ISSN: 0973-7510
E-ISSN: 2581-690X
Cutaneous tuberculosis (CTB) is the rarest case of extrapulmonary TB comprising 2% of total cases. It’s often a challenge both clinically and diagnostically. 1) To determine prevalence, age & gender-wise distribution of CTB. 2) To assess various diagnostic, microbiological modalities for the diagnosis of CTB. 76 skin biopsy specimens from suspected CTB lesions were analysed using following methods – Acid-fast Bacilli (AFB) staining (Ziehl-Neelsen method), growth of mycobacteria in culture (Lowenstein-Jensen media), and Gene Xpert MTB/RIF, Histopathological (H&E staining). Of the 76 specimens, 44 were males and 32 were females. The most commonly affected age group was 40–59 years. Infections were least common in 0-19 years age group. AFB was not seen in any of the primary smears. 10 were confirmed as CTB by the recovery of Mycobacterium in solid culture. Of the 10 culture positives, 9 were confirmed as MTB, and 1 was found to be NTM. Staining of 10 culture positive specimens revealed acid fast, beaded rods. Detection of MTB by Gene Xpert gave positive result in 9 cases with all RIF sensitive. All 9 PCR confirmed cases were also culture positive, all 9 were slow growers with a minimum of 5 weeks required for growth on the LJ slant. PCR is the test of choice and should be performed on all specimens of suspected CTB. However when coupled with the “gold standard” culture method, the diagnostic accuracy improves. Also, further, culture helps in identification and isolation of NTM’s.
Mycobacterium, Cutaneous tuberculosis, Lupus vulgaris, Acid-fast staining, Culture, GeneXpert MTB/RIF
Tuberculosis is one of the oldest of mankind’s enemies. The genus of Mycobacterium could be millions of years old. Mycobacterium tuberculosis emerged as a pathogen of our early ancestors 20,000 to 15,000 years ago in East Africa. As humans peopled the world, they took their diseases along, including tuberculosis. DNA of Mycobacterium tuberculosis has been found in both Egyptian and Peruvian mummies1. The disease (also called consumption) has been recognized in all ages and climates.
Tuberculosis is a major public health issue in terms of morbidity and mortality globally. Over 2 billion people, equal to a 1/4th of the world’s population are infected with TB. 10.4 million new cases were reported in the year 2018, with an estimated 1.5 million deaths each year. 15% of the notified cases are extrapulmonary forms of the disease2. Cutaneous tuberculosis (CTB) is the rarest case of extrapulmonary TB (EPTB) comprising 2% of the total cases of EPTB. Mycobacterium tuberculosis and Mycobacterium bovis are the agents involved in causing CTB. Atypical clinical presentation mimicking other inflammatory and neoplastic conditions makes the diagnosis of EPTB difficult and challenging. Therefore, a sharp clinical sense is required for early diagnosis and mostly, multiple techniques are necessary for the diagnosis.
Traditionally Mycobacterial diagnosis of TB has been done utilizing two cost-effective and reliable techniques, (i) Direct smear microscopy of the specimen (Ziehl-Neelsen and/or Auramine-Rhodamine stain) which although being fast, economical, simple, and mostly specific but has poor sensitivity, and (ii) Culturing of the specimen, which in spite of being considered “gold standard” for diagnosis of TB can take several weeks to give confirmation. Various other microbiological modalities are also used for the diagnosis of EPTB such as tuberculin skin test (Mantoux test), interferon-gamma release assays (QuantiFERON-TB Gold), and newer molecular methods most of which are based on nucleic acid amplification.
Aim & Objectives
- To find out the prevalence of Cutaneous TB among patients presenting with typical skin lesions.
- To find out the age & gender-wise distribution of individuals presenting with typical, suspicious skin lesions
- To assess various diagnostic, microbiological modalities for the diagnosis of Cutaneous TB.
This was a cross-sectional study carried out in the Postgraduate Department of Microbiology, division Mycobacteriology, Government medical college, and associated hospitals Srinagar. 76 skin biopsy specimens from patients with suspected cutaneous tuberculosis (CTB) lesions from April 2018 to December 2019 were analyzed using the following methods – Acid-fast Bacilli (AFB) staining (Ziehl-Neelsen method), growth of Mycobacteria in culture (Lowenstein-Jensen media), and Gene Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) [Table 6], Histopathological report (Hematoxylin and eosin staining) and clinical details were also taken into account.
Skin biopsy specimens
Skin punch biopsies were performed under all aseptic precautions from the cutaneous lesions in clinically suspected individuals for CTB and separated into two portions, one half was sent for histopathological evaluation and the other was used for microbiological processing, and examined microscopically for AFB, solid culture inoculation for isolation of Mycobacteria, and PCR (Xpert MTB/RIF assay).
Histopathological staining; Hematoxylin and eosin (H&E)
A portion of skin biopsy specimen that was sent for histopathology was received and a paraffin section was made. H&E staining was done for demonstration of granuloma. Xylene was used for dewaxing, xylene is then removed using 100% ethanol, and then hydration of section was done so that aqueous reagents penetrate the cells and tissue. Hematoxylin stain was applied to the slide for about 3 minutes, slide was then washed and weak acid alcohol was applied for differentiation. After this treatment, blueing and thorough rinsing was done, the section is now stained with a solution of eosin which acts as a counter stain. The slide was passed through several changes of alcohol to remove all traces of water, and then rinsed in several baths of xylene which “clears” the tissue and renders it completely transparent. Finally, a cover slip was applied and slide was stored for further microscopic examination. On examination, presence or absence of granuloma with or without central caseous necrosis was noted3.
Acid fast staining (Ziehl-Neelsen) and culture (LJ media)
Skin biopsy specimens obtained under aseptic precautions were transported to the Mycobacteriology laboratory inside sterile containers. The samples were broken down into small pieces and subsequently decontaminated and homogenized with sodium hydroxide – N-acetyl-L-cysteine method (NALC – NaOH). The sediment was used for making smears to be stained by Ziehl-Neelsen method for demonstration of AFB and inoculating Lowenstein-Jensen (LJ) egg based solid culture medium, The LJ slants were incubated at 37 C and 25 C and inspected for growth on weekly basis. After 8 weeks of incubation if no growth was noticed, the specimen was reported as culture negative. When a growth was observed on LJ slant, a Ziehl-Neelsen staining was performed on the specimens, and the specimens positive for AFB were reported as culture positive4.
Polymerase chain reaction (GeneXpert MTB/RIF assay)
The GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA), is a latest molecular method based on nucleic acid amplification. It’s a cartridge based test which detects MTB specific region of the rpoB gene using real time PCR (RT-PCR) with molecular beacons. It simultaneously detects Mycobacterium tuberculosis complex (MTB) DNA and resistance to first line anti-TB drug, Rifampicin. The primers incorporated in this assay amplify a small portion of the rpoB gene accommodating the 81 base pair “core” region. The probes can differentiate between the conserved wild‑type sequence and mutations in the core region that are associated with resistance to rifampicin, and it also detects mutations associated with resistance to rifampicin. An amount of 200 μl sediment prepared from skin biopsy specimens after decontamination and homogenization processes was re-suspended in phosphate‑buffered saline (PBS) to a volume of 500 μl. The sample reagent of volume 1.5 ml was then added. Then the mixture was vortexed for 30 seconds to ensure resuspension of all the bacteria. The sample was then left to stand for 15 minutes and intermittent manual shaking was done regular intervals. By using a Pasteur pipette, this solution was then transferred to the Xpert cartridge with all precautions, ensuring no spillage of the potentially highly infectious material, and the cartridge was then loaded onto the available Cepheid GeneXpert module for the run and subsequent analysis. At the end of the cycle (usually under 2 hours) results are available as MTB detected (positive) or not detected (negative). Further positive results are ranked in categories, as: Very low, low, medium, or high. Rifampicin results were reported as not detected (susceptible), detected (resistant) or indeterminate. Results can be had in less than 2 hours5. GeneXpert MTB/RIF has revolutionized the diagnosis and management of MTB infections by providing faster and accurate results by simultaneously detecting MTB complex DNA and RIF resistance. It was endorsed by the World health organization (WHO) in 2010 and is being used in over 130 countries worldwide.
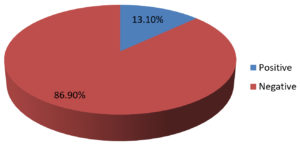
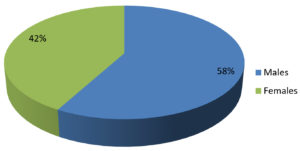
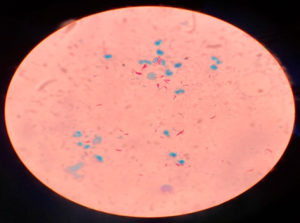
Data of 76 skin biopsy specimens was analyzed in the Mycobacteriology division of postgraduate department of Microbiology. Of the total 76 cases, 44 (58%) were males and 32 (42%) were females [Fig. 2]. The most commonly affected age group was 40–59 years (47.4%) followed by 20–39 years (27.7%). Infections were least common in the 0-19 years of age group (3.9%) [Table 3]. Of the 76 skin biopsy specimens, AFB was not demonstrated in any of the primary smears prepared by Ziehl-Neelsen method. 10 (13.1%) were found confirmed as cases of Cutaneous Tuberculosis (CTB) by the recovery of Mycobacterium in solid culture (LJ media) [Table 1, Fig.1]. Of the 10 culture positive cases, 9 (90%) were confirmed as Mycobacterium tuberculosis, and 1 (10%) was found to be Non-tuberculous mycobacteria (NTM) [Table 2]. Ziehl-Neelsen staining of 10 culture-positive specimens revealed bright red acid-fast, beaded rods (as seen in Image 1). Detection of Mycobacterium tuberculosis by Gene Xpert gave a positive result in 9 (11.8%) cases with all RIF sensitive. All 9 PCR confirmed cases were also culture-positive on LJ media, all 9 were slow growers with a minimum of 5 weeks required for appreciable, visible growth on the LJ slant. The growth on solid LJ media was discrete, raised, irregular, dry, wrinkled, buff to creamy white color colonies of mycobacterium. Clinically, Lupus Vulgaris (n=06) was the most common type, followed by Scrofuloderma (n=03) and TB verrucosa cutis (n=01) [Table 4].
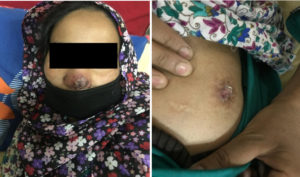
Image 1. 30 yr old female with indurated, crusted plaques with erythematous base present over (a) Nasal bridge, right nasal alae (b) Left gluteal region. Both lesions were non-tender. (Original image)
Cutaneous tuberculosis is an ancient disease. Cutaneous lesions of TB were described long before Robert Koch identified Mycobacterium tuberculosis. The first description of cutaneous TB is attributed to Laennec6 in 1826 who described his own prosector’s wart that followed an injury sustained while carrying out an autopsy on a patient with spinal TB. Mycobacterium tuberculosis was first demonstrated in tissue sections of lupus vulgaris by Demme7 in 1883. In 1886, Reihl and Paltauf8 established that the prosector’s wart was a TB lesion. Apple-jelly nodules in lupus vulgaris were first described in 18889 and tuberculids in 189610.
Table (1):
Confirmed Cutaneous TB positive cases.
Skin biopsy specimens |
N |
% |
|---|---|---|
Positive |
10 |
13.1 |
Negative |
66 |
86.9 |
Total |
76 |
100 |
Cutaneous TB appears to have been frequently encountered all around the world during the early part of this century and comprised 0.1 to 2.6 per cent of the total number of dermatology patients in various hospitals at different periods of time11-17.
In India, Cutaneous TB accounts for 0.11 to 2.5 per cent of all patients with skin diseases seen at hospitals located in different parts and this figure seems to be constant for all regions of the country18-22. In a study from Vishakhapatnam18, cutaneous TB constituted 0.025 per cent of all patients with TB and 15 per cent of all patients with extra-pulmonary TB. One study22 found that cutaneous TB was associated with TB in other organs in 22.1 per cent of patients.
Table (2):
Types of Mycobacterium recovered from positive samples.
Mycobacterium recovered |
N |
% |
|---|---|---|
Mycobacterium Tuberculosis (MTB) |
09 |
90 |
Non tubercular Mycobacterium (NTM) |
01 |
10 |
Total |
10 |
100 |
Cutaneous TB is not a well-defined entity but encompasses an extensive range of clinical manifestations23. Because of this extensive range of manifestations coupled with the rarity of the disease, a high index of clinical suspicion is required to identify skin lesions that could possibly be tubercular in origin and therefore will require Mycobacteriological evaluation. Histopathology many a times shows non-specific inflammation without granuloma formation25. The presentation is determined by the host immune response, the route of inoculation and the previous sensitization of the host to the Mycobacterium tuberculosis24. Cutaneous tuberculosis is classified according to the source of infection25 [Table 5] as; Clinical presentation varies from an ulcer to multiple plaques, papules, pustules, nodules, warts, sinus tracts and soft tissue abscesses (Image 1 showing clinical presentation of a patient in our hospital). Lupus vulgaris is the most commonly reported type from India and globally followed by TB verrucosa cutis and scrofuloderma. In our study, the most common variety was Lupus vulgaris (n=06) followed by scrofuloderma (n=03) and TB verrucosa cutis (n=01) [Table 4]. The other types are distinctly rare. The lesions of Lupus vulgaris routinely appear as “Apple jelly” nodules on diascopy and are most frequently found on the face and neck26.
Table (3):
Age and gender-wise distribution of patients with suspected lesions.
Age group (years) |
Male N (%) |
Female N (%) |
Total N (%) |
|---|---|---|---|
0 – 19 |
01 (1.3) |
02 (2.6) |
03 (3.9) |
20 – 39 |
10 (13.2) |
11 (14.5) |
21 (27.7) |
40 – 59 |
22 (29) |
14 (18.4) |
36 (47.4) |
60 & above |
11 (14.5) |
05 (6.5) |
16 (21) |
Total |
44 (58) |
32 (42) |
76 (100) |
Our study group consisted of skin biopsy specimens diagnosed as Cutaneous TB after microbiological evaluation.
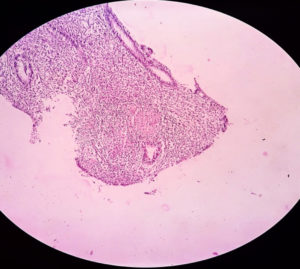
Histopathology of skin biopsy specimens, demonstrates the characteristic features (well-formed granulomas with absence of caseous necrosis, granulomas with caseous necrosis, and the presence of poorly formed granulomas with intense caseous necrosis) [Image 3 showing a granuloma in a H&E stained skin biopsy specimen). Khandka, P et al. in their study, Cutaneous Tuberculosis: Clinicopathologic Arrays and Diagnostic Challenges proposed that, the equivocal manifestation of cutaneous tuberculosis to correlate the histologic with clinical observations in an evidence-based diagnosis is imperfect and lacking pragmatics27.
Table (4):
Clinical variants and Laboratory data of samples.
S. No. |
AFB Staining |
GeneXpert |
Culture growth |
Histopathological evidence |
Clinical type |
|---|---|---|---|---|---|
1 |
Negative |
Positive / RIF sensitive |
Positive |
Granuloma seen |
Lupus Vulgaris |
2 |
Negative |
Positive / RIF sensitive |
Positive |
Granuloma seen |
Lupus Vulgaris |
3 |
Negative |
Positive / RIF sensitive |
Positive |
NIL |
Scrofuloderma |
4 |
Negative |
Negative |
Positive (NTM) |
NIL |
Lupus Vulgaris |
5 |
Negative |
Positive / RIF sensitive |
Positive |
Granuloma seen |
Lupus Vulgaris |
6 |
Negative |
Positive / RIF sensitive |
Positive |
Granuloma seen |
Scrofuloderma |
7 |
Negative |
Positive / RIF sensitive |
Positive |
NIL |
Scrofuloderma |
8 |
Negative |
Positive / RIF sensitive |
Positive |
Granuloma seen |
TB Verrucosa cutis |
9 |
Negative |
Positive / RIF sensitive |
Positive |
NIL |
Lupus Vulgaris |
10 |
Negative |
Positive / RIF sensitive |
Positive |
Granuloma seen |
Lupus Vulgaris |
AFB: Acid fast bacilli
AFB smear microscopy still remains a fast, simple, and cost-effective technique for the early diagnosis and assessment of treatment response in most countries globally. But its limitations remain overall low and variable sensitivity and the inability to differentiate between Mycobacterium tuberculosis and non-tuberculous mycobacteria28. Also, a minimum of 10,000 (104) Bacilli/ml of a specimen are required for the smear to be positive29,30. In our study, none of the smear was positive for AFB.
Table (5):
Classification of cutaneous TB (According to the source of infection).
| A) From exogenous source: 1. Primary inoculation 2. TB chancre 3. TB primary complex 4. TB verrucosa cutis 5. Warty TB 6. Verruca necrogenica 7. Prosector’s wart 8. TB cutis verrucosa |
| B) From endogenous source: 1. Scrofuloderma 2. TB colliquativa cutis 3. Orofacial TB 4. TB cutis oroficialis 5. TB ulcerosa cutis et mucosae |
| C) From hematogenous spread: 1. Lupus vulgaris 2. TB luposa cutis 3. Acute miliary TB of the skin 4. TB cutis miliaris disseminate 5. TB cutis acuta generalisata 6. TB gumma 7. Metastatic tuberculous abscess |
Culturing the specimen for the diagnosis of TB is still considered as a “gold standard” method. It has very high sensitivity and can detect as few as 10 Bacilli/ml of a specimen, making it an excellent modality for the isolation of MTB from paucibacillary specimens, as is the case with cutaneous TB and other EPTB samples. But due to slow the generation time of MTB (15-20 hrs) culturing MTB can take 4-8 weeks to yield results, which not only delays diagnosis but also the treatment in many cases31,32. Khandka, P et al. in their study, observed that, the cultures of the pathogen, Mycobacterium tuberculosis, on specific solid media or by automatic detection of its metabolites in liquid media remain the gold standard method, for identification and their drug sensitivities27. In our study, of the 76 skin biopsy specimens with suspected CTB, 10 (16.6%) were found confirmed as CTB by the recovery of Mycobacterium in solid culture (LJ media). Of the 10 culture-positive cases, 9 (90%) were confirmed as Mycobacterium tuberculosis, and 1 (10%) was found to be Non-tuberculous mycobacteria (NTM).
Table (6):
Different diagnostic modalities used.
Method |
No. of samples Tested |
No. of positive samples |
|---|---|---|
Acid fast staining (Ziehl-Neelsen) |
76 |
Nil |
Culture (L J media) |
76 |
10 |
RT-PCR |
76 |
09 |
Although the standard culture media need a long time for the growth of Mycobacterium tuberculosis, more rapid liquid culture based methods have been developed28. The rapid automated, culture techniques widely used include the radiometric BACTEC 460 TB system and the non-radiometric BACTEC MGIT 960 system which can detect growth in as early as 3–7 days, BacT/ALERT MB which can detect growth as short as 9-14 days33,34.
Conventional techniques for the diagnosis of CTB have various limitations. Difficult clinical diagnosis and a paucibacillary nature of the lesion act as added roadblocks. This has led to discovery and subsequent use of newer molecular techniques for the diagnosis. DNA PCR based techniques have been recommended for the diagnosis of various forms of CTB, but it has a variable sensitivity35. One of them commonly used worldwide, the Gene Xpert MTB/RIF. The GeneXpert MTB/RIF assay is a rapid, fully automated molecular assay with good sensitivity for CTB specimens, this assay has become a popular point of care choice for the early and accurate diagnosis of CTB. The rapidity of results (within 2 hours) combined with good sensitivity for EPTB specimens has led to incorporation of this test into routine diagnostic algorithms in India and world over. The test also provides Rifampicin sensitivity results subsequently. In PCR reactions, discrete fragments of DNA are specifically amplified from specimens in CTB36. As few as 130 Bacilli per ml of specimen can be successfully detected by the advanced molecular technique and results can be had in under 2 hours32. The GeneXpert MTB/RIF assay was applied to all suspected CTB specimens received by our lab in this study.
The GeneXpert MTB/RIF assay detects the MTB complex DNA and RIF resistance. This is achieved by the amplification of the 81 bp fragment of the MTB rpoB gene and subsequent probing of this region for mutations that are associated with resistance to rifampicin. Five probes (A-E) are used for the detection of MTB complex DNA. This assay can be completed in less than 2 hrs. It incorporates a considerable number of sequences of the mycobacterial genome (IS6110, IS986, 65 kDa, and 38 kDa) for detection of MTB complex and the RIF resistance-determining region34.
However even PCR has few limitations, of particular importance is the reduced sensitivity of the assay, as may be seen with inappropriate specimen collection, processing (loss of DNS during extraction), transport and presence of inhibitory substances37.
The performance of the Xpert MTB/RIF assay when compared with the IS6110‑based RT‑PCR assay for MTB detection in extra-pulmonary tuberculosis specimens was found to exhibit better sensitivity38. Tortoli et al. studied the utility of Xpert MTB/RIF assay in 1476 extra-pulmonary tuberculosis specimens and found it 81.3% sensitive and 99.8% specific39. A systematic review reported that Xpert MTB/RIF assay accurately detected the majority of non-respiratory samples testing smear-positive and culture-positive for Mycobacterium tuberculosis, but only two-thirds of smear-negative samples40. Qadri, M et al. in their case report about a culture negative cutaneous TB case observed that, due to the different type of lesions, correct diagnosis may be delayed because investigations fail to detect TB, as in our case where cultures were negative and TB was only detected by GeneXpert41. The ten PCR positive skin biopsy samples in our study were all culture-positive on solid LJ media.
This comprehensive study has given us a clear insight into the pattern and extend of CTB in this region. Our study in all likelihood is the first of its kind from the Kashmir region (Northern India) describing the prevalence, distribution, variants, and diagnostic modalities available for the optimum detection and recovery of the Mycobacterium in all suspected skin lesion samples.
We evaluated various laboratory diagnostic methods for Cutaneous TB and found that PCR is the test of choice that should be performed on all specimens of suspected Cutaneous TB. However, when coupled with the “gold standard” culture method, the diagnostic accuracy improves. Also, further culture helps in the identification and isolation of NTM’s. Apart from being highly sensitive & specific, Gene Xpert MTB/RIF PCR also provides rifampicin sensitivity results which is an important first-line drug in the treatment of Cutaneous TB and any resistance against it means anti – TB drugs other that first line may be needed to successfully treat the condition.
ACKNOWLEDGMENTS
We are highly thankful to the staff at Mycobacteriology division of the department of Microbiology, Government Medical College Srinagar for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors have made a substantial, direct, and intellectual contribution to the research work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This study was approved by the institutional ethical clearance committee.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Daniel TM. Tuberculosis in History: Did It Change the Way We Live? Tuberculosis and Nontuberculous Mycobacterial Infections, D. Schlossberg (7th Ed.). 2017.
Crossref - Global tuberculosis report 2018. Geneva: World Health Organisation; 2018.
- Sampias C, Rolls G. H&E Staining Overview: A Guide to Best Practices. https://www.leicabiosystems.com/knowledge-pathway/he-staining-overview-a-guide-to-best-practices
- RNTCP, Technical and Operational Guidelines for TB Control in India 2016. Annexure 1. p. 134.
- Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6(9):1067-1082.
Crossref - Laennec RTH. Traite de L’auscultation mediate et des maladies des peumons et du coevr, vol 1. Asselin and Cie, Paris, 1962: 1826, pp 649–650.
- Demme, R.: Zur diagnostischen Bedeutung der Tuberkelbacillen fuer das Kindesalter. Ben. ki. Wsch. 1883;20:217.
- Riehl, G., Paltauf, R. Tuberculosis verrucosa cutis. Vierteljahresschrift f. Dermatol. u. Syph.1886;18;19–49.
- Michelson HE. The history of lupus vulgaris. J Invest Dermatol. 1946;7(5):261-267.
Crossref - Darier MJ. Des “tuberculides” cutanees. Arch Dermatol Syph 1896;7:1431–1436
- Horwitz O. Lupus vulgaris cutis in Denmark 1895-1954: its relation to the epidemiology of other forms of tuberculosis. Acta Tuberculosea Scandinavia. 1960;49[Suppl 49]:1-142.
- Forstrom L. Frequency of other types of tuberculosis in patients with tuberculosis of the skin. Scand J Clin Lab Invest (Suppl). 1969; 23: 1-37
- Choudhury AM, Ara S. Cutaneous tuberculosis–a study of 400 cases. Bangladesh Med Res Counc Bull. 2006;32(2):60-65.
- Neves H. Incidence of skin diseases 1952-65. Trans St Johns Hosp Dermatol Soc [London] 1966;52(2):255-271.
- Mitchell PC. Tuberculosis verrucosa cutis among Chinese in Hong Kong. Br J Dermatol. 1954;66(12):444-448.
Crossref - Wong KO, Lee KP, Chiu SF. Tuberculosis of the skin in Hong Kong (A review of 160 cases). Br J Dermatol. 1968;80(7):424-429.
Crossref - Kan KS, Chung TA. A clinical study on skin tuberculosis. Korean J Dermatol, 1977;15:181-189.
- Satyanarayana BV. “Tuberculoderma” a brief review together with statistical analysis and observations. Indian J Dermatol Venereol. 1963;29(1):25-42.
- Ghosh LM. An analysis of 50,000 skin cases seen in the outpatient department of the School of Tropical Medicine, Calcutta, during the 5 years from 1942 to 1946. Ind Med Gaz. 1948;83(11):493-501.
- Mammen A, Thambiah AS. Tuberculosis of the skin. Indian J Dermatol Venereol. 1973;39(4):153-159.
- Sharma RC, Singh R, Bhatia VN. Microbiology of cutaneous tuberculosis. Tubercle. 1975;56(4):324-328.
Crossref - Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty year prospective study. Int J Tuberc Lung Dis. 1999;3(6):494-500.
- Baselga E, Barnadas MA, Margall N, de Moragas JM. Detection of M. tuberculosis complex DNA in a lesion resembling sarcoidosis. Clin Exp Dermatol. 1996;21(3):235-238.
Crossref - Beyt BE Jr, Ortbals DW, Santa Cruz DJ, Kobayashi GS, Eisen AZ, Medoff G. Cutaneous mycobacteriosis: analysis of 34 cases with a new classification of the disease. Medicine (Baltimore). 1981;60(2):95-109.
- Hill MK, Sanders CV. Cutaneous Tuberculosis. Tuberculosis and Nontuberculous Mycobacterial Infections, D. Schlossberg (7th Ed.). 2017.
Crossref - Raman M. Cutaneous tuberculosis. Chapter 25. In Tuberculosis. Sharma SK, Mohan A editors. Jaypee Brothers Medical Publishers New Delhi, 2nd ed. 2009;384-396.
- Khadka P, Koirala S, Thapaliya J. Cutaneous Tuberculosis: Clinicopathologic Arrays and Diagnostic Challenges. Dermatol Res Pract. 2018; 2018:7201973.
Crossref - Haldar S, Bose M, Chakrabarti P, et al. Improved laboratory diagnosis of tuberculosis — The Indian experience. Tuberculosis (Edinb) 2011;91(5):414-426.
Crossref - Yeager H Jr, Lacy J, Smith LR, LeMaistre CA. Quantitative studies of mycobacterial populations in sputum and saliva. Am Rev Respir Dis. 1967;95(6):998-1004.
Crossref - Ulrichs T, Lefmann M, Reich M, et al. Modified immunohistological staining allows detection of Ziehl Neelsen negative Mycobacterium tuberculosis organisms and their precise localization in human tissue. J Pathol. 2005;205(5):633-640.
Crossref - Eichbaum Q, Rubin EJ. Tuberculosis. Advances in laboratory diagnosis and drug susceptibility testing. Pathology Patterns Review. 2002;118(Suppl 1):S3 17.
Crossref - Caminero Luna JA, Casal Roman M, Ausina Ruiz V, Pina Gutieerrez JM, Sauret Valet J. Tuberculosis diagnosis. Arch Bronconeumol. 1996;32:85-99.
Crossref - Cruciani M, Scarparo C, Malena M, Bosco O, Serpelloni G, Mengoli C. Meta analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of mycobacteria. J Clin Microbiol. 2004;42(5):2321-2325.
Crossref - Almaguer Chavez J, Ocampo Candiani J, Rendon A. Current panorama in the diagnosis of cutaneous tuberculosis. Actas Dermosifiliogr. 2009;100(7):562-570.
Crossref - Suthar C, Rana T, Singh UB, et al. mRNA and DNA PCR tests in cutaneous tuberculosis. Indian J Dermatol Venereol Leprol. 2013;79(1):65-69.
Crossref - Wei CY, Lee CN, Chu CH, Hwang JJ, Lee CP. Determination of the sensitivity and specificity of PCR assays using different target dnas for the detection of Mycobacterium tuberculosis. Kaohsiung J Med Sci. 1999;15(7):396-405.
- Tan SH, Tan BH, Goh CL, et al. Detection of Mycobacterium tuberculosis DNA using polymerase chain reaction in cutaneous tuberculosis and tuberculids. Int J Dermatol. 1999;38(2):122-127.
Crossref - Miller MB, Popowitch EB, Backlund MG, Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real time PCR for Mycobacterium tuberculosis detection in clinical samples. J Clin Microbiol. 2011;49(10):3458-3462.
Crossref - Tortoli E, Russo C, Piersimoni C, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012;40(2):442-447.
Crossref - Maynard Smith L, Larke N, Peters JA, Lawn SD. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non respiratory samples: A systematic review. BMC Infect Dis. 2014;14:709.
Crossref - Qadri M, Sheikh QH, Talpur MTH et al. Case Report: Culture negative cutaneous tuberculosis. F1000Research. 2019;8:509.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.