ISSN: 0973-7510
E-ISSN: 2581-690X
The bioactive molecules found in Streptomyces are important due to their potential applications in medicine, particularly in combating infections and cancer. Studies have identified various bioactive compounds produced by different Streptomyces strains, highlighting their diverse therapeutic properties. Streptomyces albus is a prolific source of bioactive molecules, producing a diverse array of secondary metabolites with significant pharmaceutical potential. This study aimed to identify the bioactive components of Streptomyces strains isolated from marine sediment and assess their antioxidant properties. The experimental study was designed based on standard protocols to isolate Streptomyces strain from starch casein, which was further confirmed using 16S rRNA sequencing. The extracellular products from the strain were extracted using ethyl acetate and a high-efficiency vacuum evaporator to identify the active molecules using GC-MS. The antioxidant properties of the crude extract, including total phenol content, absolute antioxidant capacity, free radical neutralization power, and overall reducing power, were evaluated. All experiments were conducted in triplicate. Mean values with standard deviation were reported, and the isolated strain was identified as Streptomyces albus DR 57. In addition to eight primary active extracellular compounds, diethyl phthalate and glycyl-L-proline were detected in this strain. Research has indicated that glycyl-L-proline possesses various therapeutic potentials. The phenolic compound (22.23 ± 0.37 µg/mL) identified in this strain serves as the principal element responsible for its antioxidant characteristics. This study concluded that the identified strain demonstrated significant antioxidant capabilities. Further investigation is required to understand the mechanisms involved and to enhance the extraction of these beneficial compounds for practical applications.
Streptomyces sp., Antioxidant, Secondary Metabolites, Bioactive Molecules
Bacteria represent a significant reservoir of innovative natural compounds, with numerous bacterial natural products or their derivatives actively utilized in pharmaceuticals for both human and animal use, as well as in the food industry and crop protection.1,2 Research has extensively focused on identifying bioactive compounds of bacterial origin.3 Streptomyces species exhibit remarkable antioxidant properties, as evidenced by various studies. These studies highlight its potent free radical scavenging abilities, including activities against 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), and superoxide radicals as well as protective effects against DNA damage and cytotoxicity in cancer cell lines.4 This genus contributes to the production of 75% or a greater proportion of naturally occurring antibiotics.5,6 Numerous valuable compounds with significant applications in industries such as pharmaceuticals, healthcare, and biochemical research originate from fascinating natural resources.7 Numerous studies have documented the preference for natural antioxidants derived from plants; nevertheless, alternative studies indicate that may also serve as a credible resource for preserving natural antioxidants.8-10 Actinobacteria, as a class of microorganisms, play a significant role in microbial drug discovery due to their ability to synthesize a wide array of secondary metabolites and bioactive molecules.11 Streptomyces albus strains are considered valuable assets for applications such as biological control, secondary metabolite synthesis, and plant growth enhancement. This bacterium is predominantly found in soil. Some bioactive compounds, such as daryamides, piperazimycins, diethyl phthalate, treptokordin, rioxacarcins, and cyclohexyl ethyl ester, have antibacterial, anti-inflammatory, and anticancer activities.
Sample collection
Mangrove soil from a specific site in India was used for the research. Soil samples were collected from the Pichavaram Mangrove National Forest in Killai, Tamil Nadu, India. Soil samples were collected from a depth of 15 cm below the surface and placed in a septic containers for transportation to the working area. Actinomycetes were screened from the mangrove soil using a Starch Casein Agar (SCA) medium, comprising a mixture of 50% seawater and 50% distilled water.
Molecular characterization
A bacterial genomic DNA purification kit (HiMedia) was used to isolate total genomic DNA. Primers of 16S rRNA 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGCTACCTTGTTACGACTT-3′) were used to amplify the 1480 bp gene. PCR conditions (Prima96-HiMedia) were as follows: denaturation at 94 °C for 3 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 3 min. The amplified products were verified using a 1.2% agarose gel. The amplified products were sequenced using a Sanger Sequencing DNA Analyzer (Barcode BioSciences, Bengaluru). The 16S rRNA sequence was submitted to the NCB Inucleotide BLAST to assess its identity and similarity to sequences from other species in the database. Sequence alignment and phylogenetic tree construction were performed using MEGA 11 software.
Extraction of biologically active substances
The growth medium was prepared using 50 mL of filtered seawater and 50 mL of distilled water. Thus, the inoculum was optimized at this particular ratio. Spores from the selected actinomycetes strain were collected and inoculated into 50 mL of the growth medium, incubating on a rotary shaker at 120 rpm for 48 h at 28 °C. After this period, 100 mL of production medium was mixed with 10% inoculum and incubated at 28 °C for 7 days at 120 rpm. After fermentation, the mycelium and supernatant were separated by filtration, and centrifugation was performed at 10,000 rpm for 30 min at 4 °C. Extracellular compounds were extracted using a liquid-liquid extraction method with an equal volume of ethyl acetate, followed by rotary evaporation to concentrate the extract.
Antioxidant activity
Determination of Total Phenol
The quantification of total phenolic compounds in five fractions was performed using spectrophotometric analysis with the Folin-Ciocalteu reagent, following a slightly modified protocol from Tan et al.12 The extract was mixed with Folin-Ciocalteu reagent in a 1:1 ratio, followed by the addition of 4 mL of 1 M sodium carbonate. The resultant mixture was allowed to stand for 15 min. Optical density (OD) was measured spectrophotometrically at a wavelength of 765 nm. A standard curve was prepared using different concentrations of trihydroxybenzoic acid (100-1000 µg/mL) to quantify the total phenolic content of the extract, expressed as µg of gallic acid equivalents (GAE) per mg of extract. The assay was conducted in triplicate, and the results were averaged to enhance overall precision.
Determination of Total Antioxidant Activity
The total antioxidant activity of the fractions was evaluated following the method of Prieto et al.13 An aliquot of 0.3 mL from each fraction was mixed with 3 mL of reagent solution, which comprised 0.6 M H2SO4, 28 mM Na3PO4, and 4 mM (NH4)6MoO4. The mixture was incubated at 95 °C for 90 min in a controlled water bath and the OD of each sample mixture was measured at 695 nm. Ascorbic acid at a 100 µg/mL concentration was used as the standard control.
Quantitative Assessment of Free Radical Scavenging Activity via DPPH
The scavenging capacity of each fraction was assessed using DPPH.14 Two milliliters of DPPH solution (0.002% in methanol) was mixed with 2 mL of various concentrations (5-200 µg/mL) of each fraction and the standard (ascorbic acid) in separate tubes. The tubes were incubated in the dark at room temperature for 30 min, after which the OD was measured at 517 nm using a UV-Vis spectrophotometer. The absorbance of the DPPH control (which did not contain extract/standard) was recorded. The scavenging activity was calculated using the formula: Scavenging activity (%) = [(A – B) / A] × 100, where A is the absorbance of the DPPH control and B is the absorbance of DPPH in the presence of the extract/standard.
Total reducing power
This assay followed the procedure outlined by Subramaniam et al.15 The bioactive compound-rich fractions (100 µL) were mixed with 1% K3[Fe(CN)6] and incubated at 50 °C for 20 min; after this, 2.5 mL of 10% trichloroacetic acid was added to the mixture and centrifuged at 5,000 rpm for 10 min. The supernatant (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% FeCl3, and the resulting color intensity was measured at 700 nm. Ascorbic acid was used as the standard control.
GC-MS identification of bioactive compounds
The ethyl acetate extracts of the samples were analyzed by GC-MS using a Shimadzu QP2010 Ultra instrument, connected to a gas chromatograph and mass spectrometer. The system was equipped with an Elite-1 fused silica capillary column. Helium (99.99%) was used as the carrier gas at a constant flow rate of 1.21 mL/min, and an injection volume of 2 L was employed with a split ratio of 10. The injector and ion-source temperatures were adjusted to 160 °C and 200 °C, respectively. The oven temperature was started at 60 °C and increased to 280 °C over approximately 22 min. Readings were recorded at 70 eV with a scanning interval of 0.5 s. Chemical composition was analyzed using the NIST 14 library.16,17
Data analysis
The experiments were performed thrice, and the values were expressed as means and standard deviations. Statistical analysis was carried out using SPSS software.
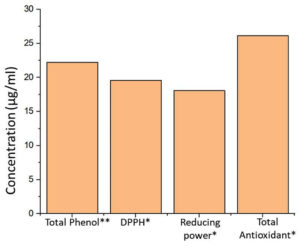
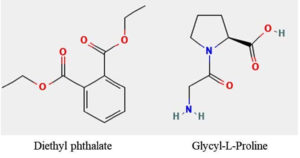
Streptomyces albus DR57 was isolated from mangroves oil sediment to confirm that the selected colony originated from SCA agar. The total genomic DNA was extracted and amplified using the 16S rRNA gene (1,500 bp). The amplified product was sequenced and subjected to BLAST search to confirm its identity. Streptomyces albus DR57 sequence was submitted to NCBI GenBank under the accession number PP329835. Additional related species were selected to construct a UPMGA tree18 (Figure 1). This study included 13 nucleotide sequences. Unclear positions were excluded from the analysis. The complete dataset comprised 1,466 locations. Phylogenetic analyses were conducted using MEGA11 software.19 The resulting phylogenetic tree illustrated the relationships between the selected strains, highlighting the distinct clustering of Streptomyces albus DR57 with closely related species. Bootstrap values indicated strong support for these groupings, reinforcing the phylogenetic significance of Streptomyces albus DR57 within its clade; Streptomyces sp. has attracted significant interest owing to its prospective applications in the pharmaceutical sector. The present study indicates that Streptomyces albus DR57 produces secondary metabolites with significant antioxidant activity. A total antioxidant capacity value of 26.13 ± 0.4 µg/mL, a total reducing power of 18.06 ± 0.51 µg/mL, and the presence of free radicals revealed in the DPPH assay indicated a 19.6 ± 0.62 µg/mL equivalent to the standard. The total phenol content quantified equivalent of trihydroxy benzoic acid was 22.23 ± 0.45 µg/mL (Figure 2). Further analysis revealed that the secondary metabolites exhibited diverse bioactivities, suggesting their potential utility in developing novel therapeutic agents. For the GCMS analysis, eight compounds were identified (Table); apart from these, diethyl phthalate and glycyl-L-proline are potentially active molecules (Figure 3). These compounds have potential therapeutic relevance due to their antibacterial, anti-inflammatory, and anticancer activities, which warrant further investigation to elucidate their mechanisms of action and efficacy in the clinical setting.
Table:
Bioactive compound identified by GCMS study from Streptomyces albus DR57
No. |
Compound Name |
Rev. Score |
Prob. % |
molecular formula |
molecular weight |
|---|---|---|---|---|---|
1 |
DiethylPhthalate |
837 |
45.4 |
C12H14O4 |
222.24 g/mol |
2 |
Phthalicacid, ethyl hex2-yn-4-ylester |
859 |
11.8 |
C16H18O4 |
274.31 g/mol |
3 |
Phthalicacid,cyclohexylethylester |
825 |
3.24 |
C16H20O4 |
276.33 g/mol |
4 |
Pyrrolo[1,2-a]pyrazine 1,4-dione, hexahydro |
710 |
43.8 |
C11H18N2O2 |
210.27 g/mol |
5 |
4-Octene,2,3,7-trimethyl-,[S-(E)] |
706 |
37 |
C11H22 |
154.29 g/mol |
6 |
Glycyl-L-proline |
662 |
8.02 |
C7H12N2O3 |
172.18 g/mol |
7 |
1,7-Dimethyluricacid |
880 |
43.05 |
C7H8N4O3 |
196.16 g/mol |
8 |
1,1-Dimethyl-2-propenylacetate |
867 |
27.81 |
C7H12O2 |
128.169 g/mol |
Streptomyces species demonstrate high antioxidant activity, with some extracts showing up to 94.84% scavenging activity in the DPPH assay. The reducing power of streptomyces extract is also significant, with some showing up to 81.83% ferric-reducing ability. This suggests that these strains can effectively donate electrons, further contributing to their antioxidant capacity.20 The DPPH assay is a reliable method for assessing free radical scavenging, with Streptomyces species exhibiting substantial inhibition percentages such as 62% and 78% for specific isolates. Bioactive compounds such as GABA and phenolic derivatives significantly improve their capacity for radical scavenging.21 Diethyl phthalate can be utilized in immuno detection processes by developing artificial coating antigens.22 It poses potential risks to aquatic life, as evidenced by studies showing its toxic effects on the hematological parameters of fish, indicating that even sub-lethal concentrations can disrupt biological functions.23 Moreover, glycyl-L-proline has been identified as a promising candidate for enhancing wound healing due to its role in collagen synthesis and cellular proliferation, highlighting the need for further research into its mechanisms of action.24 Additionally, combining these two compounds may offer novel therapeutic avenues, particularly in regenerative medicine and environmental safety, warranting comprehensive studies to explore their synergistic effects. Another potential compound, glycyl-L-proline (Gly-Pro), and its derivatives have garnered attention for their neuroprotective properties and potential therapeutic applications, particularly in neurodegenerative diseases. Kaneko et al.25 indicate that Gly-Proanditscyclicform, cyclicglycyl-L-proline, enhanced neural plasticity and facilitated learning in ischemic stroke models. Additionally, glycine-L-proline-L-glutamate analogs have shown promise in modulating oxidative stress and neuronal death, suggesting their potential in treating Alzheimer’s disease.26 Furthermore, ongoing research has elucidated their roles in cellular signaling pathways and neuroinflammation. Recent studies have also explored the synergistic effects of Gly-Pro derivatives and other neuroprotective agents, highlighting their potential to enhance the efficacy of combination therapies. As Streptomyces albus has diverse pharmaceutical applications, particularly in developing antibiotics, anticancer agents, and other therapeutic agents, exploring its metabolites has revealed promising avenues for drug development. The biosynthetic pathways of these antibiotics have been extensively studied, allowing for genetic manipulation to enhance production.27 Moreover, optimizing fermentation conditions to maximize yield and potency could lead to more effective treatments for various diseases. The Streptomyces albus DR57 strain exhibited promising antioxidant properties, and further research is needed to understand the exact mechanism and optimize these beneficial compounds for future applications. Additionally, this strain can produce secondary metabolites downstream of novel compounds under varying environmental conditions with enhanced efficacy.
The antioxidant capacity, reducing ability, and free radical scavenging potential of Streptomyces species, as partially assessed through the DPPH assay, revealed its significant potential for therapeutic applications. The findings of this study indicate that the strain Streptomyces albus DR57 exhibits notable antioxidant activity, effectively neutralizing free radicals. Bioactive compounds can be further isolated and developed for commercial applications.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Firn RD, Jones CG. Natural products-A simple model to explain chemical diversity. Nat Prod Rep. 2003;20:382-391.
Crossref - Luo Y, Cobb RE, Zhao H. Recent advances in natural product discovery. Curr Opin Biotechnol. 2014;30:230-237.
Crossref - Katz M, Hover BM, Brady S.F. Culture-independent discovery of natural products from soil metagenomes. J Ind Microbiol Biotechnol. 2016;43(2-3):129-141.
Crossref - Verma J, Attri S, Arora S. Rajesh Kumari M. Antioxidant and chemoprotectivepotential of Streptomyces levis strain isolated from human gut. AMB Expr. 2023;13:69.
Crossref - Kinkel LL, Schlatter DC, Xiao K, Baines AD. Sympatric inhibition and niche diferentiation suggest alternative coevolutionary trajectories among Streptomycetes. ISME J. 2014;8(2):249-256.
Crossref - Gozari M, Bahador N, Jassbi AR, Mortazavi MS, Eftekhar E. Antioxidant and cytotoxic activities of metabolites produced by a newmarine Streptomyces sp. isolated from the sea cucumber Holothuria leucospilota. Iran J Fish. 2018;17(2):413-426.
Crossref - Karikas GA. Anticancer and chemopreventing natural products: some biochemical and therapeutic aspects. J BUON. 2010;15(4):627-638
- Ser HL, Mutalib NSA, Yin WF, Chan KG, Goh BH, Lee LH. Evaluation of antioxidative and cytotoxic activities of Streptomyces pluripotens MUSC 137 isolated from mangrove soil in Malaysia. Front Microbiol. 2015;6:1- 11.
Crossref - Ser HL, Zainal N, Palanisamy UD, Goh BH, Yin WF, Chan KG, Lee LH Streptomyces gilvigriseus sp. nov, a novel actinobacterium isolated from mangrove forest soil. Antonie Van Leeuwenhoek. 2015 107:1369- 1378.
Crossref - Law JWF, Ser HL, Duangjai A, et al. Streptomyces colonosanans sp. nov., a novel actinobacterium isolated from Malaysia mangrove soil exhibiting antioxidative activity and cytotoxic potential against human colon cancer cell lines. Front Microbiol. 2017; 8:1-15.
Crossref - Sharma S, Shah GS. Isolation and screening of actinomycetes for bioactive compounds from the marine coast of South-Gujarat Region. Int J Innov Sci Res. 2014;1(7):345-349.
- Tan LT-H, Ser H-L, Yin W-F, Chan K-G, Lee L-H, Goh B-H. Investigation of antioxidative and anticancer potentials of Streptomyces sp. MUM256 isolated from Malaysia mangrove soil. Front Microbiol. 2015;6:1316.
Crossref - Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337-341.
Crossref - Kumar PS, Al-Dhabi NA, Duraipandiyan V, Balachandran C, Kumar PP, Ignacimuthu S. In vitro antimicrobial, antioxidant and cytotoxic properties of Streptomyces lavendulae strain SCA5. BMC Microbiol. 2014;14:1-12.
Crossref - Subramanian R, Subbramaniyan P, Raj V. Antioxidant activity of the stem bark of Shorea roxburghii and its silver reducing power. Springerplus. 2013;2(1):28.
Crossref - Kumari N, Menghani E, Mithal R, GCMS analysis & assessment of antimicrobial potential of rhizospheric Actinomycetes of AIA3 isolate. Indian J Tradit Know. 2020;19(1):111-119.
Crossref - Kumari N, Menghani E. Evaluation of antibacterial activity and identification of bioactive metabolites by GCMS technique from Rhizospheric Actinomycetes. Indian J Nat Prod Resour. (IJNPR) 2021;11(4):287-294.
Crossref - Sneath PHA,Sokal Rr, 1973.Freeman, San Fransciso; Molecular Evolutionary Genetic Analysis, Version 7.0 http://www.megasoftware.net
- Tamura K, Stecher G, Kumar S. MEGA 11: Molecular Evolutionary Genetics Analysis 2021, Version 11. Mol Biol Evol. 2021;38(7):3022-3027.
Crossref - Keerthana S, Abilasha R, Saraswathi K, Arumugam P. Antioxidant and Antibacterial Natural Products Evaluation from Terrestrial Streptomyces Species Strain KAV 2 Isolated from Rhizosphere Regions of Piper betle. J Drug Delivery Ther. 2019;9(4-A):26-37.
Crossref - Naseer N, Fatima A, Cheema MT, Hasnain S, Sajid I. Evaluation ofantioxidant, antimicrobial and antitumor compounds production potential of Streptomyces antioxidans PU-12M. Res J Biotechnol. 2022;17(11):19-28.
Crossref - Berlina AN, Ragozina MY, Komova NS, Serebrennikova KV, Zherdev AV, Dzantiev BB. Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalatein Natural Waters. Biosensors. 2022;12(11):1002.
Crossref - Sepperumal U, Saminathan S. Effect of diethylphthalate on the haematological parameters of the fresh water fish Oreochromis mossambicus (Tilapia). Eur J Zool Res, 2013;2(4):55-59.
- Smith DE, Firth CR, Mighall TM, Teasdale PA. Deglaciation and neotectonics in SE Raasay, Scottish Inner Hebrides. Scottish Journal of Geology. 2021:106-116.
Crossref - Kaneko H, Namihira M, Yamamoto S, Numata N, Hyodo K. Oral administration of cyclic glycyl-proline facilitates task learning in a rat stroke model. Behav Brain Res. 2022;417:113561.
Crossref - Turkez H, Tozlu OO, Tatar A, et al. Toxicity of Glycyl-l-Prolyl-l-Glutamate Pseudotripeptides: Cytotoxic, Oxidative, Genotoxic, and Embryotoxic Perspectives. J Toxicol. 2022;2022:3775194.
Crossref - Carratore FD, Hanko EK, Breitling R, Takano E. Biotechnological application of Streptomyces for the production of clinical drugs and other bioactive molecules. Curr Opin Biotechnol. 2022;77:102762.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.