ISSN: 0973-7510
E-ISSN: 2581-690X
Neisseria gonorrhoeae is a member of the Neisseriaceae family. They are non-spore-forming, gram-negative, encapsulated, non-motile, non-acidic, and bean-shaped bacteria. This study included 40 men that presented chronic and recurrent infection with N. gonorrhoeae virus. The initial infection of this virus was detected using a rapid bacterial antigen immunoassay and confirmed using enzyme-linked immunosorbent assay (ELISA ). The mean age of the study group was 36.71±12 (mean±) years. Men between the age of 20-49 years were the most affected by N. gonorrhoeae infection, and displayed a significantly lower average sperm count than the healthy individuals upon semen examination. The percentage of sperms with slow motility, total number of dead sperms, and abnormal- shaped sperms were some significant phenotypes observed in the infected individuals as compared to that in the healthy controls. In this study, we found that the bacterium, N. gonorrhoeae could cause erosion of the mitochondrial DNA of sperms in the semen of the infected individuals. In addition, using the gap-PCR technique, it became evident that the infected individuals portraying altered sperm characteristics as mentioned above, showed increased number of common deletion (4, 977 base pairs) in the sperm mitochondrial (mt)-DNA. Hence, our results imply that N. gonorrhoeae infection can lead to a common deletion of 4,977 bp in sperm mt–DNA, which can in turn cause male sterility.
mt-DNA, Men Sperm, N. gonorrhea
N. gonorrhoeae belongs to the Neisseriaceae family,1 that are bean-shaped, non-spore-forming, gram-negative, encapsulated, non-motile, and non-acidic bacteria.2 They grow in aerobic conditions in enriched media, such as chocolate agar medium. N. gonorrhoeae produces beta-lactamase,3 and forms non-pigmented, smooth, and tiny colonies after 18–24 hours of cultivation on chocolate agar. N. gonorrhoeae has 70 different subspecies. In fact, the rate N. gonorrhoeae infection increases dramatically owing to its enhanced antibiotic resistance capacity against several antimicrobial agents that are used to treat gonorrhea.4-6 A highly resistant N. gonorrhea strain was found in UK (2016) and later in Australia (2018), which showed resistance to the first-line therapy and double treatment with antibiotics like ceftriaxone and azithromycin (PHE, 2018 and AGDH, 2018).
Since there is no vaccine against gonococcus, the control of the infection is dependent solely on inhibition, diagnosis, and especially, treatment with antibiotics.7 N. gonorrhoeae is one of the most common causes of urinary tract infections. The mechanistic details of how genitourinary illnesses affect male fertility is unidentified. Urogenital pathogens promote provocative processes that can cause disturbances in spermatogenesis and obstruction of the vas deferens. Although the relationship between apoptosis, infection, and sperm parameters associated with inflammatory conditions is controversial, it can lead to changes in sperm parameters. 8 In the field male infertility, infections of the male genitourinary system have been studied for a long time. Previous studies show that approximately 15% of total male infertility cases are associated with reproductive tract infection. Such infections are challenging to identify due to potential contamination by other clinically asymptomatic organisms and the difficulty in culturing N. gonorrhoeae.9 N. gonorrhoeae is found in 6.5% of infertile men.10 After genitourinary infections, the typical incubation period is between two–six days followed by symptoms such as pus-filled discharge from the penis and dysuria. At times, erythematous secretions may also be present in the ear canal.
Gonococcal infection is one of the many sexually transmitted infections (STI) and is an important community health problem. Clinical studies on N. gonorrhoeae have recognized most approaches of antibiotic resistance, and confirmed the close validity of receptive generalized gonorrhea. WHO has introduced waste management recommendations to eliminate microorganisms and prevent the spread of antimicrobial-resistant gonorrhea. Worldwide, over $78 million cash, unsupervised transfers, and unlimited payment options are available to make low-cost and unloaded payments to support this initiative.11-14 The transmission process of N. gonorrhoeae infection is vastly studied. An effective pathogen, like N. gonorrhoeae associates with its human host and cannot survive outside of it. Host-to-host transmission occurs majorly via exchange of bodily fluids upon sexual intercourse from high-risk core population to marginal population with intermediate risk. At-risk groups include individuals with numerous sexual partners who have unprotected sex.15 N. gonorrhoeae is associated with men spermatozoa and can be easily transferred from infected men to their partners via ejaculation, which constitutes a large gonococcal bacterial load.16 However, the process of transfer of these bacteria from women to their sexual partners is still unclear. It is known that the external, sialic acid free layer of N. gonorrhoeae adheres to and penetrates the epithelial cells of the male ureth.7 Similarly, it is believed that the mucous, villi, and the secretion are targeted the microbes present in the cervix first and removed from the female vagina. Eventually, lipopolysaccharides (LPS) in the vagina ensure effective transmission from women to their sexual partners.17
Mitochondria are intercellular organelles responsible for energy metabolism in eukaryotic cells. For more than 30 years, it is known that it carries a second set of genomes, in most animal cells. The human mitochondrial genome is a closed circular biomolecule, 16,569 base pairs (bp) in length, present in the mitochondrial matrix. It is a double-stranded molecule containing 37 genes that encode for 22 tRNAs, 2 rRNAs, and 13 proteins of the four respiratory enzyme complexes. ND1, ND2, ND3, ND4, ND5, and ND6 are subunits of complex 1. Cytochrome b is a subunit of complex 3. CO1, CO2, and CO3 are subunits of complex 4. ATPases 6 and 8 are subunits of complex 5. mtDNA also encodes the remaining units and proteins that make up or regulate the mitochondria. mtDNA is a molecule that replicates independently and does not undergo recombination. It reproduces quickly and is devoid of any of the proposed mechanisms for efficient DNA reading and repair. Its unique feature is the complete absence of intronic sequences in all the genes. Both the DNA strands are duplicated to form the functional machinery for the mitochondrial protein synthesis. In recent years, more than 24 mtDNA mutations associated with human diseases have been identified.18 One of the most common mutations is a deletion of 4,977 bp in mtDNA, which results in the loss of approximately one-third of mtDNA, and includes a deletion of the structural genes: ATPase-6, ATPase-8, COX III, ND-3, ND-4L, and ND4 along with five types of mobile RNA. The 4,977 bp mtDNA deletion is situated between positions 8,469 and 13,447 of the mtDNA.19 Sperm produces reactive oxygen species (ROS) such as nitric oxide (NO) and superoxide anions, which are critical factors in the sperm condensation, acromial reaction, and fusion with the egg. As the mitochondrial genome is not protected by histones, this could result in multiple mutations, like the 4,977 bp deletion. Previous studies indicate the role of mtDNA in male infertility.20-22 Among sperm characteristics, sperm motility is an important indicator of fertility. Low sperm motility is largely related to infertility.23 Since the sperm requires a large amount of ATP to propagate using the flagellar system, disruption of the mitochondrial respiratory system results in decreased motility and fertility.24
Samples collection
Forty semen samples were collected from patients suffering from gonorrhea for a long time, who visited the urology clinic, after obtaining the consent of the patient and in accordance with the ethics of scientific research. Initial detection was performed using a rapid immunoassay to detect the antigen of Neisseria gonorrhea in the semen swabs of the patients.
Gonorrhea Rapid Test
A swab sample was taken from the urethra/genital area for examination. Then, the tip of the swab was thoroughly swirled against the walls of the extraction tube containing buffer (composed of two extraction solutions ). Two drops of this sample were added to the sample well in the laboratory. The test detects N. gonorrhea antigen in the urethral/genital specimen using the first Neisseria gonorrhea- conjugated colloidal gold, an antibody that binds to N. gonorrhea antigens, and the antigen is a visual “marker” of the antibody. The region with stable antibodies that capture the compound, causes the visible markers to produce a visible pink band that confirms the presence of N. gonorrhea and hence, the patient tests positive for gonorrhea. If the pink band is absent, Neisseria is absent, indicating a negative result for gonorrhea. It takes 10 min to obtain the results.
Human Anti Neisseria Gonorrhea ELISA Kit Cat. No: MBS3803564
The ELISA kit ( Catalog # MBS3803564) is a colorimetric detection system for N. gonorrhea antigens.
General Seminal fluid analysis
It is accredited by the standards adopted by the World Health Organization.11
Sperm extraction and DNA isolation from semen
Seminal samples were liquefied at 35°C to 37°C. After at least 25 min, the sperms were detached from the seminal plasma by centrifugation at 700 × g for 10–15 min. DNA with an absorbance ratio of 1.8 or higher at 260 nm and 280 nm, was used. The extracted DNA was stored at 4°C. Using NCBI-SNP databases and Primer Gap-PCR, primers were designed online using BatchPrimer3 v1.0. A Gap-PCR primer for common deletion of 4,977 bp was designed in the current study. These primers were ordered from Scientific Researcher. Co. Ltd. Iraq and are listed in the Table 1.
Table (1):
Wild type and mtDNA 4,977 bp deleted primer with their sequence and product length for Gap-PCR technique.
| Primers | Amplified region | 5′ ⇒ 3′ | product |
|---|---|---|---|
| Primer WF | T1 1,257–1,279 | TATACCGCCATCTTCAGCAAAC | 177 b p |
| Primer WR | T2 1,411- 1,433 | ACTGCTAAATCCACCTTCGAC | |
| Primer DF | D1 8,416- 8,437 | CCTTACACTATTCCTCATCACC | 127 b p |
| Primer DR | D2 13,498-13,519 | GTGGTCTTTGGAGTAGAAACC |
Statistical analysis
The statistical analysis was performed by using SPSS software V.25 in order to obtained the means and the percentages value of parameters as well as the comparison was done among parameters at probability level (P< 0.05) and (P< 0.001).
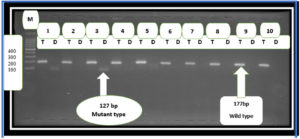
The mean age of the group included in this study was 36.71 years, where the age group between 20-29 years showed the highest number of N. gonorrhoeae infections, while the age group between 30-39 years had the lowest number of patients with gonorrhea (Table 2). Our results revealed significant differences (P< 0.001) in sperm parameters between healthy controls and patients with gonorrhea ( Table 3; Figure 2). We also observed a significant difference (p< 0.001) in the patients with gonorrhea and deletion of the 4,977 bp region compared to patients with gonorrhea and without common deletion (Table 4). Here, three (7.5%) patients with N. gonorrhoeae infection carried the common deletion of 4,977 bp in their sperm mt-DNA (Figure 1).
Table (2):
Patients age group infected with N. gonorrhea detected by rapid test and ELISA.
| Age group | Number of patients | percentage |
|---|---|---|
| 20-29 | 16 | 40 % |
| 30-39 | 5 | 12.5 % |
| 40-49 | 13 | 32.5 % |
| 50≥ | 6 | 15 % |
| Total | 40 | 100 % |
| Mean | 36.71 | |
Table (3):
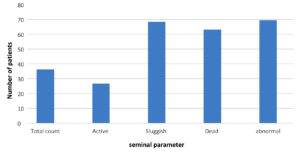
Seminal fluid parameter in patient with N. gonorrhea.
Patients =40 |
Healthy control =20 |
|
|---|---|---|
Total of sperm count*106 |
||
mean |
36.275 |
96.88 |
range |
17-59 |
80-95 |
std |
9.31 |
2.45 |
Active sperm motility |
||
mean |
26.8 |
89.94 |
range |
12-48 |
75-98 |
std |
8.8295 |
1.49 |
sluggish sperm motility % |
||
mean |
68.425 |
20.11 |
range |
44-85 |
20-28 |
std |
10.92449 |
1.22 |
Dead % |
||
mean |
63.2 |
15.1 |
range |
45-82 |
15-20 |
std |
8.075271 |
2.33 |
Abnormal sperm morphology% |
||
mean |
69.575 |
20.341 |
range |
49-84 |
10-15 |
std |
7.442 |
3.91 |
Table (4):
Seminal parameter in sperm mt-DNA common deletion 4,977bp in N. gonorrhea patients Patients With common deletion.
| Total count*106 | Active % | Sluggish % | Dead % | Abnormal % | |
|---|---|---|---|---|---|
| mean | 19.66 | 13 | 45.33 | 49.66 | 54.66 |
| range | 17-22 | 12-15 | 44-47 | 45-53 | 49-59 |
| Patients Without common deletion | |||||
| mean | 37.62 | 27.91 | 70.78 | 64.29 | 70.29 |
| range | 22-59 | 16-48 | 49-85 | 54-82 | 59-84 |
Figure 1. Agarose gel electrophoresis image that showed the Gap-PCR product analysis of mtDNA common deletion 4,977 bp positive Toxoplasma gondii of patient’s sperm samples. M: marker (1500-100bp). Lanes (T) wild type at (177bp) PCR product. Lanes (D) deletion(mutant) type at (127bp) PCR product. The positive results of mutant type in lane 1 AND 3
Gonorrhea is a disease that is caused by Neisseria gonorrhea infection and can be sexually transmitted. This infection is a significant disease problem, and more than 106 million novel cases are identified each year, globally.11 Higher rate of gonorrhea infection was found in men in the age group between 15-24 years,25 which corelates with the highest male sexual activity window.26 During transmission, N. gonorrhoeae are often unprotected from semen and must become accustomed to the forthcoming environmental changes. Previous studies have shown that spermicides facilitate the movement of Neisseria gonorrhoeae and alter its interactions with epithelial cells. It was found that exposure to sperm reduces adhesion to the surface of gonococcus independently of OP or type IV retraction of the pilus. It was also found that semen caused the spread of bacteria that had previously adhered to the epithelial surfaces. Despite reduced surface adhesion, the interaction of bacteria with seminal plasma is enhanced by cell-cell interaction. The increased interaction between bacteria leads to the formation of small colonies, an essential phase in the process of N. gonorrhoeae infection. Sperm also improved bacterial accumulation in the arrangement of 3D slip-resistant biofilms. These findings highlight the significance of gonococcal adaptation to the flow of semen inside the genitals. Further investigation into the response of N. gonorrhoeae to the seminal fluid will improve our understanding of the mechanisms underlying infection and transmission to naïve hosts.27 Our studies showed that N. gonorrhoeae motility is driven by the semen plasma (SP), as bacterial motility shows a strong correlation with the presence of semen.28 The present results revealed degenerative effects like impaired sperm parameters in patients with bacterial infection, as opposed to healthy uninfected controls. Sperm survival rates in infected patients were lower than those in healthy, uninfected men. Sperm concentration (106/ml ), motility (68.42%), survival (63.2%) and abnormal morphology (69.59%) were significantly impaired in infected samples than in healthy uninfected men, similar to a previous study.29
As the results indicated, there was a decrease in all sperm variables in the sperm of infected individuals, including vitality, motility, morphology, and DNA integrity of the sperm 29. Recent evidence has shown that both sperm and leukocytes can directly or indirectly distress the properties of sperm, depending on the type of bacterial infection 30. It has been proven that sperm morphology and motility are important factors in sperm transactions. Disorders of spermatogenesis are observed in patients with infections of the genitourinary system 31. Male reproductive tract infection (MRI ) can affect sperm parameters and impair adrenal gland function. Therefore, it is considered a possible source of male infertility that could be corrected. Nevertheless, the physiological and epidemiological effects of infection on adrenal gland function in men remain controversial 32. The male accessory glands secrete many factors essential to maintain sperm physiology, such as prostaglandins, fructose, bicarbonate, citric acid, and alpha-glucosidase 33. Infection of the male reproductive system considerably reduces the semen and plasma levels of fructose, alpha-glucosidase, and zinc, which counteracts the secretory purpose of the seminal vesicles, epididymis, and prostate gland.34,35
The current study has proposed that N. gonorrhea infection can cause mutations in mt-DNA of sperm in 7.5% of patients. An explanation for it may be that N. gonorrhoeae employ a variety of strategies to prevent complement activation and counteract complement-mediated bacterial degradation.34,36 In most cases, gonococcal cervicitis or symptomatic urethritis manifests as an acute inflammatory reaction with the secretion of purulent mucus rich in neutrophils. Therefore, we suggest that N. gonorrhoeae may also have the potential to prevent and possibly suppress the induction of adaptive immune responses.37 Bacteriospermia, and subsequently leukospermia, can deleteriously affect male fertility through diverse mechanisms, including impaired spermatogenesis, impaired sperm function, and reproductive system dysfunction.38 In addition, sperm leukocytes can influence sperm function by stimulating cytokine and ROS production. In the seminal plasma, increased levels of ROS are related to the oxidation of lipids in the plasma membrane of sperm and can prime the degradation of sperm DNA.39 ROS can prevent or stimulate many enzymes, thereby promoting their capacity. Highly responsive radical oxygen ions bind directly to phosphokinase C (PKC), stimulating its translocation to the plasma membrane via phosphotyrosine-binding sites.40,41
When infected, the male genitourinary system reflects two types of changes: increased diameter and decreased speed of sperm flow.42 This results in increased ROS levels. The causative agent of gonorrhea, N. gonorrhoeae lives on the mucous membrane of the genitals, rectum, and nasopharynx Upon trauma, among the gram-negative bacteria, N. gonorrhoeae is unique as it reaches enormous sizes thereby, increasing the peptidoglycan levels. N. gonorrhoeae can activate TLR2, TLR4, and cellular receptor binding oligonucleotides erectile domain (NOD )-1 and NOD2 to regulate the innate immune response against antigenic bacteria.43
Altogether, this stimulates the transcription factor (NFkB) and multiple replications of serine-threonine receptor-interacting protein kinase 2 (RIPK2). NOD receptor activation leads to stimulation of not only cytokines (IL-16) and chemokines (CCL17 and CCL22), but also growth factors-1 (CSF1), and other protein recognition receptors such as TLR2 and TLR4.44 In addition, IL-1 induces sperm apoptosis through beta cell proliferation and differentiation to stimulate the production of neutrophils and monocytes with chemotherapy such as IL-8.42 Expression of IL-1 and IL-8 in N. gonorrhoeae significantly affects the clinical outcomes.45
A study showed that there is a negative relationship between the oxidative stress and sperm concentration, function, and motility.46 Cytokines may also affect sperm quality and lead to male sterility.42 In addition, one study reported that elevated levels of IL-1 were related to decreased sperm movement and increased seminal levels of ROS and malondialdehyde (MDA), a byproduct of lipid oxidation.47 Several studies have also shown that sperm DNA integrity is a key factor in maintaining male fertility.48,49 Sperm DNA condensation appears to play a critical role in male fertility, and early embryonic development is affected by the integrity of sperm DNA. Previous studies have shown that the overproduction of both semen leukocytes and ROS after bacteriuria leads to the degradation of sperm DNA.39 In fact, during a bacterial infection, SRM levels may be elevated in patients with urogenital tract infections.50 The effect of bacteria on sperm parameters may be related to their ability to produce specific inflammatory mediators and attract leukocytes, thereby increasing ROS levels. Increasing the level of reactive oxygen species with a gradually increasing number of leukocytes in semen affects the degradation of sperm DNA.30
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by Ethical Scientific Research Ethics Committee and College of Biotechnology, Al-Qasim Green University, Iraq.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of Clinical Microbiology. 9th Edition, American Society for Microbiology, Washington, DC, 2007.
- Ryan KJ, Ray CG, Sherris JC. Medical Microbiology: An Introduction to Infectious Diseases (4th ed., pp. 327-341). United States: McGraw Hill. 2004.
- Holder NA. Gonococcal infections. Pediatrics in Review. 2008;29(7):228-234.
Crossref - Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med. 2012; 366(6):485-7.
Crossref - Hook EW 3rd, Kirkcaldy RD. A Brief History of Evolving Diagnostics and Therapy for Gonorrhea: Lessons Learned. Clin Infect Dis. 2018; 67(8):1294-1299.
Crossref - Scheld W, Hughes M, Whitley J. Emerging infections. John Wiley & Sons. 2020; (10).
- Suay-García B, Pérez-Gracia MT. Drug-resistant Neisseria gonorrhoeae: latest developments. Eur J Clin Microbiol Infect Dis. 2017; 36(7): 1065-1071.
Crossref - Gimenes F, Souza RP, Bento JC, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. 2014, 11(12):672-87.
Crossref - Purvis K, Christiansen E. Male infertility: current concepts. Ann Med. 1992; 24(4):259-72.
Crossref - Abusarah EA, Awwad ZM, Charvalos E, Shehabi AA. Molecular detection of potential sexually transmitted pathogens in semen and urine specimens of infertile and fertile males. Diagn Microbiol Infect Dis. 2013; 77(4):283-6.
Crossref - World Health Organization (WHO). laboratory manual for the examination and processing of human semen. 5th edit. 2010.
Crossref - Carmona-Gutierrez D, Kainz K, Madeo F. Sexually transmitted infections: old foes on the rise. Microb Cell. 2016; 3(9):361-362.
Crossref - Unemo M, Golparian D, Sánchez-Busó L, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization.
J Antimicrob Chemother. 2016; 71(11): 3096-3108.
Crossref - Newman L, Rowley J, Vander Hoorn S, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One. 2015; 10(12):e0143304.
Crossref - Papp JR, Schachter J, Gaydos CA, & Van Der Pol B. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae-2014. MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports/Centers for Disease Control. 2014; 63: 1.
- Harvey HA, Porat N, Campbell CA, et al. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol Microbiol. 2000; 36(5):1059-70.
Crossref - Ketterer MR, Rice PA, Gulati S, et al. Desialylation of Neisseria gonorrhoeae lipooligosaccharide by cervicovaginal microbiome sialidases: the potential for enhancing infectivity in men. The Journal of infectious diseases. 2016; 214(11): 1621-1628.
Crossref - Adler M, Shieh PB. Metabolic Myopathies. Semin Neurol. 2015;35(4):385-97.
Crossref - Guo ZS, Jin CL, Yao ZJ, Wang YM, Xu BT. Analysis of the Mitochondrial 4977 Bp Deletion in Patients with Hepatocellular Carcinoma. Balkan J Med Genet. 2017; 20(1):81-86.
Crossref - Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013; 288(1):191-9.
Crossref - Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online. 2003;7(1):65-70.
Crossref - Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab. 2008; 93(8):3199-207.
Crossref - Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D. The association of age and semen quality in healthy men. Hum Reprod. 2003; 18(2):447-54.
Crossref - Davila MP, Muñoz PM, Bolaños JM, et al. Mitochondrial ATP is required for the maintenance of membrane integrity in stallion spermatozoa, whereas motility requires both glycolysis and oxidative phosphorylation. Reproduction. 2016; 152(6):683-694.
Crossref - Centers for Disease Control and Prevention (CDC). Gonorrhea – CDC Fact Sheet. Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. 2022.
- Centers for Disease Control and Prevention (CDC). Sexually Transmitted Diseases Surveillance: Gonorrhea. 2009.
- Anderson MT, Byerly L, Apicella MA, Seifert HS. Seminal Plasma Promotes Neisseria gonorrhoeae Aggregation and Biofilm Formation. J Bacteriol. 2016; 198(16):2228-35.
Crossref - Anderson MT, Dewenter L, Maier B, Seifert HS. Seminal plasma initiates a Neisseria gonorrhoeae transmission state. mBio. 2014; 5(2):e01004-13.
Crossref - Eini F, Kutenaei MA, Zareei F, Dastjerdi ZS, Shirzeyli MH, Salehi E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with Leukocytospermia. BMC Mol Cell Biol. 2021; 22(1):42.
Crossref - Fraczek M, Kurpisz M. Mechanisms of the harmful effects of bacterial semen infection on ejaculated human spermatozoa: potential inflammatory markers in semen. Folia Histochem Cytobiol. 2015; 53(3):201-17.
Crossref - Menkveld R, Huwe P, Ludwig M, Weidner W. Morphological sperm alternations in different types of prostatitis. Andrologia. 2003; 35(5):288-93.
Crossref - Weidner W, Wagenlehner FM, Marconi M, Pilatz A, Pantke KH, Diemer T. Acute bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: andrological implications. Andrologia. 2008; 40(2): 105-12.
Crossref - Dohle GR. Inflammatory-associated obstructions of the male reproductive tract. Andrologia. 2003; 35(5):321-4.
Crossref - Marconi M, Pilatz A, Wagenlehner F, Diemer T, Weidner W. Impact of infection on the secretory capacity of the male accessory glands. Int Braz J Urol. 2009; 35(3):299-308.
Crossref - Ram S, Cullinane M, Blom AM, et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001; 193(3): 281-95.
Crossref - Lewis LA, Burrowes E, Rice PA, Ram S. Interactions of Neisseria with complement, p. 123–144. In Genco CA and Wetzler L, Neisseria: molecular mechanisms of pathogenesis. Caister Academic Press, Norfolk, United Kingdom;2010.
- Hedges SR, Mayo MS, Mestecky J, Hook EW 3rd, Russell MW. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect Immun. 1999; 67(8):3937-46.
Crossref - Esmailkhani A, Akhi MT, Sadeghi J, et al. Assessing the prevalence of Staphylococcus aureus in infertile male patients in Tabriz, northwest Iran. Int J Reprod Biomed. 2018; 16(7):469-474.
Crossref - Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018; 50(11):e13126.
Crossref - Signorelli J, Diaz ES, Morales P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res. 2012; 349(3):765-82.
Crossref - De Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008; 1784(1):106-15.
Crossref - Azenabor A, Ekun AO, Akinloye O. Impact of Inflammation on Male Reproductive Tract. J Reprod Infertil. 2015;16(3):123-9.
- Château A, Seifert HS. Neisseria gonorrhoeae survives within and modulates apoptosis and inflammatory cytokine production of human macrophages. Cell Microbiol. 2016; 18(4):546-60.
Crossref - Mavrogiorgos N, Mekasha S, Yang Y, Kelliher MA, Ingalls RR. Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response. Innate Immun. 2014; 20(4):377-89.
Crossref - Singer M, Ouburg S. Effect of cytokine level variations in individuals on the progression and outcome of bacterial urogenital infections–a meta-analysis. Pathog Dis. 2016; 74(2):ftv126.
Crossref - Kemal Duru N, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000; 74(6):1200-7.
Crossref - Koçak I, Yenisey C, Dündar M, Okyay P, Serter M. Relationship between seminal plasma interleukin-6 and tumor necrosis factor alpha levels with semen parameters in fertile and infertile men. Urol Res. 2002; 30(4):263-7.
Crossref - Haidl F, Haidl G, Oltermann I, Allam JP. Seminal parameters of chronic male genital inflammation are associated with disturbed sperm DNA integrity. Andrologia. 2015; 47(4):464-9.
Crossref - Rusz A, Pilatz A, Wagenlehner F, et al. Influence of urogenital infections and inflammation on semen quality and male fertility. World J Urol. 2012; 30(1): 23-30.
Crossref - Gallegos G, Ramos B, Santiso R, Goyanes V, Gosálvez J, Fernández JL. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil Steril. 2008; 90(2):328-34.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.