ISSN: 0973-7510
E-ISSN: 2581-690X
The global textile industry is significant and presents great business potential, particularly for emerging nations. On the other hand, because of their erratic and quick expansion, these industrial clusters are to blame for the adverse environmental impacts. Different chemicals, salts, and dyes are all mixed together in wastewater resulting from the textile printing business. This causes it to be brightly colored, have an alkaline pH, and have substantially lower levels of dissolved oxygen, all of which have an impact on the surrounding environment. The study collects wastewater from the textile industry at each stage of the process and analyses it to identify its parameters. These parameters include pH (Analytical value is 6.6 to 13.1), BOD (Analytical Value is 432 to 1840mg/l), COD (Analytical Value is 635 to 4459 mg/l), Total Dissolved Solids – TDS (Analytical Value is 6530 to 21989 mg/l ), TSS (Analytical Value is 275 to 1189), and Ammonium Nitrogen (Analytical Value is 34.2 to 49.4 ), Since these are all baseline variables, the natural ecological system is deteriorating. This allows for the deduction of the state authorities’ final alleviation standards for the ensuing treatment process.
Textile Industries, Wastewater, Physicochemical Parameters, Deteriorate, Natural Ecological System
The textile industry sectors are necessary for the economic development1 of every country worldwide. In the textile industry, many reactive dyes are used, consisting of various chemical structures of aromatic compounds2 connected with azo bonds.3 About 40 to 50 percent of the mixture of all dyes used in the process will come out with effluent from the industries.4 The manufacturing process will create different types of pollutants with the dyes5 and lack of treatment knowledge and unavailability of economically viable treatment facilities6 will adversely affect the natural environment as this partially treated wastewater is discharged into natural waters for irrigation purposes and sewerage.7,8 The raw fabrics that come from the weaving mills cannot be used directly for the printing process due to the various types of impurities that are present with the fabrics during the weaving process to give them strength and smoothness of the fabric.9 So, processing the fabrics to make them printable is a multi-step process and requires different chemical treatments.10
The many process processes before and after the printing process, such as desizing, washing, bleaching, mercerizing, dyeing, printing, and washing, are all a part of the textile printing business. Desizing is facilitated by oxidizing raw fabric waste, which can be accomplished using hydrogen peroxide,11 hypochlorite,12 clorine dioxide, enzymes, and mineral acids.13 lipase, cellulase, and amylase are good enzymes for the desizing process.14 In scouring the desized fabrics becomes clean and grease-free. The fabric is then washed out with detergents and solvents,15 containing caustic soda &/or enzymatic agents. Bleaching increases the whiteness and absorption capacity of materials for dyes used in the printing process16 and can be achieved with hydrogen peroxide.17 The Mercerization process increases strength and ability to absorb dyes, ultimately helping to reduce the amount of dye used in the printing process.18 This is done by caustic lye and successive washings with acid and water to render the cotton fiber neutral.19 Dyeing and printing are similar processes, where the dyeing of cotton fabrics is mostly monochromatic20 and the printing consists of multiple dyes, binders, and polymer resin, plasticizers. Defoamers and resins are used to increase color fastness.21 Lastly, Washing is performed with laundry detergents to remove the dye and other chemicals that cannot be absorbed by the cotton fabrics.
The goal of the current study was to evaluate the actual physico-chemical characteristics of industrial wastewater. Various salts, acids, enzymatic ingredients, dyes of various colors, and other chemicals are used during the entire process.22 All of the leftover materials are eventually flushed into the water supply as industrial effluent. This indicates that the textile printing business produces a lot of wastewater.23 The status of the generated effluent should be clarified before discharge into the natural environment24. It also helps to improve the effluent quality with chemical or biological treatment if needed.
Study area
Jetpur is located in the Saurashtra region of Gujarat state on National Highway 8B and is about 70 km from Rajkot on the way to Junagadh. It is one of the largest centers for screen printing, block printing, and yarn dyeing in the country. The city is built on the river bank of BHADAR. Famous for the cotton saree industry, the Jetpur is a major exporter of Khanga and Kitenge, also known as African print. More than 1500 cotton printing industrial units operate in Jetpur and liters of wastewater are generated daily from these industries. In the Saurashtra region including Jetpur, the rainfall rate is much lower which means it is a water-scare area.
Collection and preservation of the sample
Each of the analysis methods is highly dependent on the samples, where they are collected from, and how they are stored after collection. The sample collected may deteriorate if not kept accurately. Therefore, if possible, neither biological activity nor destruction of the chemical component should occur. At the time of sampling, it should be thoroughly rinsed with the samples collected in 10-liter plastic containers, and the sample analysis will be started within 24 hours after the sampling time. The wastewater was collected from the textile printing industry every month from September 2019 to February 2019, when the whole process (i.e. bleaching, mercerizing, printing the white fabrics, and washing the printed fabrics) was done in the industry. All the sample was collected separately for each process and also a representative sample coming from the collection tank of the ETP from Western Overseas (which is at 21°43’25.20″ N and longitude 70°34’10.27″ E), located in the village: Jetalsar Taluka: Jetpur.
Analysis of physicochemical parameters for the characterization of wastewater
The analysis of wastewater quality offers information on the extent to which it may degrade natural ecosystems if released into the environment as is.25 After examining the wastewater, the company can determine whether to adequately treat it using physical, chemical, or biological techniques26 in order to reuse it for industrial uses or dump it into the environment.
pH is measured using Aquasol Digital Meter Model No. AM-AL-01 (Rakiro Biotech Systems Private Limited). Before measuring the pH value of the wastewater sample, the pH meter should be calibrated using standard buffer solutions of pH 4.0, 7.0, and 9.18. The pH measurement and the calibration of the pH meter are carried out at room temperature.
Total Dissolved Solids are the sum of the cations and anions in water, and more specifically, these are the solids containing all inorganic and organic substances present in water and/or wastewater that can pass a 2-micron filter. It is measured by gravimetric analysis according to Standard Methods for the Testing of Water and Wastewater, 22nd Edition, Part: 254°C.27
Total suspended solids: These measure particles larger than 2 microns in a water system. It contains both inorganic and organic components. It contributes to water turbidity and also indicates solids that may settle to the bottom of the liquid system. It is measured according to the Standard Methods for the Analysis of Water and Wastewater 22nd Edition, Part: 2540 D.27
Biological Oxygen Demand is a measure of the number of oxidizable substances in a water sample that can lower the DO concentration. It is a bioassay method that measures the oxygen consumed by bacteria from the decomposition of organic matter, measured according to Standard Methods for the Analysis of Water and Wastewater, 22nd Edition, Part: 5210 B.27
Chemical oxygen demand represents the amount of oxygen consumed to digest organic matter present in the wastewater under aerobic conditions (oxygen is present) at a given temperature. Ultimately, this is an important chemical parameter for measuring organic matter present in the fluid system.28 It is measured according to the Standard Methods for the Testing of Water and Wastewater 22nd Edition, Part: 5220 B.27
Ammonical Nitrogen(NH3-N)
It describes measuring the amount of ammonia, which is a toxic pollutant present in industrial wastewater. Ammonia could be a direct poison to living things and upset the balance of environmental systems. It is measured according to the Standard Methods for the Testing of Water and Wastewater 22nd Edition, Part: 4500 – Norg B.27
The characterization of the wastewater from the textile printing industry is of paramount importance for further primary, secondary, and tertiary treatment in order to reuse it in industry or for agricultural processes. As such, if it discharges in the open environment, then its effect will be much more severe; for this purpose, the physico-chemical parameters are analyzed and compared with the standards issued by the state pollution control office and the central pollution control office.
Characterization of wastewater
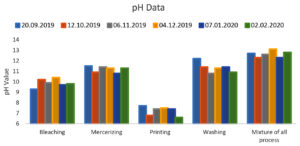
The pH range of the collected wastewater from the bleaching process is 9.3 to 10.4, from the mercerizing process is 10.8 to 11.5, from the printing process is 6.6 to 7.7, from the washing process is 10.8 to 12.2, and the mixture of all processes is 12.3 to 13.1. The pH of the effluent from the printing process wastewater pH was much higher than the range set by the governmental agencies, which should be between 6.5 to 8.5 for the discharge according to the State and Central Environmental Control Agency. The pH data of the collected wastewater is expressed in Figure 1. It’s important to note that the specific alkaline chemical used, its concentration, and the conditions of its application will determine the effects observed. The application of alkaline chemicals should be carried out with caution and consideration of environmental and safety aspects, as their misuse or mishandling can have adverse consequences. To achieve the ultimate discharge Standards, the effluent should be neutralized with the appropriate acidic materials that aid in subsequent treatment, such as primary, secondary, and tertiary treatment.
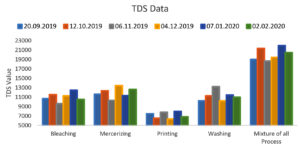
Total Dissolved Solids(TDS) range of collected wastewater from the bleaching process is 9648 to 12460 mg per liter, from mercerizing Process is 10340 to 12645 mg per liter, from the printing process is 6530 to 7820 mg per liter, from the washing process is 10224 to 13232 mg per liter and a mixture of all the process is 18720 to 21989 mg per liter which is much higher than the range specified by the governmental agencies (2100 mg per Liter) which indicates a much higher value than compared to other processing industries. The Data of the TDS is represented in the form of Graph as shown in Figure 2. It might be due to the use of highly salty chemical materials for the fixing of the dyes, bleaching agents, and dyeing agents used in each stage of the printing process29. And so these kinds of wastewater should not suggest for drinking and agricultural purposes. And in further treatment processes like reverse osmosis and all, it can be removed from the water and it can be allowed to use in the agricultural process.
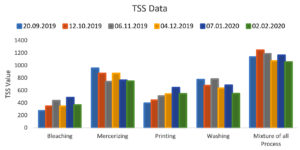
Total Suspended Solids (TSS) range of collected wastewater from the bleaching process is 275 to 487 mg per liter, from mercerizing Process is 742 to 955 mg per liter, from the printing process is 394 to 645 mg per liter, from the washing process is 548 to 783 mg per liter and a mixture of all the process is 1056 to 1189 mg per liter which is much higher than the range specified by the governmental agencies (100 mg per Liter). The Graphical representation of the TSS is presented in Figure 3. As the TSS comes up with the turbidity, it will be going to affect the penetration of the lights in the natural water bodies as well as lower down the dissolved oxygen29. This can be lowered with the help of biological treatments.
The Biological Oxygen Demand (BOD) range of collected wastewater from the bleaching process is 582 to 736 mg per liter, from mercerizing Process is 634 to 842 mg per liter, from the printing process is 432 to 785 mg per liter, from the washing process, is 845 to 986 mg per liter and a mixture of all the process is 1146 to 1840 mg per liter which is much higher than the range specified by the governmental agencies (30 mg per Liter) to discharge for the irrigation purposes after proper treatment. The BOD data of the collected wastewater is mentioned in Figure 4. This is a result of the variety of chemicals employed in the production processes to produce the printed cloth. Limits for further reusing excellent quality water that is determined by the BOD level should be set,30 which can be achieved by aerobic digestion or the anaerobic fermentation process. The biological processes of aerobic digestion31 and anaerobic fermentation can both be utilized to break down organic waste. Their conditions and final goods, though, vary. In conclusion, anaerobic fermentation produces digestate and biogas while aerobic digestion results in the generation of compost. The system’s specific objectives and needs for waste management or energy production determine which process should be used.
The range of chemical oxygen demand (COD) range of the collected effluent from the bleaching process is 1276 to 1576 mg per liter, from the mercerizing Process is 1498 to 1680 mg per liter, from the printing process is 635 to 763 mg per liter and from the washing process is 1246 to 1456 mg per liter and a mixture of all the processes is 3926 to 4449 mg per liter which is much higher than the range given by government agencies (100 mg per liter), and therefore this water is not recommended for drinking or for the agricultural process. The COD Data of the collected wastewater is mentioned in Figure 5. The higher COD value shows the harmful condition of the water;32 therefore, the COD level should be reduced in the further treatment stages with the help of chemicals or biological agents.
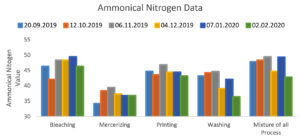
The ammoniacal nitrogen range of collected effluent from the bleaching process is 42.1 to 49.4 mg per liter, from the mercerization Process is 34.2 to 39.4 mg per liter, from the printing process is 43.2 to 46.8 mg per liter, from the washing process is 36.4 to 44.6 mg per liter and a mixture of all the processes is 42.8 to 49.4 mg per liter which is within the range set by the government agencies (50 mg per Liter) but due to other Parameter is not within the specified limits, this water can not be directly discharged into natural water bodies or use for agricultural purposes. Figure 6 represents the data of the ammonical Nitrogen of the selected wastewater. In dyeing and printing operations, auxiliary chemicals like ammonium salts like ammonium sulfate or ammonium chloride are frequently utilized. They may aid in improving the colors’ absorption and attachment onto the fabric fibers. Salts of ammonium function as mordants or dye helpers, enhancing colour fastness and dyeing efficiency.33 It’s important to keep in mind that the results seen will depend on the precise kind and quantity of ammonium salts utilised, as well as the application technique. Ammonium salts or any other compounds must be handled, dosed, and in accordance with all applicable environmental and safety requirements when used in the textile processing industry.34 It can be decreased chemically or biologically through the denitrification process. The biological procedure may be feasible and profitable because chemical treatment will result in an increase in the chemical loads in the effluent.
In order to establish the impact of the textile sector on water pollution, wastewater was gathered and examined. The pH increases up to 13.1, BOD is increasing up to 1840 mg/l, COD is increasing up to 4449 mg/l, TSS increased up to 1189 mg/l, and TDS is increasing up to 21989 mg/l, values of the textile wastewater significantly higher than the recommended maximum value. This is due to the high levels of organic compounds, including dyes, dye intermediates, surfactants, and other organic chemicals used in dyeing, printing, and finishing processes. The study’s findings highlight the current water quality of industrial effluents discharged into surface waters and their potential effects on human health and aquatic life. In order to reduce water pollution and promote ecological and economic development, it is necessary to enforce water quality regulations for businesses installing sewage treatment facilities and to create straightforward, affordable, and environmentally friendly treatment methods for textile effluent remediation.
Recommendations
According to the study conducted, wastewater from the textile industry needs proper treatment before it can be safely discharged into water bodies. The industry should set up its independent wastewater treatment plants (ETP) and keep them running effectively to ensure water quality for future generations. A separate drainage system must be built to prevent industrial effluent from being discharged directly into water bodies. The landfills for industrial and municipal waste should be away from the residential areas of the city. Strict implementation of environmental regulations is necessary. Overall, there is an urgent need to raise public awareness of the sources, extent and prevention of water pollution, and the impact of pollution on human health. The present study provides the basic data and the methodology for the assessment of various physico-chemical properties of textile wastewater. Regular monitoring should be carried out to check the increase in water pollution for sustainable development in the study area.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Stark BL. Long-term economic change: Craft extensification in the Mesoamerican cotton textile industry. J Anthropol. Archaeol. 2020;59:101194.
Crossref - Kumar D, Patel Z, Pandit P, et al. Textile Industry Wastewaters From Jetpur, Gujarat, India, Are Dominated by Shewanellaceae, Bacteroidaceae, and Pseudomonadaceae Harboring Genes Encoding Catalytic Enzymes for Textile Dye Degradation. Front Environ Sci. 2021;9:1-15.
Crossref - Ozdemir O, Armagan B, Turan M, Celik MS. Comparison of the adsorption characteristics of azo-reactive dyes on mezoporous minerals. Dye Pigment. 2004;62(1):49-60.
Crossref - Maqbool Z, Hussainn S, Ahmad T, et al. Use of RSM modeling for optimizing decolorization of simulated textile wastewater by Pseudomonas aeruginosa strain ZM130 capable of simultaneous removal of reactive dyes and hexavalent chromium. Environ Sci Pollut Res. 2016;23(11):11224-11239.
Crossref - Ajao A, Adebayo GB, Yakubu SE. Bioremediation of textile industrial effluent using mixed culture of Pseudomonas aeruginosa and Bacillus subtilis immobilized on agar-agar in a bioreactor. J Microbiol Biotechnol Res Sch Res Libr J Microbiol Biotech Res. 2011;1:50-56.
- Suhartini S, Pangestuti MB, Dewanti BS, Hidayat N. Textile wastewater treatment: Biodegradability on aerobic and anaerobic process. IOP Conf Ser Earth Environ Sci. 2019;230.
Crossref - Modi HA, Rajput G, Ambasana C. Decolorization of water soluble azo dyes by bacterial cultures, isolated from dye house effluent. Bioresour Technol. 2010;101(16):6580-6583.
Crossref - Keharia H, Patel H, Madamwar D. Decolorization screening of synthetic dyes by anaerobic methanogenic sludge using a batch decolorization assay. World J Microbiol Biotechnol. 2004;20:365-370.
Crossref - Hassabo A, Othman H, Ebrahim S. Natural thickener in textile printing (A Mini Review). J Text Color Polym Sci. 2021.
Crossref - Kamaruddin MA, Yusoff MS, Aziz HA, Akinbile CO. Recent Developments of Textile Waste Water Treatment by Adsorption Process: A Review. Int J Sci Res Knowl. 2013;1(4):60-73.
Crossref - Leggett MJ, Mcdonnell G, Denyer SP, Setlow P, Maillard JY. Bacterial spore structures and their protective role in biocide resistance. J Appl Microbiol. 2012;113(3):485-498.
Crossref - Khan S, Malik A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ Sci Pollut Res. 2018;25(5):4446-4458.
Crossref - Babu BR, Parande AK, Raghu S, Kumar TP. Cotton Textile Processing/ : Waste Generation and Effluent Treatment. J Cotton Sci. 2007;11:141-153.
- Madhu A, Chakraborty JN. Developments in application of enzymes for textile processing. Journal of Cleaner Production. 2017; 145: 114-133.
Crossref - Vilaseca M, Gutierrez M-C, Lopez-Grimau V, Lopez-Mesas M, Crespi M. Biological Treatment of a Textile Effluent After Electrochemical Oxidation of Reactive Dyes. Water Environ Res. 2010;82(2):176-182.
Crossref - Carmen Z, Daniel S. Textile Organic Dyes – Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents – A Critical Overview. Org Pollut Ten Years After Stock Conv – Environ Anal Updat. 2012.
Crossref - Deo HT, Chinta SK. Effluent treatment in textile processing: Part I-bleaching of cotton fabric. Indian J Fibre Text Res. 2000;25(1):75-81.
- Wang X, Jiang J, Gao W. Reviewing textile wastewater produced by industries: characteristics, environmental impacts, and treatment strategies. Water Sci Technol. 2022;85(7):2076-2096.
Crossref - Brahma S, Islam MR, Dina RB. Role of Mercerizing Condition on Physical and Dyeing Properties of Cotton Knit Fabric Dyed with Reactive Dyes. Int J Curr Eng Technol. 2018;8(4):152-157.
Crossref - Ammayappan L, Jose S, Raj AA. Sustainable production processes in textile dyeing. Environmental Footprints and Eco-Design of Products and Processes. 2016;185-226.
Crossref - Zeydan M, Toga G. Reducing Variation of Dyeing Process in Textile Manufacturing Industry. Int J Mater Text Eng. 2011;5:2279-2287.
- Mia R, Selim M, Shamim AM, et al. Review on various types of pollution problem in textile dyeing & printing industries of Bangladesh and recommandation for mitigation. J Text Eng Fash Technol. 2019;5(4):220-226.
Crossref - Mittal S, Nagar B, Choudhary M. Characterization of textile industrial waste water in Sanganer Area in Jaipur City. Int J Sci Technol Res. 2020;9(3):5372-5375.
- Wei F, Shahid MJ, Alnusairi GSH, et al. Implementation of floating treatment wetlands for textile wastewater management: A review. Sustainability. 2020;12(14):5801.
Crossref - Ragab MM, Othman HA, Hassabo AG. An Overview of Printing Textile Techniques. Egypt J Chem. 2022;65(8):749-761.
- Bidu JM, van der Bruggen B, Rwiza MJ, Njau KN. Current status of textile wastewater management practices and effluent characteristics in Tanzania. Water Sci Technol. 2021;83(2):2363-2376.
Crossref - Brandi J, Wilson-Wilde L. Standard Methods. Encyclopedia of Forensic Sciences. 2013:522-527.
Crossref - Kolb M, Bahadir M, Teichgraber B. Determination of chemical oxygen demand (COD) using an alternative wet chemical method free of mercury and dichromate. Water Res. 2107;122:645-654.
Crossref - Abu Bakar N, Othman N, Yunus ZM, et al. Physico-Chemical Water Quality Parameters Analysis on Textile. IOP Conf Ser Earth Environ Sci. 2020;498:012077.
Crossref - Tianzhi W, Weijie W, Hongying H, Khu ST. Effect of coagulation on bio-treatment of textile wastewater: Quantitative evaluation and application. J Clean Prod. 2021;312:127798.
Crossref - Yurtsever A, Sahinkaya E, Cinar O. Performance and foulant characteristics of an anaerobic membrane bioreactor treating real textile wastewater. J Water Process Eng. 2020;33:10188.
Crossref - Boguniewicz-Zablocka J, Klosok-Bazan I, Naddeo V, Mozejko CA. Cost-effective removal of COD in the pre-treatment of wastewater from the paper industry. Water Sci Technol. 2020;81(7):1345-1353.
Crossref - Yaseen DA, Scholz M. Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Technol. 2019;16:1193-1226.
Crossref - Pani N, Tejani V, Anantha-Singh TS, Kandya A. Simultaneous removal of COD and Ammoniacal Nitrogen from dye intermediate manufacturing Industrial Wastewater using Fenton oxidation method. Appl. Water Sci. 2020;10:66.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.