ISSN: 0973-7510
E-ISSN: 2581-690X
Endophytic fungi have attracted considerable attention because of their diverse bioactive compounds and potential pharmaceutical applications. In this study, we investigated the antioxidant activity of endophytic fungi isolated from green and brown algae. The isolates were obtained from various algal species, and their antioxidant potential was assessed using standard assays. The DPPH assay and reducing power assay were used to measure the antioxidant properties of fungal strains (Fug 03-07) against a specific biochemical process. The inhibitory potential of these strains was assessed using optical density (OD) at 517 nm and compared to the control group. Fug 05 consistently displayed the strongest inhibitory effect across all concentrations, suggesting its most potent influence on the process. Ascorbic acid mirrored these low OD values, confirming its inhibitory efficacy. Fug 09 displayed the highest percentage inhibition at lower concentrations, while Fug 07 emerged as the leader at higher concentrations. The observed concentration-dependent trend suggests that the inhibitory effects of fungal strains strengthen with increasing concentration, highlighting their potential for various applications. The strong inhibitory effect of ascorbic acid aligns with its known antioxidant properties. Further research is needed to understand the underlying mechanisms and explore potential applications. The results indicate that these endophytic fungi possess significant antioxidant properties, making them promising candidates for further research in the field of natural antioxidants.
Endophytic Fungi, Antioxidant, Radical Scavenging Assay, DPPH Assay
In recent years, there has been a growing interest in the exploration of bioactive compounds produced by endophytic fungi associated with marine organisms.1 This interest is driven by the recognition that marine ecosystems, particularly algae, represent a bountiful source of natural products with diverse pharmacological properties.2 Oxidative stress arises from an imbalance between the generation of reactive oxygen species (ROS) and the body’s capacity to counteract them using antioxidants.3 Algae, which include both green and brown varieties, are renowned for their resilience in challenging marine environments and have evolved an impressive repertoire of defence mechanisms against oxidative stress.4 Their primary strategy involves the synthesis of secondary metabolites, including phenolic compounds, alkaloids, and terpenoids, which play a pivotal role in safeguarding algae from environmental stressors and predators.5 It is hypothesized that endophytic fungi residing within these algae may partake in the synthesis of these bioactive compounds, thereby augmenting the overall antioxidant capacity of their host organisms.6 Endophytic fungi have long been acknowledged for their potential to produce bioactive molecules with various biological activities, including antimicrobial, anti-inflammatory, and antioxidant properties.7 These fungi establish mutually beneficial relationships with host organisms by inhabiting their internal tissues, often resulting in the synthesis of unique secondary metabolites. These metabolites have been a subject of intensive research, holding promise for the discovery of novel antioxidants with therapeutic potential.8 Reactive oxygen species (ROS) are highly reactive molecules capable of inflicting oxidative damage to cellular components, contributing to the onset of various diseases and the ageing process.9 Natural antioxidants derived from both plant and microbial sources have emerged as promising therapeutic agents for mitigating oxidative stress-related disorders.10 In particular, endophytic fungi residing within the tissues of plants and algae have gained recognition as prolific sources of bioactive compounds, including antioxidants. In the context of this study, we aim to investigate the antioxidant activity of endophytic fungi isolated from green and brown algae. However, despite the growing body of literature on endophytic fungi, their role in the production of antioxidants within marine algae remains relatively unexplored territory. This research endeavour seeks to address this knowledge gap by isolating endophytic fungi from green and brown algae and conducting a comprehensive evaluation of their antioxidant activity. Our primary objective is to shed light on the degree to which endophytic fungi contribute to the overall antioxidant capacity of their algal hosts. Furthermore, this study carries the potential to unveil novel antioxidant compounds with prospective applications in the realms of pharmaceuticals and nutraceuticals.
This study examines the antioxidative properties of endophytic fungi extracted from green and brown algae. The results offer insights into the potential health advantages associated with these microorganisms found in marine environments. Ultimately, this research could establish a basis for creating novel antioxidant treatments and enhance our comprehension of the complex relationship between endophytic fungi and their host algae.
Sample collection
Green and brown algae were collected from coastal regions. The samples were carefully transported to the laboratory to prevent contamination and degradation.
Isolation of endophytic fungi
The endophytic fungi were isolated from the collected algae using the surface sterilization method. Briefly, the algae were washed with distilled water, followed by surface sterilization using 70% ethanol and 2% sodium hypochlorite.11 After several rinses with sterile distilled water, small tissue sections were cut, plated on to potato dextrose agar (PDA), and incubated at 25 °C for 7-10 days until fungal growth was observed.
Identification of endophytic fungi
The isolated endophytic fungi were identified based on morphological characteristics and molecular analysis using ITS (Internal Transcribed Spacer) sequencing.12 In the process of molecular identification of endophytic fungi, the fungal cultures were cultivated on PDA slants at a controlled temperature of 28 ± 2 °C for a duration of 7 days.13 Following this incubation period, the fungal mat was meticulously separated and subsequently suspended in a lysis buffer solution. The extraction of DNA was executed utilizing the Expure Microbial DNA Isolation Kit. Following the successful isolation of DNA, the primers ITS 1 (5′-TCC GTA GGT GAA CCT GCGG-3′) and ITS 4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) primers were employed for the purpose of DNA amplification of the fungal genome.14 The purified PCR product thus obtained was utilized for Sanger Sequencing, which was carried out at the Regional Facility for DNA Fingerprinting located at the Rajiv Gandhi Centre for Biotechnology in Thiruvananthapuram, India. Subsequently, the obtained raw data in ATB file format was processed to derive FASTA sequences, and further analysis was conducted using the Basic Local Alignment Search Tool (BLAST Analysis) to facilitate the accurate identification of fungal species.
Preparation of extracts
The endophytic fungi were grown in liquid culture media (e.g., Sabouraud dextrose broth) for 7-10 days. The mycelium was harvested, freeze-dried, and then extracted using solvents such as ethyl acetate.
Antioxidant activity assays
The antioxidant activity of the fungal extracts was evaluated using in vitro assays DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay.15-19 The assays were performed in triplicate and ascorbic acid was used as a positive control. The DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, widely employed in natural product antioxidant investigations, relies on the principle that substances capable of donating hydrogen atoms function as antioxidants by scavenging free radicals.20-22 The simplicity and sensitivity of this method from its measurement of radical scavenging compounds. DPPH, a stable and commercially available organic nitrogen radical, serves as the center piece of this assay. The degree of antioxidant activity is directly related to the reduction in DPPH levels within test samples. Monitoring DPPH through a UV spectrometer is the favoured approach due to its ease and precision, with DPPH exhibiting a distinct absorption peak at 517 nm, initially appearing purple and transitioning to yellow as it absorbs hydrogen from antioxidants. This reaction is stoichiometric, permitting the straightforward evaluation of antioxidant efficacy by tracking the reduction in UV absorption at 517 nm during the procedure shown in Figure 1.
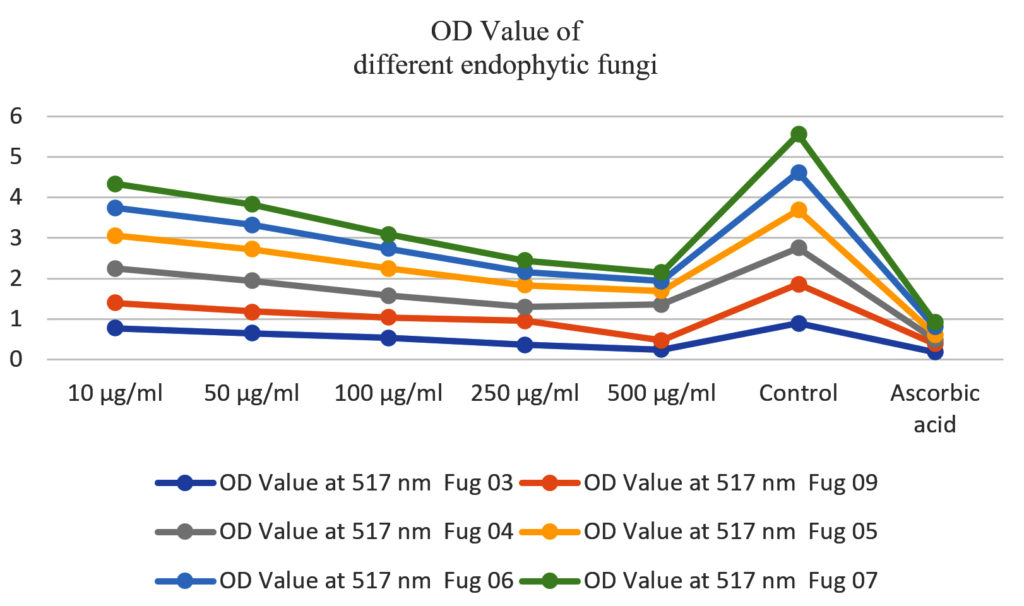
The isolation and identification process yielded a diverse range of endophytic fungi from both green and brown algae. The antioxidant activity of the fungal extracts was evaluated using different assays.17-20 The results revealed that many isolated endophytic fungi exhibited potent antioxidant activity. The DPPH significant scavenging capacities, while the reducing power assay showed the ability of the extracts to donate electrons. The presented Table 1 and Table 2 encapsulates the results of a comprehensive experiment aimed at investigating the inhibitory potential of different concentrations (10 μg/ml, 50 μg/ml, 100 μg/ml, 250 μg/ml, and 500 μg/ml) of Fungi strains (Fug 03, Fug 09, Fug 04, Fug 05, Fug 06, and Fug 07) in a specific biochemical process, evaluated through optical density (OD) values measured at 517 nm.
Table (1):
Represent the OD value of different fungi in various concentration
| Tested sample concen. (μg/ml) | OD Value at 517 nm | |||||
|---|---|---|---|---|---|---|
| Fug 03 | Fug 09 | Fug 04 | Fug 05 | Fug 06 | Fug 07 | |
| 10 μg/ml | 0.776 | 0.6236 | 0.844 | 0.8173 | 0.6836 | 0.5976 |
| 50 μg/ml | 0.647 | 0.536 | 0.7626 | 0.78 | 0.6036 | 0.4996 |
| 100 μg/ml | 0.536 | 0.4996 | 0.5396 | 0.6783 | 0.4866 | 0.3526 |
| 250 μg/ml | 0.361 | 0.5953 | 0.3433 | 0.5336 | 0.3276 | 0.278 |
| 500 μg/ml | 0.244 | 0.2213 | 0.8926 | 0.336 | 0.2446 | 0.2016 |
| Control | 0.897 | 0.9693 | 0.8926 | 0.9336 | 0.93566 | 0.932 |
| Ascorbic acid | 0.186 | 0.1986 | 0.1113 | 0.1126 | 0.1986 | 0.1113 |
Table (2):
Represent the percentage of inhibition of different fungi in various concentration
| Tested sample concen. (μg/ml) | Percentage of Inhibition | |||||
|---|---|---|---|---|---|---|
| Fug 03 | Fug 09 | Fug 04 | Fug 05 | Fug 06 | Fug 07 | |
| 10 μg/ml | 13.4522 | 35.6381 | 5.38117 | 12.3973 | 26.8806 | 35.8727 |
| 50 μg/ml | 27.7964 | 44.6852 | 14.4993 | 16.3987 | 35.4367 | 46.3877 |
| 100 μg/ml | 40.1709 | 51.5308 | 39.4993 | 27.2955 | 47.9501 | 62.1602 |
| 250 μg/ml | 59.6804 | 63.8459 | 61.5097 | 42.801 | 64.9554 | 70.1717 |
| 500 μg/ml | 72.7239 | 77.1586 | 72.571 | 63.9871 | 73.8324 | 78.3619 |
| Ascorbic acid | 79.1899 | 79.4978 | 87.5187 | 87.9243 | 78.7522 | 88.0544 |
Additionally, the Table 1 furnishes the corresponding percentages of inhibition in relation to both a control group and a positive control group treated with ascorbic acid. The discussion of these results delves into the implications and nuances of this data.1-22 The OD values at 517 nm, serving as a proxy for the extent of light absorption by the test samples, provide crucial insights into the degree of inhibition exhibited by the various Fungi strains. Lower OD values indicate a more pronounced inhibition of the biochemical process under scrutiny. A consistent trend across all concentrations emerges, with Fug 05 consistently registering the lowest OD values among the Fungi strains. This suggests that Fug 05 has the highest inhibitory influence on the biochemical process under investigation. In contrast, the control group displays the highest OD values, indicating that the process unfolds without any hindrance in the absence of fungi strains or ascorbic acid. Notably, ascorbic acid, employed as the positive control, mirrors the low OD values of the Fungi strains, affirming its efficacy in inhibiting the biochemical process. The percentage of inhibition stands as a pivotal metric in gauging the effectiveness of Fungi strains in suppressing the targeted biochemical process.
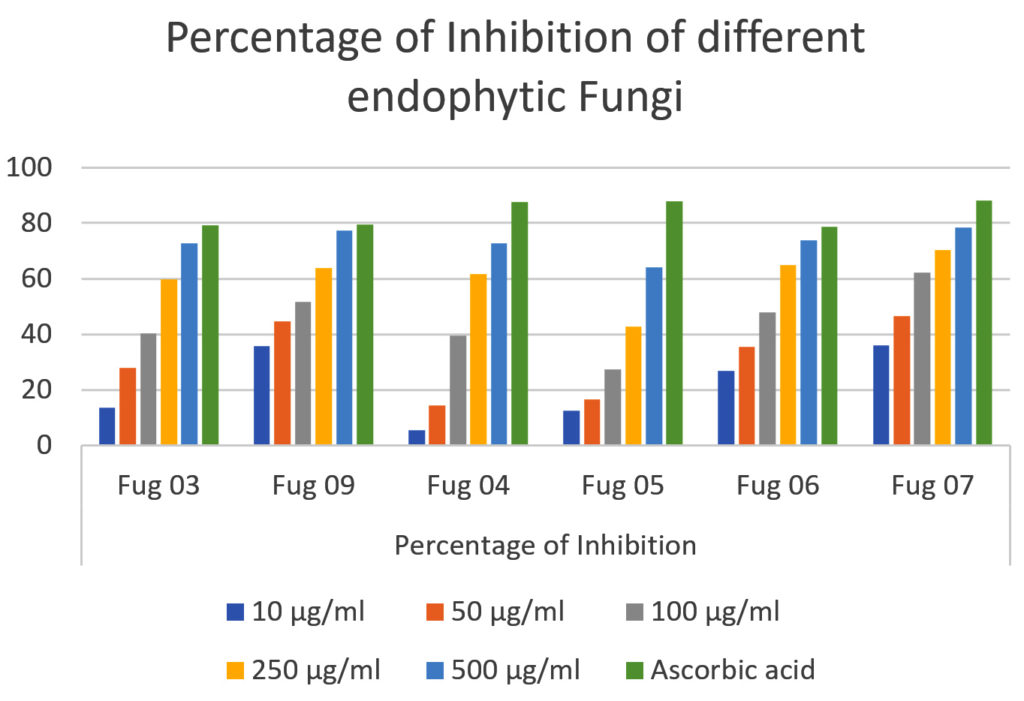
At lower sample concentrations (10 μg/ml and 50 μg/ml), Fug 09 surfaces as the frontrunner, boasting the highest inhibition percentages among the Fungi strains. As the sample concentration escalates, Fug 07 consistently outperforms its counterparts, displaying the highest percentage of inhibition among the Fungi strains which is represented in Figure 2 and Figure 3. Predictably, ascorbic acid consistently exhibits elevated inhibition percentages across all concentrations, underscoring its robust inhibitory prowess in relation to the process under scrutiny. Furthermore, the variety of sample concentrations explored, ranging from 10 μg/ml to 500 μg /ml, provides insight into the concentration-dependent dynamics of inhibition induced by Fungi strains. The outcomes reveal that as the concentration of Fungi strains increases, so too does their inhibitory impact on the biochemical process. This concentration-dependent trend sheds light on the potential scalability of the inhibitory effects exhibited by Fungi strains, hinting at their versatility in various applications. Comparative analysis among the Fungi strains emphasizes that Fug 05 and Fug 07 consistently manifest more potent inhibitory effects across different concentrations. These strains exhibit a consistent ability to curtail the biochemical process.
In contrast, the control group, bereft of Fungi strains or ascorbic acid, does not show inhibitory activity. Ascorbic acid, used as the positive control, shows remarkable inhibitory effects, surpassing most Fungi strains in its ability to obstruct the target process. Biologically, these findings imply that Fungi strains, particularly Fug 05 and Fug 07, harbour the potential to significantly inhibit the biochemical process studied. In addition, in-depth research is imperative to elucidate the precise mechanisms underpinning this inhibition and to explore the practical applications of these strains in various domains. Equally noteworthy is the demonstrated efficacy of ascorbic acid as a potent inhibitor, in line with its well-known antioxidant properties.
In conclusion, both studies reveal the potential of natural sources for beneficial applications. The first study highlights the antioxidant activity found in endophytic fungi isolated from green and brown algae, indicating their potential as valuable sources of natural antioxidants. The antioxidant capacities of the endophytic fungal cultures were significantly correlated with their total phenolic contents, suggesting that phenolics were also the major antioxidant constituents of the endophytes. Further investigations are essential to identify and characterize the specific bioactive compounds responsible for these antioxidant properties. The use of these endophytic fungi as antioxidants may contribute to the development of innovative therapies against oxidative stress-related conditions. In Huang et al studies report comparatively higher activity of antioxidants in endophyte colonized hosts resulting from abiotic stress and propose antioxidants are another currency via which mutualistic interactions between fungal endophytes and their hosts can occur. Endophytic fungi from medicinal plants are a valuable source for bioprospecting endophytes, representing a chemical reservoir for new compounds such as anticancer, immunomodulatory, antioxidant, antiparasitic, antiviral, antitubercular, insecticidal, making it a treasure worth searching. Endophytic fungi have a special relationship with medicinal plants, influencing the formation of metabolic products and enhancing growth, which can be exploited to produce better drugs.
Meanwhile, the second study sheds light on the inhibitory abilities of various strains of fungi and ascorbic acid in modulating a specific biochemical process. These results emphasise the concentration-dependent nature of inhibition, showcasing the promising potential of fungi strains, especially Fug 05 and Fug 07, in scenarios where inhibition is desired. Further in-depth investigations are required to unravel the intricacies of these inhibitory mechanisms and their broader implications in both scientific and practical domains. Both studies, in their unique ways, underscore the importance of harnessing natural resources for various applications in medicine and beyond.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Huang WY, Cai YZ, Xing J, Corke H, Sun M. A Potential Antioxidant Resource: Endophytic Fungi from Medicinal Plants. Eco Bot. 2007;61(1):14-30.
Crossref - Chandra H, Kumari P, Prasad R, Gupta SC, Yadav S. Antioxidant and antimicrobial activity displayed by a fungal endophyte Alternaria alternata isolated from Picrorhiza kurroa from Garhwal Himalayas, India. Biocatal Agric Biotechnol. 2021;33:101955.
Crossref - Huang WY, Cai YZ, Hyde KD, Corke H, Sun M. Endophytic fungi from Nerium oleander L (Apocynaceae): Main constituents and antioxidant activity. World J Microbiol Biotechnol. 2007;23(9):1253-1263.
Crossref - Kaul S, Gupta S, Ahmed M, Dhar MK. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem Rev. 2012;11(4):487-505.
Crossref - Lee JC, Hou MF, Huang HW, et al. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013;13(1):55.
Crossref - Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401(1):1-11.
Crossref - Pavia H, Toth GB. Inducible Chemical Resistance to Herbivory in the Brown Seaweed Ascophyllum nodosum. Ecology. 2000;81(11): 3212-3225.
Crossref - Strobel GA. Endophytes as sources of bioactive products. Micro and Infec. 2003;5(6):535-544.
Crossref - Schulz B, Boyle C, Draeger S, Rommert AK, Krohn K. Myco Res. 2002;106(9):996-1004.
Crossref - Kamat S, Kumari M, Taritla S, Jayabaskaran C. Endophytic Fungi of Marine Alga From Konkan Coast, India-A Rich Source of Bioactive Material. Front Mari Sci. 2020;7.
Crossref - Gasparovic AC. Free Radical Research in Cancer. Antioxidant. 2020;9(2):157.
Crossref - Haniffa HM, Dharmaratne HRW, Mohammad MY. Bioactive cyclo-(S-Pro-R-Leu) from Aspergillus flavus, the marine endophytic fungus from brown alga, Dictyota kunthi. J Natn Sci Foundation Sri Lanka. 2022;50(3):717-721.
Crossref - Bastos EGP, Aguiar AA, de Oliveira AIT, da Silva JFM, Pimenta RS. Antimicrobial evaluation of endophytic fungi extracts isolated from Casearia sylvestris. J Med Plan Res. 2017;11(43):683-689.
Crossref - Ma H, Liu R, Zhao Z, et al. A Novel Peptide from Soybean Protein Isolate Significantly Enhances Resistance of the Organism under Oxidative Stress. Plos One. 2016;11(7):e0159938.
Crossref - Septiana E, Yadi Y, Simanjuntak P. Antioxidant Activity of Endophytic Fungi Isolated from Turmeric Flowers. Biosaintifika. 2021;1212(2):268-273.
Crossref - Habisukan UH, Elfita E, Widjajanti H, Setiawan A, Kurniawati AR. Diversity of endophytic fungi in Syzygium aqueum. Biodiversitas. 2021;22(10):4572-4582.
Crossref - Lavanya N, Mani VM, Nachimuthu S, Deepakkumar R, Preethi K. Endophytic fungal isolation from Blumea axillaris: Identification and biological activity of secondary metabolites. Notulae Scientia Biologicae. 2021;13(2):10953.
Crossref - Meshram V, Kapoor N, Saxena S. Muscodor kashayum sp. nov. – a new volatile anti-microbial producing endophytic fungus. Mycology. 2013;4(4):196-204.
Crossref - Vargas-Asensio G, Pinto-Tomas A, Rivera B, et al. Uncovering the cultivable microbial diversity of costa rican beetles and its ability to break down plant cell wall components. PLoS ONE. 2014;9(11):e113303.
Crossref - Li W, Zhao X, Sun X, Zu Y, Liu Y, Ge Y. Evaluation of Antioxidant Ability In Vitro and Bioavailability of trans-Cinnamic Acid Nanoparticle by Liquid Antisolvent Precipitate. J Nanomater. 2016;2016(1):1-11.
Crossref - Sisay MA, Mammo W, Yaya EE. Phytochemical studies of Melilotus officinalis. Bulle Chem Soc Ethio. 2021;35(1):141-150.
Crossref - Cui JL, Guo TT, Ren ZX, Zhang NS, Wang ML. Diversity and antioxidant activity of culturable endophytic fungi from alpine plants of Rhodiola crenulata, R. angusta, and R. sachalinensis. Plos One. 2015;10(3):e0118204.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.