol. 2018;1;13(1):38-43.
Crossref - Malaviya AN, Sawhney S, Mehra NK, Kanga U. Seronegative arthritis in South Asia: an up-to-date review. Curr Rheumatol Rep. 2014;16(4):413.
Crossref - Malaviya AN. Spondyloarthritis in India. Indian J Rheumatol. 2020 ;15(5):2-5.
Crossref - Umamaheshwari V. Frequency of HLA-B27 antigen among seronegative spondyloarthropathy patients in comparison with healthy controls. Doctoral dissertation. 2018.
- Mishra MN, Singai V. Human leukocyte antigen B27 in 453 Asian Indian patients with seronegative spondyloarthropathy. Iran J Immunol. 2010;7(4):252-256.
- Nessa A, Tabassum S, Sultana S. HLA-B27 antigen frequency among suspected Spondyloarthropathy patients attaining a tertiary level hospital of Bangladesh. Bangladesh Med Res Counc Bull. 2014;40(3):102-106.
Crossref - Banicioiu-Covei S, Vreju AF, Rosu A, Ciurea PL. The Importance of HLA-B27 in the Evolution of Reactive Arthritis. Curr Health Sci J. 2019;45(4):345-352.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.