ISSN: 0973-7510
E-ISSN: 2581-690X
Carbapenem-resistance in Gram-negative bacteria poses a global threat, necessitating vigilant surveillance for informed policy decisions. Our study investigated clinically significant, carbapenem-resistant Enterobacterales infections. The analysis involved documenting data on clinical specimens received, antibiogram, treatment given and relevant clinical details sourced from medical records. Statistical analyses aimed to understand the association between risk factors and mortality outcomes. Out of 280 cases, most were males (71.1%), aged over 65 (49.6%), with a mortality rate of 14.6%, significantly linked to age (p < 0.001*). Skin/soft tissue (36.7%) and respiratory (34.29%) infections exhibited the highest CRE isolation rates, mainly attributed to Klebsiella pneumoniae (69.2%). Infection type significantly influenced mortality (p < 0.05). Factors like prior hospitalization/surgery, antibiotic exposure, chemotherapy, COVID status, ICU duration, mechanical ventilation, and indwelling devices were associated with mortality (p < 0.05) on univariate analysis. Multivariate analysis for infection type, clinical sample, organism, and prior carbapenem use was significant (p < 0.05). Cotrimoxazole, aminoglycosides and tigecycline displayed sensitivity in a substantial portion of CRE cases. Betalactams/Betalactam inhibitor monotherapy was the most commonly administered empiric antibiotic (survival rate-76.19%). Majority (76.4%) received combination therapy (Beta-lactam inhibitor with colistin/tigecycline/carbapenem), with 87.85% survival rate. Thus, continuous monitoring and review of the risk factors and antibiogram of CRE cases, would help to adapt to the changing antibiotic resistance patterns. The action plan could prioritize the use of Cotrimoxazole and aminoglycosides for empirical treatment. Specific pathogen driven, targeted combination therapy using aminoglycoside and Polymyxin B-based regimens showed no recorded instances of mortality.
Carbapenem-resistance, Enterobacterales, Risk Factors, Outcome, Empirical Therapy, CRE
Multidrug-resistant (MDR) Gram-negative organisms: Carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Pseudomonas aeruginosa (CRPA) and Carbapenem-resistant Enterobacterales (CRE) occupies the top tier of the WHO list of antibiotic-resistant “priority pathogens”.1 Carbapenem-resistance in Gram-negative bacteria is a global epidemic, that needs to be identified by active ongoing surveillance.2
Antibiotic resistance in Enterobacterales has significant clinical and socioeconomic impacts with increased morbidity and mortality.1,2 The possible reason could be administration of antibiotics with suboptimal or no activity against these organisms.3
The potential impact of the COVID-19 pandemic on the incidence of CRE infections is unknown. The interruptions in supplies and health services could have led to the undetected propagation of CRE. On the other hand, the stricter infection control measures contributed to the prevention of the spread of other nosocomial pathogens including CRE.4
To address the global concern about carbapenem-resistant Enterobacteriaceae (CRE), the existing literature have focused on risk factors and data from high-resource settings.5,6 The absence of localized data restricts the development of targeted strategies for prevention and stewardship. This study aims to address these gaps by identifying and analyzing key risk factors for CRE infection in our setting, with implications for clinical practice. Hence this retrospective observational study was undertaken to investigate the infections, antibiogram, risk factors, treatment and outcome of CRE infections.
The Retrospective cohort study on patients with positive CRE culture was conducted in the Microbiology laboratory of a tertiary care center situated in South India during January 2022 – June 2023. The study was conducted after obtaining clearance from the Institutional Ethics Committee.
Inclusion criteria
Clinically significant, nonrepetitive Enterobacterales isolates resistant to carbapenem group of antibiotics.
Exclusion criteria
All isolates other than Enterobacterales and isolates which were sensitive to carbapenems.
All the clinical specimens received, processed and with isolation of CRE were noted. Antimicrobial susceptibility was determined by Automated Vitek 2 Compact system and interpreted as per CLSI guidelines.7 The antibiogram, antibiotics used during treatment, mortality and other clinical data were collected from medical records. Data were grouped into: demographic data, age, gender, year, ICU stay, diagnosis of hospitalization (morbidity) and the number of other comorbidities. Other risk factors such as recent previous hospitalization, use of invasive devices (central venous catheter, catheter vesical and intubation), prior surgery/hemodialysis, previous antimicrobial use, were noted.
Statistical analysis was performed using SPSS version 25.0 for Windows. Categorical variables were evaluated using the Χ2 test or Fisher exact test. Logistic regression models were used to analyse risk factors for CRE infection and mortality. Univariate and multivariate analysis of various risk factors vs mortality was done. A p-value of ≤0.05 was considered statistically significant. The reference category was the patients who survived. The chi-square statistics was obtained by comparing the -2 log-likelihoods of the final model and reduced model. The reduced model was formed by omitting an effect from the final model. The null hypothesis was that the omitted effect (the specific risk factor) had no significant impact on the outcome. The chi-square statistic in this context helps us to assess whether omitting a specific risk factor from the final logistic regression model significantly reduces the ability to explain mortality.
Majority of the patients belonged to age group of >65 years (n = 139, 49.6%) and 16-65 years (n = 136; 48.6%), followed by age groups of >5 years (n = 4; 1.4%) and 1-5 years (n = 1; 0.4%) [Χ2= 15.667, p-value <0.001*]. Of the 280 cases of CRE, majority were males (n = 199; 71.1%) and the rest were females (n = 81; 28.9%) [Χ2 = 0.636, p-value = 0.425].
280 CRE isolates were from different sources: Skin and Soft Tissue (n = 103; 36.79%); Respiratory samples (n = 96; 34.29%); Urine (n = 33; 11.79%); Blood (n = 31; 11.07%); Other Sterile Body Fluids (n = 15; 5.36%) and Cerebrospinal Fluid Samples: (n = 2; 0.71%) (Χ2 = 5.936; p-value = 0.051). Table 1 shows the spectrum of CRE (Χ2 = 13.9,10, p-value = 0.053)
Table (1):
Spectrum of carbapenem-resistant Enterobacterales
| Organism (n = 280) | Dead (41) | Alive (239) | χ2 value | p-value |
|---|---|---|---|---|
| Escherichia coli (n = 23) | 7 (30.43%) | 16 (69.57%) | 13.910 | 0.053 |
| Klebsiella pneumoniae (n = 194) | 32 (16.49%) | 162 (83.51%) | ||
| Proteus spp. (n = 21) | 0 | 21 (100%) | ||
| Enterobacter spp. (n = 12) | 2 (16.67%) | 10 (83.33%) | ||
| Serratia marcescens (n = 6) | 0 | 6 (100%) | ||
| Citrobacter spp. (n = 11) | 0 | 11 (100%) | ||
| Morganella morganii (n = 13) | 1 (7.7%) | 12 (92.3%) | ||
| Providencia rettgeri (n = 1) | 0 | 1 (100%) |
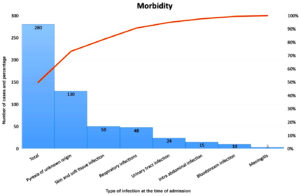
The associated morbid conditions of the 280 patients at the time of admission is shown in Figure 1. The mortality rate was 14.6% (n = 41). Univariate analysis of morbidity vs mortality and various risk factors vs mortality are shown in Tables 2 and 3, respectively. Multivariate analysis of the risk factors as predictors for mortality (Table 4). The empirical antibiotics and the patient outcome were noted in Table 5 [Χ2 = 37.747; p-value = 0.031, Significant].
Figure 1. Pareto chart showing the morbid condition of the patients at the time of admission.*
*Frequencies mentioned represent numerical value (number of cases out of a total 280 cases)
Table (2):
Morbidity at the time of admission
| Morbidity | Dead | Alive | χ2 value | p-value |
|---|---|---|---|---|
| Pyrexia of unknown origin (n = 130) | 6 (4.62%) | 124 (95.38%) | 61.420 | <0.001 * |
| Skin and soft tissue infection (n = 50) | 4 (8%) | 46 (92%) | ||
| Respiratory infection (n = 48) | 19 (39.58%) | 29 (60.42%) | ||
| Urinary tract infection (n = 24) | 7 (29.17%) | 17 (70.83%) | ||
| Intra-abdominal infection (n = 15) | 3 (20%) | 12 (80%) | ||
| Bloodstream infection (n = 10) | 2 (20%) | 8 (80%) | ||
| Meningitis (n = 3) | 0 | 3 (100%) |
p-value (<0.05) statistically significant
Table (3):
Univariate analysis of risk factors for mortality
Risk Factor |
Dead (41) |
Alive (239) |
χ2 value |
p-value |
|---|---|---|---|---|
Presence of Surgical intervention during admission (n = 82) |
14 (17.07%) |
68 (82.93%) |
0.548† |
0.462 |
Prior Hospitalization/Surgery during preceding 12 months (n = 73) |
20 (27.40%) |
53 (72.60%) |
12.852† |
less than 0.001* |
Prior use of Multiple antibiotics (n = 64) |
16 (25%) |
48 (75%) |
17.128 |
less than 0.001 * |
Prior use of carbapenems (n = 15) |
9 (60%) |
6 (40%) |
25.955† |
less than 0.001 * |
Prior use of Beta-lactam antibiotics (n = 74) |
19 (25.68%) |
55 (74.32%) |
9.796† |
less than 0.001 * |
Hypertension (n = 58) |
13 (22.41%) |
45 (77.59%) |
3.534 |
0.06 |
Diabetes mellitus (n = 79) |
18 (22.78%) |
61 (77.22%) |
5.837 |
0.016 |
Chemotherapy (n = 14) |
7 (50%) |
7 (50%) |
14.740† |
less than 0.001* |
Organ/stem cell transplant |
0 |
2 (100%) |
0.346 |
1 |
Covid-positive (n = 39) |
14 (35.90%) |
25 (64.10%) |
16.377 |
less than 0.001 * |
Duration of hospital stay
| 30 (12.82%) |
204 (87.18%) |
3.785† |
0.067 |
Duration of hospital stay >7 days (n = 46) |
11 (23.91%) |
35 (76.09%) |
||
ICU admission (n = 80) |
33 (41.25%) |
47 (58.75%) |
63.438 |
less than 0.001 * |
Mechanical ventilation (n = 84) |
35(41.67%) |
49 (58.33%) |
70.115 |
less than 0.001 * |
Indwelling device (n = 134) |
36 (26.87%) |
98 (73.13%) |
30.718† |
less than 0.001 * |
*p-value (<0.05) statistically significant; † Fischer’s exact test value
Table (4):
Multivariate analysis of risk factors as predictors for mortality
| Effect | Likelihood Ratio test | Parameter estimates | |||
|---|---|---|---|---|---|
| Exp(B) (Odds Ratio)a | Lower Bound | 95% Confidence Interval for Exp(B)b Upper Bound | |||
| Chi-Square value | Sig. | ||||
| Age | 6.607 | 0.037† | 2.078 | 0.051 | 84.042 |
| Gender | 0.564 | 0.453 | 1.500 | 0.522 | 4.311 |
| Type of infection/Diagnosis | 16.343 | 0.022* | 0.049 | 0.000 | 5.271 |
| Sample type | 4.608 | 0.032* | 2.319 | 1.077 | 4.990 |
| Organism isolated | 4.218 | 0.040* | 0.504 | 0.222 | 1.146 |
| Surgical intervention during admission | 0.064 | 0.801 | 1.216 | 0.266 | 5.565 |
| Total Duration of Hospital stay | 1.022 | 0.312 | 0.401 | 0.065 | 2.471 |
| Prior Hospitalisation/Surgery during preceding 12 months | 0.000 | 0.989 | 0.990 | 0.189 | 5.183 |
| Prior use of multiple antibiotics | 3.312 | 0.191 | 1.445 | 0.210 | 9.948 |
| Prior carbapenem use | 3.907 | 0.048† | 6.844 | 0.943 | 49.677 |
| Prior betalactam use | 0.279 | 0.598 | 0.551 | 0.060 | 5.072 |
| Hypertension | 0.120 | 0.730 | 1.303 | 0.291 | 5.842 |
| Diabetes | 0.753 | 0.386 | 1.972 | 0.416 | 9.360 |
| Chemotherapy Immunosuppression | 0.497 | 0.481 | 2.552 | 0.186 | 35.040 |
| Mechanical ventilation | 2.346 | 0.126 | 5.335 | 0.538 | 52.921 |
| COVID status | 0.540 | 0.462 | 1.733 | 0.399 | 7.524 |
| ICU stay | 3.479 | 0.062 | 6.942 | 0.842 | 57.266 |
| Duration of ICU stay | 0.684 | 0.408 | 0.416 | 0.051 | 3.380 |
| Indwelling device | 0.337 | 0.562 | 2.728 | 0.084 | 89.062 |
| History of organ/stem cell transplant | 0.000 | 0.995 | 0.036 | 0.036 | 0.036 |
aExp(B) (Odds Ratio) represents the multiplicative change in the odds of the outcome (patient expiring) for a one-unit change in the predictor variable. b95% Confidence Interval for Exp(B): This provides the range within which the odds ratio is likely to fall with 95% confidence. *p-value (<0.05) statistically significant; † Significant (p < 0.05). The wide confidence interval suggests that the true effect of age/prior carbapenem use on the outcome is uncertain and could vary widely
Table (5):
Patient outcome and empirical antibiotic therapy
| Empirical antibiotic | Patient outcome | |
|---|---|---|
| Expired | Survived | |
| No antibiotic given empirically (n = 133) | 6 (4.51%) | 127 (95.49%) |
| β-lactams/β-lactam inhibitors (n = 70) | 19 (27.14%) | 51 (72.86%) |
| Clindamycin (n = 21) | 4 (19.05%) | 17 (80.95%) |
| Aminoglycosides (n = 15) | 3 (20%) | 12 (80%) |
| 3rd generation cephalosporins + Clindamycin + Aminoglycosides (n = 9) | 1 (11.11%) | 8 (88.89%) |
| 3rd generation cephalosporins + Clindamycin (n = 7) | 2 (28.57%) | 5 (71.43%) |
| Cotrimoxazole (n = 7) | 0 | 7 (100%) |
| β-lactams/β-lactam inhibitors + Aminoglycosides (n = 6) | 2 (33.33%) | 4 (66.67%) |
| 3rd generation cephalosporins+ metronidazole (n = 5) | 2 (40%) | 3 (60%) |
| Quinolones (n = 4) | 1 (25%) | 3 (75%) |
| Polymyxins (n = 3) | 1 (33.33%) | 2 (66.67%) |
| Total (n = 280) | 41 (14.64%) | 239 (85.36%) |
PCT-Q was elevated in 22 of the 31 blood culture isolates of CRE (>2 ng/ml). Future prospective studies are necessary to validate severity scoring systems to analyze the burden of CRE.
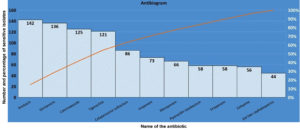
Figure 2. Pareto chart showing antibiotic sensitivity pattern of the 280 CRE isolates*
*Frequencies mentioned represent numerical value (number of cases out of a total 280 cases)
The antibiogram of the 280 CRE isolates are shown in Figure 2. Culture driven pathogen specific antibiotic therapy with patient outcome is shown in Table 6.
Table (6):
Patient outcome and the antibiotic/antibiotic combination used for treatment
| Antibiotics used in the treatment of CRE infections | Patient outcome | |
|---|---|---|
| Expired (n,%) | Survived (n,%) | |
| β-lactam inhibitor (Piperacillin-tazobactam, Cefaperazone-sulbactum, ceftazidime-avibactum) with tigecycline/colistin/carbapenem (n = 214) | 26 (12.15%) | 188 (87.85%) |
| β-lactam inhibitor with aminoglycosides (n = 7) | 0 | 7 (100%) |
| Carbapenem with tigecycline (n = 34) | 11 (32.35%) | 23 (67.65%) |
| Carbapenem with colistin (n = 15) | 4 (26.67%) | 11 (73.33%) |
| Carbapenem with Polymyxin B (n = 2) | 0 | 2 (100%) |
| Cotrimoxazole with aminoglycoside (n = 6) | 0 | 6 (100%) |
| Cotrimoxazole with Polymyxin B (n = 2) | 0 | 2 (100%) |
| Total (n = 280) | 41 | 239 |
The retrospective analysis of the variables influencing the acquisition of CRE and their association with mortality is studied.
Univariate analysis of age and gender vs mortality, age had significant impact on mortality (p-value <0.001*). Similar to our study findings, there are reports of median age of patients with CRE-66 years (interquartile range of 57 to 80), with upto 45.2% being males (70 out of 155 patients).8
The distribution of CRE across diverse clinical sample types highlights the importance of monitoring. Increased rate of CRE isolation was from skin and soft tissue infection (36.7%) and respiratory infection (34.29%). Univariate analysis of clinical sample type with mortality was not statistically significant (p-value = 0.051). The result is in par with a study by Wang et al, in which no statistically significant variance in infection types was observed amongst CRE groups.9
Klebsiella pneumoniae was the commonest CRE isolated (n = 194, 69.2%), but there was no significant association of the organism isolated with mortality rate. (p-value = 0.053). Sekar et al. study and a study from South India highlighted an increase in resistance to carbapenem, notably in Klebsiella species.10 Research focusing on the pediatric demographic in rural areas of Southern India reported notably high rates of carbapenem-resistant Klebsiella spp.11 PUO, skin and soft tissue infection and respiratory infections account for majority of cases (over 80%) in our setup. The rising occurrence of CRE have been reported from skin and soft tissue infections.12 In a study by Rebold et al., respiratory infections accounted for 38%, followed by urinary infections at 20%, intra-abdominal at 16%, and primary bacteremia at 14% of the CRE infections.13
Research conducted by Guarnera et al. revealed that the colonization and subsequent infection of multidrug-resistant organisms commonly occurs and poses a challenging obstacle to eliminate in patients undergoing various stages of treatment for acute myeloid leukemia and/or Fever of unknown origin. This presence significantly impacts long-term outcomes.14
The type of morbidity had a significant association with mortality as shown in Table 2. (p-value <0.05). As per review article by Qin Hu et al, mortalities due to CRE infection ranged from 10% to 59% in 12 studies with combination therapies and from 8.3% to 64.7% in 11 studies with monotherapies.15 A study by Mariappan et al. found that comparing infections caused by susceptible strains of the same species to those caused by multiple antibiotic-resistant bacteria reveals significantly more severe consequences. These include extended hospitalization and heightened rates of both morbidity and mortality. This study did not isolate any specific factor as an independent risk for acquiring carbapenemase-producing enterobacterales (CPE) infection. However, through multivariate analysis, it became apparent that patients with CPE infections face an elevated risk of mortality when they require ventilation and rely on indwelling medical devices for support.16,17
Our study showed that risk factors such as prior Hospitalization/Surgery during preceding 12 months, prior use of Multiple antibiotics/carbapenems, Chemotherapy, COVID status, Duration of ICU stay, Mechanical ventilation and presence of indwelling devices were significantly associated with mortality (p-value <0.05). These findings are on par with the findings by Kong et al. in China, in which the independent predictors of in-hospital death included age over 65 years (OR: 3.19), prior exposure to carbapenems (OR: 3.54), and the presence of a central venous catheter (OR: 4.19).18 A retrospective cross-sectional analysis conducted in Penang over a 3-year period (January 2021-December 2023), demonstrated a strong positive association between carbapenem utilization and the incidence of carbapenem-resistant Enterobacterales (CRE).19
Multivariate analysis was done to analyze the relationship between mortality and multiple independent risk factors. The Table 4 depicts information about the significance levels, odds ratios and confidence intervals for the predictors in the model. Using the logistic regression model, the factors: age, gender, >7 days-duration of hospital stay, and COVID status were considered as covariates, while the factors that were statistically significant on univariate analysis: infection type, prior hospitalization/surgery in 12 months, prior use of multiple antibiotics/carbapenems/Beta lactams, chemotherapy, mechanical ventilation, indwelling device were considered as variates.
The type of infection, clinical sample, organism and prior carbapenem use, maintained statistical significance on multivariate analysis. (p-value <0.05). Prior use of carbapenems was statistically significant (p-value of 0.048) with a high odds ratio of 6.844, suggesting a substantial impact on the outcome. The significant p-value suggests that these predictors included in the final model are important for explaining the variation in mortality.
Other predictors, such as Gender, Surgical intervention, presence of indwelling device, and most of the medical history-related variables as mentioned in the Table 4, do not seem to significantly impact mortality since it is not statistically significant in predicting the outcome. This suggests that these variables may not be strongly associated with the outcome.
The established risk factors for carbapenem-resistant Enterobacteriaceae (CRE) infection included advanced age, the use of ventilation devices, and prior exposure to antimicrobials (multiple and carbapenems). The findings of this study, thus have implications for clinical practice, particularly in the areas of infection control and antimicrobial stewardship.
Elderly patients are generally at higher risk due to age-related immunosenescence and the presence of multiple comorbidities, necessitating closer monitoring and timely intervention. The association between ventilation devices and CRE necessitates the need for strict adherence to infection prevention protocols, such as proper hand hygiene, regular equipment disinfection, and care of devices.
Prior antimicrobial exposure, especially to broad-spectrum antibiotics, was found to be a significant contributor to the development of CRE. This highlights the critical importance of antimicrobial stewardship programs to ensure the judicious use of antibiotics, reduce unnecessary prescriptions, and promote the use of targeted therapies based on culture and sensitivity results.
The interventions to mitigate the risks associate with CRE may include early screening for CRE colonization upon admission, especially in intensive care units, and implementation of isolation protocols for colonized or infected individuals to prevent spread in healthcare settings.20 Additionally, strengthening antimicrobial stewardship programs to limit the unnecessary use of broad-spectrum antibiotics is critical. Tailoring empirical therapy based on local resistance patterns and enforcing de-escalation strategies can also reduce selection pressure and curb resistance. Collectively, these interventions can help healthcare facilities control CRE transmission and improve patient outcomes. By addressing these modifiable risk factors, healthcare providers can reduce the incidence of CRE infections and enhance patient outcomes, while also contributing to the global effort against antimicrobial resistance.21
A six-year study by Lin et al. revealed a 7%-13% decline in susceptibility rates to Beta lactams/Beta-lactam inhibitor combinations among initial CRE isolates. This trend may be attributed to higher antibiotic consumption, the acquisition of resistance determinants, or shifts in the prevalence of carbapenemase types within the Southeast region.22
The antibiotic susceptibility pattern of our study of 280 cases of CRE in the decreasing order of frequency is as shown in Figure 2. The antibiotics Cotrimoxazole (n = 125, 45%), aminoglycosides {Amikacin (n = 142, 51%) and gentamicin (n = 136, 49%)}, tigecycline (n = 121, 43%) were sensitive in a substantial portion of CRE cases. Over 70% of the total CRE cases were sensitive to either of these antibiotics as shown in Figure 2. Hence, these antibiotics which showed lower rates of resistance, may be relied upon for empirical treatment in the present setup in contrast to the to 3rd generation cephalosporins, beta-lactam inhibitor drugs and carbapenems.
The antibiotics cefaperazone sulbactam, imipenem and meropenem were not as sensitive as aminoglycosides/cotrimoxazole/tigecycline, but they were still sensitive in 86 cases (31%), 73 cases (26%) and 66 cases (24%) of CRE respectively. Antibiotics piperacillin tazobactam (n = 58, 21%), ertapenem (n = 58, 21%), cefepime (n = 56, 20%), 3rd gen cephalosporins (n = 44, 16%) were least sensitive against CRE. Hence, they may not be relied upon as first-line treatments, and their use should be carefully considered in case of a suspected CRE infection. Our research outcomes align with Mohamed et al. findings, which investigated the antibiogram of CRE isolates and observed varying levels of resistance to different antibiotics. Specifically, concerning cephalosporins, the resistance rates for CRE were notably high, ranging from 69% to 80% for 3rd generation cephalosporins, 89% for cefepime, 97% for imipenem and 97% for meropenem.23,24
Our study findings would guide the clinicians to make use of the local hospital and ward level susceptibility data to help their antibiotic treatment decisions when their patients are at risk of being infected by a carbapenem-resistant pathogen. Out of the total 280 patients, 147 patients (52.5%) received empirical antibiotics, and among them, 112 patients (76.19%) survived. This highlights the importance of early diagnosis and quick start of empirical antibiotic therapy in improving the patient outcome. Interestingly, patients who did not receive any antibiotic therapy had a lower mortality rate (4.51%) compared to those who received. This could be attributed to the good practice of restricted antibiotic use, antibiotic stewardship and subsequent culture driven specific antibiotic therapy. Ohnuma
et al.’s research demonstrated that the use of suitable initial empirical therapy differed based on pathogens, often being less common for resistant organisms. Surprisingly, multiple studies have shown that inadequate empirical antibiotic therapy did not correlate with increased mortality. Consequently, the selection of appropriate empirical therapy continues to pose a significant challenge.25
Beta-lactams/Beta-lactam inhibitor monotherapy was the most commonly administered empiric antibiotic (n = 70) as shown in Table 5. The combination of 3rd generation cephalosporins + Clindamycin + Aminoglycosides category had a lower mortality rate (11.11%) compared to other categories, probably due to the fact that amikacin and gentamicin were sensitive in majority of CRE isolates (n = 142, 51% and n = 136, 49%) respectively, while the combination of third generation cephalosporins + metronidazole had a relatively higher mortality rate (40%). Other empirical antibiotics had varying success rates in treating patients as shown in the Table 5. This study thus helps to assess the effectiveness of these empirical antibiotic treatments in managing patient outcomes, so as to guide in making informed decisions regarding empirical therapy.
Higher doses of Tigecycline, Carbapenem, Colistin are found to be associated with better clinical outcomes and another strategy is to combine these drugs though evidence for the latter is more obscure. In our study, as shown in Table 6, the effectiveness of different antibiotic treatments in terms of patient survival and mortality when dealing with CRE infections is described. As shown in Table 6, a majority (n = 214,76.4%) of CRE infected patients received combination of Beta-lactam inhibitor (Piperacillin-tazobactam, Cefaperazone-sulbactum, ceftazidime-avibactum) with colistin/tigecycline/carbapenem, out of which 87.85% survived.
Among the total of 34 patients who received carbapenem with tigecycline, 67.65% survived and among the 15 patients who received carbapenem with colistin, 73.33% survived. Thus, our study supports the role of combination therapy vs monotherapy. As indicated by Sheu et al. study, the use of combination therapy is associated with reduced mortality rates compared to the use of monotherapy.26
None of the patients in the categories who received beta-lactam inhibitor with aminoglycosides, carbapenem with Polymyxin B, Cotrimoxazole with aminoglycoside and Cotrimoxazole with Polymyxin B, expired (0% mortality). This difference in mortality maybe attributed to the fact that cotrimoxazole (45%) amikacin and gentamicin were sensitive in majority of CRE isolates (45%, 51% and 49% respectively). According to Sahitya et al. study on CRE infections, the effectiveness in addressing CRE has been established through the utilization of combination therapies that encompass carbapenems, polymyxin, tigecycline, aminoglycoside, and Fosfomycin.27 Conversely, a recently developed antibiotic involves ceftazidime avibactam alone or avibactam, often combined with carbapenem-containing regimens, showcasing a newer addition to the treatment options available for addressing CRE infections.28 Current evidence indicates that ceftazidime-avibactam demonstrates superior efficacy and safety over polymyxins in the management of infections caused by carbapenem-resistant Enterobacteriaceae.29 Determining drug MICs through phenotypic susceptibility testing is key to tailoring treatment, particularly in refractory cases or infections at difficult-to-access sites. When carbapenem-resistant Gram-negative bacteria are strongly suspected such as in severe sepsis, septic shock, or immunocompromised hosts empirical combination therapy with two or more active agents is recommended, with antibiotic selection guided by local resistance trends and antibiogram data.30
In countries with limited resources, obstacles to enhancing the management of CRE infections include the absence of advanced molecular diagnostics capable of offering a deeper insight into resistance patterns related to CRE infections. Additionally, the scarcity of new antibiotics poses a challenge, hindering the potential for improved outcomes and decreased mortality among critically ill patients suffering from CRE infections.31 The study’s constraint was the absence of statistical analysis correlating empirical and definitive treatment methods with mortality rates. Despite investigating the various risk factors and mortality, the study did not capture patient outcomes regarding average duration of hospitalization and reduced functional status at discharge.
Continuous monitoring, regular update and review of the risk factors and antibiogram of CRE cases, would help to adapt to the changing antibiotic resistance patterns. The factors such as age, infection type, the specific isolated organism, and prior carbapenem use were significant on multivariate analysis. This emphasizes the importance of considering these variables in clinical assessments and treatment strategies. The action plan could prioritize the use of Cotrimoxazole, Gentamicin and Amikacin as the primary antibiotics for empirical treatment of CRE cases. Specific pathogen driven, targeted combination therapy using aminoglycoside and Polymyxin B based regimen showed no recorded instances of mortality. However, individual patient factors and resistance patterns should still guide antibiotic choices. It should be kept in mind that antibiotic selection should always be guided by culture and sensitivity testing, and the advice of infectious disease specialists would ensure the most appropriate and effective treatment for individual patients.
The recommendations to mitigate the risk associated with CRE will include prompt identification of high-risk patients (elderly, ventilated, or prior surgery or use of broad-spectrum antibiotics), judicious use of antibiotics including using culture-based therapy and vigilant monitoring of patients on invasive devices and strict adherence to hand hygiene and contact precautions. At the institutional level, CRE screening for high-risk populations, strengthening infection prevention programs, including isolation protocols for colonized or infected patients and robust antimicrobial stewardship programs are useful.
ACKNOWLEDGMENTS
The authors are grateful to Manipal Academy of Higher Education for the constant support.
CONFLICT OF INTEREST
All authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
The authors listed have made a substantial direct and intellectual contribution to the work and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Kasturba Medical College, Mangalore (IEC KMC MLR 10-2021/322).
- Asokan GV, Ramadhan T, Ahmed E, Sanad H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med J. 2019;34(3):184-193.
Crossref - Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl 7):S521-8.
Crossref - He G, Huang J, Huang S, et al. Risk Factors Affecting Clinical Outcome in Patients with Carbapenem-Resistant K. pneumoniae: A Retrospective Study. Med Sci Monit. 2020:26:e925693.
Crossref - Dong LT, Espinoza HV, Espinoza JL. Emerging superbugs: The threat of Carbapenem Resistant Enterobacterales. AIMS Microbiol. 2020;6(3):176-182.
Crossref - Rebold N, Lagnf AM, Alosaimy S, et al. Risk Factors for Carbapenem-Resistant Enterobacterales Clinical Treatment Failure. Microbiol Spectr. 2023;11(1):e0264722.
Crossref - Ranjbar R, Alam M. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Evid Based Nurs. 2023.
Crossref - CLSI, Performance Standards for antimicrobial susceptibility testing standards M100. 33rd ed. Wayne PA: Clinical Laboratory Standards Institute; 2023.
- Moghnieh R, Abdallah D, Jadayel M, et al. Epidemiology, risk factors, and prediction score of carbapenem resistance among inpatients colonized or infected with 3rd generation cephalosporin resistant enterobacterales. Sci Rep. 2021;11(1):14757.
Crossref - Wang Q, Zhang Y, Yao X, et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacterales nosocomial infections. Eur J Clin Microbiol. 2016;35(10):1679-1689.
Crossref - Sekar R, Srivani S, Amudhan M, Mythreyee M. Carbapenem resistance in a rural part of southern India: Escherichia coli versus Klebsiella spp. Indian J Med Res. 2016;144(5):781
Crossref - Sekar R, Mythreyee M, Srivani S, Sivakumaran D, Lallitha S, Saranya S. Carbapenem-resistant Enterobacterales in pediatric bloodstream infections in rural southern India. Indian Pediatr. 2017;54(12):1021-1024.
Crossref - Russo A, Trecarichi EM, Torti C. The role of gram-negative bacteria in skin and soft tissue infections. Curr Opin Infect Dis. 2021;35(2):95-102.
Crossref - Pérez-Galera S, Bravo-Ferrer JM, Paniagua M, et al. Risk factors for infections caused by carbapenem-resistant Enterobacterales: an international matched case-control-control study (EURECA). E Clinical Medicine. 2023;57:101871.
Crossref - Guarnera L, Trotta GE, Boldrini V, et al. Fever of unknown origin and multidrug-resistant organisms colonization in AML patients. Mediterr J Hematol Infect Dis. 2023;15(1):e2023013.
Crossref - Hu Q, Chen J, Sun S, Deng S. Mortality-related risk factors and novel antimicrobial regimens for carbapenem-resistant Enterobacterales infections: A systematic review. Infect Drug Resist. 2022;15:6907-26.
Crossref - Mariappan S, Sekar U, Kamalanathan A. Carbapenemase-producing Enterobacterales: Risk factors for infection and impact of resistance on outcomes. Int J Appl Basic Med Res. 2017;7(1):32-39.
Crossref - Kedišaletše M, Phumuzile D, Angela D, Andrew W, Mae NF. Epidemiology, risk factors, and clinical outcomes of carbapenem-resistant Enterobacterales in Africa: A systematic review. J Glob Antimicrob Resist. 2023;35:297-306.
Crossref - Kong H, Hu Z, Zhang L, et al. Clinical risk factors and outcomes of carbapenem-resistant Escherichia coli nosocomial infections in a Chinese teaching hospital: a retrospective study from 2013 to 2020. Microbiol Spectr. 2024;12(7):e04228-23.
Crossref - Arulappen AL, Khan AH, Hasan SS, et al. The correlation between antibiotic usage and antibiotic resistance: a 3-year retrospective study. Front Cell Infect Microbiol. 2025;15:1608921.
Crossref - Sharma K, Tak V, Nag VL, Bhatia PK, Kothari N. An observational study on carbapenem-resistant Enterobacterales (CRE) colonisation and subsequent risk of infection in an adult intensive care unit (ICU) at a tertiary care hospital in India. Infect Prev Pract. 2023;5(4):100312.
Crossref - Yu H, Gonzalez AH, Torres GE, et al. A retrospective study of risk factors, mortality, and treatment outcomes for infections with carbapenemase-producing Enterobacterales in a tertiary hospital in Havana, Cuba. Antibiotics (Basel). 2022;11(7):942.
Crossref - Lin CK, Page A, Lohsen S, et al. Rates of resistance and heteroresistance to newer β-lactam/β-lactamase inhibitors for carbapenem-resistant Enterobacterales. JAC Antimicrob Resist. 2024;6(2):dlae048.
Crossref - Ting MH, Martens M, Albazzaz H, Wolesensky C, Kobic E. Risk factors for clinical treatment failure regarding carbapenem-resistant Enterobacterales in the southwestern United States. Infect Dis Now. 2025;55(3):105052.
Crossref - Mohamed T, Yousef LM, Darweesh EI, Khalil AH, Meghezel EM. Detection and characterization of carbapenem resistant enterobacteriacea in Sohag University Hospitals. Egypt J Med Microbiol. 2018;27(4):61-69.
Crossref - Ohnuma T, Chihara S, Costin B, et al. Association of appropriate empirical antimicrobial therapy with in-hospital mortality in patients with bloodstream infections in the US. JAMA Network Open. 2023;6(1):e2249353.
Crossref - Sheu C-C, Chang Y-T, Lin S-Y, Chen Y-H, Hsueh P-R. Infections caused by carbapenem-resistant Enterobacterales: An update on therapeutic options. Front Microbiol. 2019;10:80.
Crossref - Sahitya DSK, Jandiyal A, Jain A, et al. Prevention and management of carbapenem-resistant Enterobacterales in Haematopoietic Cell Transplantation. Ther Adv Infect Dis. 2021;8:204993612110534.
Crossref - Tilahun M, kassa Y, Gedefie A, Ashagire M. Emerging carbapenem-resistant Enterobacterales infection, its epidemiology and novel treatment options: A Review. Infect Drug Resist. 2021;14:4363-4374.
Crossref - Yang P, Li Y, Wang X, Chen N, Lu X. Efficacy and safety of ceftazidime-avibactam versus polymyxins in the treatment of carbapenem-resistant Enterobacteriaceae infection: a systematic review and meta-analysis. BMJ Open. 2023;13:e070491.
Crossref - Krishna V. Management of carbapenem resistant Gram-negative infections. Indian J Pediatr. 2025;92(7):757-764.
Crossref - Madney Y, Aboubakr S, Khedr R, et al. Carbapenem-resistant Enterobacterales (CRE) among children with cancer: Predictors of mortality and treatment outcome. Antibiotics. 2023;12(2):405.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.