ISSN: 0973-7510
E-ISSN: 2581-690X
Today world is trying to cope with the biggest pandemic caused by Coronavirus disease 2019 (COVID-19). The disease is graded as mild, moderate, serious and critical illness. Very few studies are done with methemoglobin along with other parameters for the assessment of the severity of COVID-19 disease. The objectives of the study were to estimate methemoglobin (Met-Hb), hemoglobin (Hb), ferritin and lactate dehydrogenase (LDH) levels in patients with COVID-19 disease and to investigate the interaction between these parameters and the severity of the disease. This observational study was conducted in three groups of COVID-19 patients- moderate, severe and critical, each group containing 30 patients, between June 2021 and September 2021 in the biochemistry department of a tertiary care hospital. For all patients, Met-Hb, Hb, ferritin, and LDH levels were estimated on the 2nd-3rd day of hospital admission. Patients in the critical group were older and had significantly high values of Met-Hb, ferritin and LDH and significantly low values of Hb (P<0.05). In multivariate ordinal regression analysis, older age (OR-3.08; 95%CI:1.19-7.19;P-0.019), higher values of LDH (OR-8.66; 95%CI:2.53-29.5; P-0.001) and ferritin (OR-3.08; 95%CI:1.09-8.7;P-0.033) were independently associated with severity of the disease. A cut-off value of 410.50 U/L for LDH predicted the severity of the disease with 90% sensitivity and 88.3% specificity. In conclusion, higher levels of LDH and ferritin were related to the severity of the disease in COVID-19 cases. Although Met-Hb showed a minimal increase without any association with severity, it may be an underlying cause of hypoxia that may go unnoticed. So, monitoring of all these parameters should be done at intervals.
COVID-19, Methemoglobin (Met-Hb), Hemoglobin(Hb), Ferritin, Lactate Dehydrogenase (LDH)
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has given rise to highly contagious Coronavirus disease (COVID-19), with hypoxia as one of the main complications. The disease is graded as mild, moderate, serious and critical illness.1 Majority of the patients affected by the virus show the respiratory disease of mild to moderate variety and does not need any specific treatment. However, hospitalization is needed in some seriously ill patients. Serious illness is most likely to occur in old persons and having previous as well as unrevealed medical conditions like diabetes mellitus, cardiovascular disease, chronic respiratory disease or cancer etc.2
Today COVID-19 is a concern to the health and well-being of people worldwide. The cardinal findings in the report by the Chinese Center for Disease Control and Prevention were as follows: the mild disease was present in 81% of patients. Hospitalization was needed in 14% with severe disease. Intensive care admission was needed in 5% with very severe conditions mainly for ventilator support. Mortality was seen in 4% of the diseased patients.3
Methemoglobin (Met-Hb) is one of the important derivatives of hemoglobin. It forms when iron in hemoglobin gets oxidized from ferrous (Fe2+) to the ferric (Fe3+) state. Met-Hb is produced by free radicals, some oxidizing drugs or chemicals. In the study by T Schuerholz et al. raised Met-Hb level was shown to be related to the severity of sepsis.4 Alamdari et al. detected higher Met-Hb levels in patients with COVID-19 disease than in healthy individuals.5
Human body stores iron in the form of ferritin. Also, in acute and chronic inflammatory processes ferritin is increased as an acute-phase protein and used as a prognostic marker.6 According to Gomez-Pastora et al. high ferritin levels in SARS-CoV-2 infection suggest an inflammatory condition. The magnitude of the cytokine storm and severity of COVID-19 disease can be best predicted by ferritin.7
In tissue injury, necrosis, hemolysis, hypoxia, or malignancies elevated levels of serum LDH has been seen.8 In severe infections, cytokine-mediated tissue damage may occur with the release of LDH.9 A pooled analysis by Brandon Michael Henry et al.10 comparing severe and non-severe groups discovered that odds of developing severe disease increase by 6-fold with an increase in LDH levels.
Does methemoglobin play any role in the COVID-19 disease process? Does it have any correlation with hemoglobin, ferritin and lactate dehydrogenase? Very few studies are done with methemoglobin along with other parameters for the assessment of the severity of COVID-19 disease. To find out the answers, in this study we hypothesized that methemoglobin was associated with hemoglobin, ferritin and lactate dehydrogenase in COVID-19 patients as it is part of hemoglobin.
This observational study was conducted on COVID-19 patients admitted to Krishna Hospital and Medical Research Center, Karad, Maharashtra from June 2021 to September 2021. Ethical clearance was taken from Institutional Ethics Committee, KIMSDU, Karad, Maharashtra, India [Ref. No. KIMSDU/IFC/04/2020 Protocol no. 424/2019-2020].
Participants
The sample size was calculated as follows: To obtain a mean difference in LDH level of 147.1 U/L (408.4± 175.3U/L vs.261.3 ±100.3U/L) as values taken from a study by Begum Okem et al.11 among COVID-19 patients with and without the need for intensive care with 10% permissible error, 95% confidence interval and 80% power; sample size calculated as minimum 20 in each group. The formula used was:
n= (SD12 + SD22) (Z 1-α/2 + Z 1-β )2 /d2
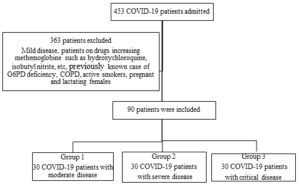
The Covid-19 patients were diagnosed by reverse transcription-polymerase chain reaction (RT-PCR) or clinical and high-resolution computed tomography (HR-CT) findings and patients admitted to the hospital with age >30 years were included in the study. Group 1 included 30 COVID-19 patients with moderate disease (adolescent or adult with clinical signs of pneumonia (fever, cough, dyspnoea, fast breathing) but without signs of severe pneumonia, including SpO2 ≥ 90% on room air).1 Group 2 included 30 COVID-19 patients with severe disease (a person with clinical signs of pneumonia with one of the following: respiratory rate> 30breaths /min; severe respiratory distress; or SpO2 < 90% on room air).1 Group 3 included 30 COVID-19 patients with the critical disease having the following: a person with acute respiratory distress syndrome with oxygen impairment {Mild ARDS: 200mmHg < PaO2/FiO2 ≤ 300 mmHg (with PEEP or CPAP ≥ 5cmH2O). Moderate ARDS: 100mmHg < PaO2/FiO2 ≤ 200 mmHg (with PEEP or CPAP ≥ 5cmH2O). Severe ARDS: 200mmHg < PaO2/FiO2 ≤ 100 mmHg (with PEEP or CPAP ≥ 5cmH2O)} with sepsis, septic shock or acute thrombosis.1
Following patients were excluded: COVID-19 patients with mild disease, patients on drugs causing methemoglobinemia such as hydroxychloroquine, chloroquine, isobutyl nitrite, sodium nitrate, sodium valproate, etc, patients on treatment with immunosuppressive agents and previously known case of glucose 6 phosphate dehydrogenase (G6PD) deficiency, severe renal failure, cirrhosis, hepatitis, COPD, active smokers, pregnant and lactating females, etc.
For the selection of patients, a purposive sampling method was used. According to inclusion & exclusion criteria, patients were selected. After explaining the study to every patient, informed written consent was taken. Demographic data, history and clinical data from the patient’s file were filled in a proforma for every patient.
Biochemical investigation
From each patient 4 ml venous blood sample was collected. In the EDTA vacutainer, 1ml blood was collected and in the plain vacutainer, 3ml blood was collected on the 2nd or 3rd day of admission to the hospital. Blood processing was done in the Biochemistry laboratory of KIMSU, Karad. The centrifuge was used for serum separation. Serum LDH in Units per liter (U/L)estimation was done on Erba 360 auto analyzer by Deutsche Gesellschaft Fur Klinische Chemie (DGKCH) method (reference range- 200-400U/L).12 The kit contains LDH-P reagent which uses pyruvate. Serum ferritin in nanogram per milliliter (ng/ml) estimated by two sites immunoenzymometric assay on Tosoh AIA System analyser (reference range 20-274 ng/ml).13 Met-Hb in percentage (%) estimated by the method described by Sato on Spectronic 20 spectrophotometer (reference range- 0-1.5%). 14Hb in grams per deciliter (gm/dl) was measured by an automated hematology analyzer by spectrophotometry (reference range-12-15gm/dl).
Statistical analysis
Variables were expressed as numbers (percentages) or as the mean ± standard deviation (SD). With the Kolmogorov Smirnov test, the normal distribution of the data was assessed. Categorical variables were analyzed by the chi-square significance test. Continuous variables were evaluated by Kruskal Wallis Test. Correlation analysis was done with the Spearman correlation test and was expressed as Spearman’s correlation coefficient. For cut-off points for laboratory data, we used laboratory reference levels. Not all cut-off points were consistent with cut-off values of ROC analysis. The ordinal regression model was fit for severity of COVID-19 disease as dependant variable and all significant demographic and laboratory variables were included as independent variables. Laboratory variables were converted into dichotomous variables based on cut-off values. To assess the cut-off value of LDH, Ferritin, Met-Hb and Hb for severity, a receiver operating characteristic (ROC) analysis was conducted. IBM SPSS Statistics, version 20 was used for data analysis. P-value ≤ 0.05 was considered statistically significant.
As per the inclusion and exclusion criteria in the study, out of 453 patients diagnosed with COVID-19 disease admitted to the hospital 90 patients were studied from June 2021 to September 2021 (Figure 1).
General characteristics
On analyzing the general characteristics of the COVID-19 patients (Table 1), gender-wise three groups were similar without significant differences. The patients with the critical disease were significantly older (64.70 ± 14.33years), had a significantly high frequency of comorbidities (96.7%) and duration of hospitalization(18.36 ± 13.06 days) with low survival (26.7%).
Table (1):
General characteristics of the COVID-19 patients.
Characteristics |
Group 1 a (n-30) |
Group 2 b (n-30) |
Group 3 c (n-30) |
P-value |
|---|---|---|---|---|
Gender |
||||
Male |
15(50%) |
20(66.7%) |
13(43.3%) |
0.175 |
Female |
15(50%) |
10(33.3%) |
17(56.7%) |
|
Age (years, mean ± SD) |
43.13 ± 9.32* |
56.90 ± 11.73** |
64.70 ± 14.33*** |
0.000 * |
≤ 55 |
25(83.3%) |
14(46.7%) |
8(26.7%) |
0.000 * |
>55 |
5(16.7%) |
16(53.3%) |
22(73.3%) |
|
Comorbidity |
||||
Yes |
12(40%) |
24(80%) |
29(96.7) |
0.001* |
No |
18(60%) |
6( 20) |
1(3.3%) |
|
Duration of hospitalization (days, mean ± SD) |
11.20 ± 2.07* |
15.53 ± 6.75 |
18.36 ± 13.06*** |
0.009 * |
Survived |
||||
Yes |
30 (100.0%) |
28 (93.3%) |
8 (26.7%) |
0.000 * |
No |
0(0%) |
2(6.7%) |
22(73.3%) |
COVID-19: Coronavirus disease-19
aCOVID-19 patients with moderate disease, b COVID-19 patients with severe disease,
c COVID-19 patients with critical disease.
*P<0.05.
*Significance of difference between group I and group II: P<0.05
**Significance of difference between group II and group III: P<0.05
***Significance of difference between group I and group III: P<0.05
Laboratory findings
As per the laboratory findings (Table 2), patients in the critical group had significantly higher Met-Hb (1.60 ± 0.51%), LDH (736.7 ± 248.45 U/L) and ferritin (556.66 ± 325.88 ng/ml) levels and statistically significantly lower Hb (8.70 ± 2.95gm/dl) levels as compared to moderate group (P=0.000). The critical group had a significantly high frequency of cases with low levels of Hb <12gm/dl, high levels of Met-Hb>1.5%, LDH >400U/L and ferritin >274ng/ml.
Table (2):
Laboratory findings in the COVID-19 patients.
Characteristics |
Group 1 a (n-30) |
Group 2 b (n-30) |
Group 3 c (n-30) |
P-value |
|---|---|---|---|---|
Hb (gm/dl) |
12.39 ± 1.76* |
10.83 ± 2.46** |
8.70 ± 2.95*** |
0.001* |
≥12 |
18(60.0%) |
11(36.7%) |
4(13.3%) |
0.001* |
<12 |
12(40.0%) |
19(63.3%) |
26(86.7%) |
|
Met-Hb(%) |
1.09 ± 0.20* |
1.49 ± 0.35 |
1.60 ± 0.51*** |
0.000* |
≤1.5 |
29(96.7%) |
14(46.7%) |
13(43.3%) |
0.000* |
>1.5 |
1(3.3%) |
16(53.3%) |
17(56.7%) |
|
LDH(U/L) |
307.8 ± 108.97* |
598.3 ± 202.71** |
736.7 ± 248.45*** |
0.000* |
≤400 |
27(90.0%) |
7(23.3%) |
4(13.3%) |
0.000* |
>400 |
3(10.0%) |
23(76.7%) |
26(86.7%) |
|
Ferritin (ng/ml) |
162.97 ± 133.8* |
436.02 ± 346.83 |
556.66 ± 325.88*** |
0.000* |
≤274 |
22(73.3%) |
11(36.7%) |
6(20.0%) |
0.000* |
>274 |
8(26.7%) |
19(63.3%) |
24(80.0%) |
COVID-19: Coronavirus disease-19
aCOVID-19 patients with moderate disease, b COVID-19 patients with severe disease,
c COVID-19 patients with critical disease.
*P<0.05.
*Significance of difference between group I and group II: P<0.05
**Significance of difference between group II and group III: P<0.05
***Significance of difference between group I and group III: P<0.05
Correlation analysis
Correlation analysis in COVID-19 patients is detailed in Table 3. In the moderate group, no significant correlation was found between the parameters. In the severe and critical group, a significant strong positive correlation between Met-Hb and LDH (ρ = 0.805) (P-0.000) & ( ρ =0.698)(P-0.002)was found. Between Met-Hb and ferritin, a strong positive correlation (ρ =0.612) (P-0.000) & (p =0.619)(P-0.001) was found. Also, a significant negative correlation between Met-Hb and Hb (p = -0.372) ((P-0.043) & (p = -0.609)(P-0.001) was found in these two groups.
Table (3):
Correlation analysis in COVID-19 patients.
Group 1 a (n-30) |
Group 2 b (n-30) |
Group 3 c (n-30) |
||
|---|---|---|---|---|
Met-Hb with LDH |
Spearman’s ρ (rho) |
0.121 |
0.805 |
0.698 |
P- value |
0.523 |
0.000* |
0.000* |
|
Met-Hb with ferritin |
Spearman’s ρ(rho) |
0.323 |
0.612 |
0.619 |
P– value |
0.082 |
0.000* |
0.000* |
|
Met-Hb with Hb |
Spearman’s ρ(rho) |
0.022 |
-0.372 |
-0.609 |
P– value |
0.906 |
0.043* |
0.000* |
COVID-19: Coronavirus disease-19
aCOVID-19 patients with moderate disease, b COVID-19 patients with severe disease,
c COVID-19 patients with critical disease.
*P<0.05.
Receiver operating characteristic (ROC) curve analysis
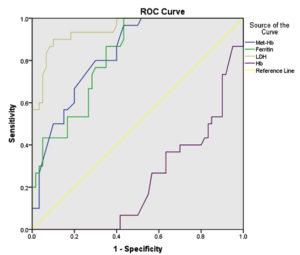
ROC analysis for the severity of COVID-19 disease is detailed in Table 4. In the ROC analysis, LDH was identified as the best predictive marker of severity of COVID-19 disease than ferritin, Met-Hb and Hb. Serum LDH level >410.50U/L showed the best combination of sensitivity (90%) and specificity (88.3%) with AUC-0.948 (Figure 2). It had a 76% positive predictive value and 94% negative predictive value. Also, a positive likelihood ratio (LR) of 7.69 for LDH indicated a 7.69 times increase in the odds of having the severe disease in the patient with LDH levels >410.50U/L. Ferritin level >331.50 ng/ml had sensitivity-86.7%, specificity-60 % and AUC-0.818 which is inferior to LDH. Other parameters had low positive LR so small probability of having severe disease, given a positive test.
Table (4):
Receiver operating characteristic (ROC) curve analysis.
| Parameter | AUC | P value | Optimal cut-off value | Sensitivity (%) | Specificity (%) | PPV | NPV | Likelihood ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| Met-Hb% | 0.831 | 0.000* | 1.30 | 80 | 70 | 60 | 89 | 2.66 | 0.28 |
| Ferritin ng/ml | 0.818 | 0.000* | 331.50 | 86.7 | 60 | 56 | 87 | 2.16 | 0.22 |
| LDH U/L | 0.948 | 0.000* | 410.50 | 90 | 88.3 | 76 | 94 | 7.69 | 0.11 |
| Hb gm/dl | 0.771 | 0.000* | 10.45 | 73.3 | 58.3 | 54 | 78 | 1.75 | 0.45 |
AUC- Area under the curve
PPV- Positive predictive value
NPV- Negative predictive value
*P < 0.05
Figure 2. Receiver operating characteristic (ROC) curves of LDH, Ferritin, Hb and Met-Hb for predicting the disease severity in COVID-19 patients.
Ordinal regression analysis of variables and severity of COVID-19
Multivariate ordinal regression analysis showed severity of COVID-19 disease was related with age > 55 years (OR: 3.080; 95% CI: 1.19–7.19; P – 0.019), Ferritin >274ng/ml (OR: 3.088; 95% CI: 1.09–8.7; P – 0.033) and LDH>400U/L(OR: 8.662; 95% CI: 2.53-29.5; P – 0.001) as shown in Table 5.
Table (5):
Ordinal regression analysis of variables and severity of COVID-19.
| Factors | Multivariate analysis | ||
|---|---|---|---|
| Odds ratio (OR) |
95% Confidence interval (CI) | P value | |
| Gender | |||
| Male | Reference | ||
| Female | 1.503 | (0.50- 4.5) | 0.468 |
| Age | |||
| ≤55 years | Reference | ||
| >55 years | 3.080 | (1.19-7.19) | 0.019* |
| Met-Hb % | |||
| ≤1.5 | Reference | ||
| >1.5 | 1.076 | (0.33-3.46) | 0.902 |
| Ferritin (ng/ml) | |||
| ≤274 | Reference | ||
| >274 | 3.088 | (1.09- 8.7) | 0.033* |
| LDH (U/L) | |||
| ≤400 | Reference | ||
| >400 | 8.662 | (2.53-29.5) | 0.001* |
| Hb (gm/dl) | |||
| ≥12 | Reference | ||
| <12 | 2.190 | (0.63-7.55) | 0.215 |
*P < 0.05
Research is going on about the diagnosis, treatment and prevention of COVID-19 which is a global issue. Identifying factors related to the severity of the disease may help in the early detection of at-risk patients. Also, some of the factors can be modified by therapeutic measures. In this observational study, 90 COVID-19 patients were studied with differences in severity. The general characteristics and laboratory findings were compared within the groups. Correlation, ROC and multivariate ordinal regression analysis were done.
Patients in the critical group in our study were significantly older age without the gender difference, with a significantly higher frequency of comorbidities and low survival. With multivariate ordinal regression analysis, older age (>55 years) was significantly related to the severity of COVID-19 disease. A systemic review by Ana Karla G et al.15 reported that older age, male patients and associated comorbidities were probable threats leading to severe cases. This may be related to low immunity in older age that further declines with associated comorbid conditions.
The two meta-analyses demonstrated that low Hb was associated with disease severity in COVID-19.16,17 Lower Hb levels may be due to coagulation abnormality, underlying medical condition or anemia related to an infection and/or a systemic inflammatory reaction.18,19 Probable mechanisms increasing the risk of severity in COVID-19 infection with low Hb are- i) decreased oxygen delivery to organs and tissues aggravating hypoxemia,20 ii) interaction of SARS-CoV-2 virus with Hb causing hemolysis,21 iii) increased oxidative stress due to hepcidin- mimetic action of SARS-coV-2.21
On the other hand, the meta-analysis by Ana Karla G et al.15 did not find any relation between low Hb levels and severity in COVID- 19 patients. In the present study, Hb was significantly low in the critical group, but on multivariate ordinal regression analysis low Hb was not significantly related to the severity of the disease.
The reference range of Met-Hb is 0.67±0.33% for healthy non-smoker individuals.22 Alamdari et al. found higher Met-Hb levels in COVID-19 patients as compared to healthy individuals.5 Another study by Begum Oktem et al. showed no statistically significant increase in Met-Hb levels in both the groups requiring intensive care and in the cases with the mortal course.11
Alunno A et al. suggested that activation of macrophages was caused by the SARS-CoV-2 infection generating a large number of inflammatory molecules in COVID-19 patients leading to a cytokine storm.23 Also, it is known that upon macrophage activation overproduction of reactive oxygen species (ROS) occurs24 leading to Oxidative stress. Oxidative stress causes increased oxidation of iron in hemoglobin from the ferrous (Fe2+) to the ferric form (Fe3+) i.e., increased methemoglobin formation.25 The oxidation decreases the capability of hemoglobin to carry oxygen26 and release oxygen to tissues leading to tissue hypoxia. The availability of Hb for oxygen binding and transport decreases with the production of Met-Hb. So, one of the probable reasons for hypoxia in COVID-19 patients is increased methemoglobin.26 Also, in some viral infections increase in methemoglobin has been noticed.27
The present study showed that Met-Hb is significantly high in the critical group but on multivariate ordinal regression analysis, high Met-Hb was not related to the severity of the disease. Felix S et al. suggested that in COVID-19 patients, Met-Hb appears to be increased with the disease progression.28 So, Met-Hb may not be necessarily increased when the Met-Hb samples were taken at the beginning of the disease as in the present study and study by Begum Oktem et al.11
In acute anemia, there is an increase in nitric oxide (NO) signalling along with an increase in MetHb as a by-product due to increase NO-based oxidation of Hb to MetHb.29 This can explain an increase in Met-Hb with a decrease in Hb in severe COVID-19 disease as shown in our study.
In the present study, ROC analysis confirmed LDH > 410 U/L was more likely (+LR 7.69) to develop the severe form of the disease. This suggests LDH is a valuable predictor of the severity of COVID-19 disease. Similarly, a 6 times increase in odds of developing the severe disease with increased LDH levels was reported by Brandon Michael Henry et al.10 Szarpak L et al.30 did a meta-analysis with 28 studies that showed significantly high LDH in the severe group as compared to the non-severe group. This suggested that LDH could be used as a marker for severity and survival in COVID-19 disease. In COVID-19 disease, an increase in LDH levels may be due to the release of isozyme LDH 3 present in lung tissue after developing a severe form of interstitial pneumonia due to cytokine storm, 10 or maybe due to multiple organ injuries including heart,31 liver and kidney.32
A systemic review by Ana Karla G. Melo et al.15 reported that during the first days of COVID-19 illness, ferritin and LDH were good indicators of both severity and survival. A systemic review by Linlin Cheng 1 et al.33 found higher ferritin levels in severe disease compared to less severe COVID-19 disease. In the study by Hou et al., ferritin was selected as a predictive marker of severe COVID-19 by multivariate logistic regression analysis and the AUC to differentiate critical from the mild patient was 0.812.34 In the present study, ferritin was significantly high in the critical group. The multivariate ordinal regression analysis showed high ferritin (>278ng/ml) was significantly related to the severity of COVID-19 disease. Also, on ROC analysis ferritin >331 ng/ml is the second-best predictor of the severity of COVID-19 disease with AUC was 0.818 and + LR was 2.16.
A systematic review by Pastora J et al.7 found elevated ferritin levels (ferritin>400ng/ml) about 1.5 to 5.3 times higher in patients with severe disease on admission. One study suggested that those macrophages which generate cytokines might be accountable for the production of serum ferritin.35 Collected data have reported the function of ferritin as a signaling molecule and direct facilitator of the immune system.35 Elevated ferritin levels may play an important role by participating in cytokine storm causing pulmonary edema and ARDS.36
Our study demonstrated a significant positive correlation between Met-Hb, LDH and Ferritin. However, by applying multivariate ordinal regression analysis, only older age (>55 years), high LDH (>400U/L) and high ferritin (>278ng/ml) were significantly related to the severity of COVID-19 disease. This indicates that Met-Hb is increased in severe and critical patients and although not alarming, yet might have an additive effect on the pathogenesis of the disease.
Limitations of the study
This study evaluated parameters in the blood drawn on the 2nd -3rd day of hospitalization, we did not evaluate the effect of serial levels over the period and how alterations in levels of parameters could forecast the outcomes. Another limitation of the study was a smaller number of patients which may have affected the statistical significance of our findings.
Even though Met-Hb, Hb, ferritin and LDH levels were significantly different between the disease groups, only high LDH and high ferritin levels were significantly associated with the severity of COVID-19 disease. LDH and ferritin levels may play a role in predicting COVID-19 severity. Although Met-Hb showed a minimal increase without any association with severity, it may be an underlying cause of hypoxia that may go unnoticed. So, monitoring of all these parameters should be done at intervals. To confirm our results, further studies with a large study population are needed.
ACKNOWLEDGMENTS
The authors would like to thank authorities of Krishna Hospital and KIMSDU, Karad for encouraging and supporting the research. Also thanks to all patients for participation in the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
VSP, AS and VR contributed in research design. VSP and DAJ contributed in the performance of the research. VSP and SP performed data analysis. VSP, AS and SP wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was funded by Krishna Institute of Medical Sciences, “Deemed to be University”, Karad, Maharashtra, India with Reference number KIMSDU/DR/ER/96/2020.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee, KIMSDU, Karad, Maharashtra, India with Reference Number KIMSDU/IFC/04/2020, Protocol no. 424/2019-2020.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- World Health Organization. COVID-19 clinical management: living guidance, 25 January 2021. World Health Organization. 2021. https://apps.who.int/iris/handle/10665/338882
- Health topic – Coronavirus disease (COVID-19). https://www.who.int / Health topic / Coronavirus disease (COVID-19). Accessed: 01 January 2022
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239-1242.
Crossref - Schuerholz T, Irmer J, Simon TP, Reinhart K, Marx G. Methemoglobin level as an indicator for disease severity in sepsis. Critical Care. 2008 Apr;12(2):1-1.
- Alamdari DH, Moghaddam AB, Amini S, et al. Application of methylene blue-vitamin C-N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur J Pharmacol. 2020;885:173494.
Crossref - Ahmed S, Ahmed ZA, Siddiqui I, Rashid NH, Mansoor M, Jafri L. Evaluation of serum ferritin for prediction of severity and mortality in COVID-19-A cross-sectional study. Ann Med Surg. 2021;63:102163.
Crossref - Gomez-Pastora J, Weigand M, Kim J, et al. Hyperferritinmia in critically ill COVID-19 patients-is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249.
Crossref - Duman A, Akoz A, Kapci M, et al. Prognostic value of neglected biomarker in sepsis patients with the old and new criteria: predictive role of lactate dehydrogenase. Am J Emerg Med. 2016;34(11):2167-2171.
Crossref - Martinez-Outschoorn UE, Prisco M, Ertel A, et al. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell Cycle. 2011;10(8):1271-1286.
Crossref - Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722-1726.
Crossref - Oktem B, Uzer F, Guven FM, Guven FMK, Topal M. Role of methemoglobin and carboxyhemoglobin levels in predicting COVID-19 prognosis: an observational study. Med Gas Res. 2020;10(4):174-178.
Crossref - Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostics, 5th edition. Elsevier Health Sciences; 2012.
- ST AIA-PACK FER – Tosoh Bioscience. Available at: https://www.diagnostics.eu.tosohbioscience.com›IFU PDF. Accessed: 6 May 2021
- Sato K. Methemoglobin. In Drugs and Poisons in Humans 2005 (pp. 655-657). Springer, Berlin, Heidelberg.
- Melo AK, Milby KM, Caparroz AL, et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PloS One. 2021;16(6):e0253894.
Crossref - Alnor A, Sandberg MB, Gils C, Vinholt PJ. Laboratory tests and outcome for patients with coronavirus disease 2019: a systematic review and meta-analysis. J Appl Lab Med. 2020;5(5):1038-1049.
Crossref - Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42(2):116-117.
Crossref - Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis. 1997;25(4):888-895.
Crossref - Ganz T. Anemia of inflammation. N Eng J Med. 2019;381(12):1148-1157.
Crossref - Hemauer S, Kingeter AJ, Han X, et al. Daily lowest hemoglobin and risk of organ dysfunctions in critically ill patients. Crit Care Med. 2017;45(5):e479.
Crossref - Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clinics and Practice. 2020;10(2):1271.
Crossref - Borland C, Harmes K, Cracknell N, Mack D, Higenbottam T. Methemoglobin Levels in Smokers and Non-Smokers. Arch Environ Health. 1985;40(6):330-333.
Crossref - Alunno A, Carubbi F, Rodriguez-Carrio J. Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin. RMD Open. 2020;6(1):e001295.
Crossref - Shehat MG, Tigno-Aranjuez J. Flow cytometric measurement of ROS production in macrophages in response to FcγR cross-linking. J Vis Exp. 2019;145:e59167.
Crossref - Gutmann C, Siow R, Gwozdz AM, Saha P, Smith A. Reactive oxygen species in venous thrombosis. Int J Mol Sci. 2020;21(6):1918.
Crossref - Alamdari DH, Moghaddam AB, Amini S, Alamdari AH, Damsaz M, Yarahmadi A. The application of a reduced dye used in orthopedics as a novel treatment against coronavirus (COVID-19): a suggested therapeutic protocol. Arch Bone Jt Surg. 2020;8(suppl1):291.
- Au WY, Ngai CW, Chan WM, Leung RY, Chan SC. Hemolysis and methemoglobinemia due to hepatitis E virus infection in patients with G6PD deficiency. Ann Hematol. 2011;90(10):1237-1238.
Crossref - Scholkmann F, Restin T, Ferrari M, Quaresima V. The Role of Methemoglobin and Carboxyhemoglobin in COVID-19: A Review. J Clin Med. 2021;10(1):50.
Crossref - Hare GM, Tsui AK, Crawford JH, Patel RP. Is methemoglobin an inert bystander, biomarker or a mediator of oxidative stress-The example of anemia? Redox Biol. 2013;1(1):65-69.
Crossref - Szarpak L, Ruetzler K, Safiejko K, et al. Lactate dehydrogenase level as a COVID-19 severity marker. Am J Emer Med. 2021;45:638-639.
Crossref - Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943.
Crossref - Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Resp Med. 2020;8(4):420-422.
Crossref - Cheng L, Li H, Li L, Liu C, Yan S, Chen H, Li Y. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal. 2020;34(10):e23618.
Crossref - Hou H, Zhang B, Huang H, et al. Using IL-2R/lymphocytes for predicting the clinical progression of patients with COVID-19. Clin Exp Immunol. 2020;201(1):76-84.
Crossref - Rosario C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y. The hyperferritinemia syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11(1):185.
Crossref - Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401-409.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.