ISSN: 0973-7510
E-ISSN: 2581-690X
Methicillin-resistant Staphylococcus aureus (MRSA) infections of soft tissues and deep and superficial wounds affect morbidity, mortality, and healthcare resources in Saudi Arabia. The growing frequency of these infections underscore the need for effective antimicrobial stewardship projects and robust infection control. This study included patients from various age groups and was conducted in a tertiary care facility in Riyadh. Clinical specimens from different body parts were cultured, as part of the study protocol. Samples showing significant Staphylococcus aureus (S. aureus) growth were identified phenotypically and tested for microbial susceptibility. Antibiotics were evaluated and analyzed according to Clinical and Laboratory Standards Institute (CLSI) guidelines. MRSA isolates were screened using cefoxitin discs. Data was gathered and analyzed over one year, from January to December 2023. This study included 11,837 culture specimens, of which 3,795 yielded positive cultures. S. aureus was isolated in 333 cases, accounting for 2.8% of all specimens, and 8.8% of positive cultures. Most patients were male, comprising 121 study participants (36%). The highest MRSA prevalence was observed between 12 and 40 years. The prevalence of MRSA in the local healthcare environment was 3.3%. Hospitalized patients showed a slightly higher incidence of MRSA infection. MRSA prevalence was significantly associated with the specimen type (p < 0.05). Vancomycin and linezolid were 100% effective against all S. aureus strains, followed by tetracycline and gentamicin. The D-test was used to assess 307 S. aureus strains to assess inducible clindamycin resistance, 28 tested positive. The high MRSA infection load and new MRSA strains highlight the need for ongoing surveillance, stringent infection control, and targeted antibiotic stewardship programs. Our findings could be used to guide antibiotic treatment and combat antibiotic resistance in local hospitals.
Methicillin-resistant Staphylococcus aureus, Antimicrobial Susceptibility Testing, Resistance
Methicillin-resistant Staphylococcus aureus (MRSA) is a strain of S. aureus that develops resistance to methicillin and other beta-lactam antibiotics.1 The emergence of MRSA dates back to the early 1960s, shortly after the introduction of methicillin, when certain strains of S. aureus began exhibiting resistance to this antibiotic.2 MRSA quickly became a significant concern in healthcare settings because of its ability to cause infections that are challenging to treat. Despite the introduction of methicillin and other semisynthetic penicillins as advancements in the treatment of S. aureus infections, the emergence of methicillin-resistant strains, such as MRSA, within just a couple of years has highlighted a major setback in combating these bacteria.2 Over time, MRSA resistance mechanisms have evolved, resulting in strains resistant to methicillin and other classes of antibiotics, posing a significant challenge in clinical management.3
Distinguishing healthcare-associated MRSA (HA-MRSA) and community-associated MRSA (CA-MRSA) is crucial for understanding the epidemiology and transmission dynamics of these strains.4 While HA-MRSA is often linked to healthcare settings and nosocomial infections, CA-MRSA has emerged as a distinct entity that affects otherwise healthy individuals in community settings.5 The rise of CA-MRSA has raised concerns about its potential to spread rapidly among the general population, as it can be transmitted through close contact or shared items in schools, gyms, and households. Unlike HA-MRSA, which is often acquired in healthcare facilities, CA-MRSA infections can occur in individuals without any previous healthcare exposure. This shift in MRSA epidemiology highlights the importance of targeted prevention strategies and surveillance efforts to control the spread of resistant bacteria in healthcare and community settings.
Globally, the spread of MRSA remains a serious concerns, with studies reporting rising prevalence across diverse populations and environments.6 MRSA’s ability to colonize different niches, including the nasal cavity, skin, and wounds, has contributed to its persistence and dissemination.7 Its evolutionary history is shaped by the acquisition of resistance determinants and genetic elements that confer survival advantages under antibiotic pressure. The antimicrobial susceptibility patterns of MRSA strains vary geographically, necessitating region-specific strategies for antimicrobial stewardship and prevention of infection. MRSA is a serious issue in Saudi Arabia, where reports of its prevalence vary by geography and hospital setting.8-10 Some studies have reported MRSA prevalence as high as 82.5% in certain healthcare facilities in Saudi Arabia.8 Furthermore, studies have highlighted the emergence of new clonal complexes and MRSA strains in the country.11,12 This highlights the dynamic nature of MRSA epidemiology in Saudi Arabia and emphasizes the need for continuous surveillance and monitoring.12 MRSA is a significant concern in Saudi Arabia, with varying prevalence rates and the emergence of new strains. This dynamic nature emphasizes the importance of ongoing surveillance and monitoring to combat the spread of MRSA in healthcare facilities effectively. Region-specific antimicrobial stewardship and infection prevention are crucial for addressing this serious issue and reducing the impact of MRSA on public health in Saudi Arabia.
Saudi Arabia has a high rate of methicillin-resistant S. aureus (MRSA) infection, which has a major effect on healthcare costs, morbidity, and mortality.13,14 MRSA infections have been linked to several clinical disorders such as hospital-acquired infections and infections of soft tissues, wounds, and skin.14 In addition, the frequency of MRSA infections has steadily increased in the country.13 These findings highlight the importance of implementing effective infection control measures and antimicrobial stewardship programs to mitigate the spread of MRSA in healthcare settings.9,15 These programs can help reduce the incidence of MRSA infections, improve patient outcomes, and reduce healthcare costs. To combat the rising trend of MRSA in Saudi Arabia, it is crucial for healthcare facilities to prioritize infection control practices such as proper hand hygiene, disinfection protocols, and appropriate use of antibiotics. By proactively addressing these issues, healthcare providers can improve the overall health and well-being of the Saudi Arabian population.
Continued surveillance and monitoring of antimicrobial resistance patterns is essential for guiding treatment decisions and preventing the spread of resistant strains. Healthcare professionals should prioritize infection prevention measures such as hand hygiene and proper disinfection practices to minimize the risk of MRSA transmission in healthcare settings. Collaborative efforts among healthcare providers, public health authorities, and researchers are crucial for developing and implementing effective strategies to address the growing threat of MRSA and other antimicrobial-resistant infections in Saudi Arabia. Moreover, studies have identified distinct antimicrobial resistance patterns of MRSA, highlighting the need for comprehensive, coordinated responses to this public health challenge.14,16,17 The emergence of MRSA strains with reduced susceptibility to glycopeptides further complicates clinical management of MRSA infections.18
Study design and settings
This retrospective study included patients from our 450-bed tertiary care hospital. The hospital established standards for culture, sensitivity of sample collection, and aseptic transport to the laboratory. All patient data were entered into the hospital’s electronic system. Microbiological and antimicrobial susceptibility testing were performed by the Microbiology Department of MD Lab, Riyadh, Saudi Arabia.
Ethics approval
This study was approved by the Research Committee of Dr. Sulaiman Al Habib Medical Group Research Center, Riyadh, Saudi Arabia, vide reference number RC24.05.13.
Data collection
Data was collected and analyzed retrospectively from January to December 2023.
Inclusion criteria
This study included all non-duplicate samples submitted for sensitivity and culture from patients of all ages who were registered inpatients or outpatients at this institution.
Exclusion criteria
This study excluded all duplicate, repeat, or follow-up cultures performed within 7 days of the initial culture request.
Statistical analysis
Statistical analysis was performed using Excel and IBM SPSS version 20. Statistical significance was set at p < 0.05 significant.
Study protocol
Clinical samples from various body sites, including pus, blood, urine, sputum, body fluids, wound swabs, tracheal aspirates, were plated on appropriate media following the standard operating procedures (SOPs) to process each sample. Culture plates were incubated at 37 °C for 24 to 48 hours. Using Vitek 2, all cultures with significant S. aureus growth were phenotyped and tested for antibiotic susceptibility. Antimicrobial susceptibility testing was performed using the Standard Kirby-Bauer disc diffusion method as per the M100 CLSI criteria. All S. aureus isolates were screened for MRSA using a cefoxitin (FOX 30 µg) disc. This was further confirmed by measuring oxacillin resistance using the Vitek 2 system.
Antibiotics tested were penicillin (PG 10 units), oxacillin (OX 1 µg), ciprofloxacin (CIP 5 µg), levofloxacin (LEV 5 µg), erythromycin (E 15 µg), clindamycin (CD 2 µg), trimethoprim/ sulfamethoxazole (TS 1.25/23.75 µg), gentamicin (GM 10 µg), fusidic acid (FC 10 µg), tetracycline (T 30 µg), vancomycin (VA 30 µg), and linezolid (LZD 30 µg).
During the study period, 11,837 culture specimens were collected. Of these, 3795 (32%) yielded positive bacterial cultures with susceptibility results. S. aureus was detected in 333 cases, representing 2.8% of culture requests and 8.8% of positive cultures. The overall frequency of S. aureus isolation in males was 180 (54.1%), whereas in females it was 153 (45.9%). Among the 333 cases, 259 (77.8%) were recorded as outpatients in various OPD clinics, while the remaining 74 patients (22.2%) were admitted as inpatients. Patient ages ranged from 1 day to 105 years, with a mean of 39.5 ± 25.41 standard deviation (SD). The highest frequency of S. aureus isolation pertained to individuals with ages 12 to 40 years, with 121 cases (36%), followed by those older than 61 years (80 cases; 24%). The isolates were most commonly detected in pus and wound specimens (n = 207, 62.2%), followed by ear swabs (n = 31, 9.3%), sputum/tracheal aspirates, and urine (n = 26, 7.8% each).
The prevalence of MRSA was found to be 3.3% in our local healthcare settings. Among the 333 S. aureus samples, the isolation of MRSA was 124 (37.2%), while the isolation of MSSA accounted for 209 (62.8%). Table 1 shows the association of MRSA with patients’ sex, age, registration in the hospital, and the type of specimen collected. The prevalence of MRSA in male patients was 34.4% compared to 40.5% in female patients, with a p-value = 0.258. No significant correlation was observed between MRSA isolation and sex. MRSA isolation was highest in 12-40 year olds (48 cases). This was followed by 30 cases in the 41-60-year age group. MRSA isolation was not significantly associated with age (P = 0.475).
Table (1):
Association of MSSA and MRSA isolates (n = 333) with gender, age, patient registration and specimen type
| Variables | MSSA Count (%) | MRSA Count (%) | P-value | |
|---|---|---|---|---|
| Gender | Male | 118 (65.5%) | 62 (34.4%) | 0.258 |
| Female | 91 (59.4%) | 62 (40.5%) | ||
| Age group | Less than 12 years | 40 (65.5%) | 21 (34.4%) | 0.475 |
| 12 to 40 years | 73 (60.3%) | 48 (39.6%) | ||
| 41 to 60 years | 41 (57.7%) | 30 (42.2%) | ||
| More than 61 years | 55 (68.7%) | 25 (31.2%) | ||
| Patient reg. | OP | 164 (63.3%) | 95 (36.6%) | 0.685 |
| IN | 45 (60.8%) | 29 (39.1%) | ||
| Specimen | Blood | 12 (75%) | 4 (25%) | <0.001 |
| Body fluids | 8 (72.7%) | 3 (27.2%) | ||
| Conjunctival swabs | 1 (100%) | 0 (0%) | ||
| Ear swabs | 26 (83.8%) | 5 (16.1%) | ||
| HVS | 1 (100%) | 0 (0%) | ||
| Nasal swabs | 8 (57.1%) | 6 (42.8%) | ||
| Sputum/tracheal aspirates | 13 (50%) | 13 (50%) | ||
| Pus and wound | 122 (58.9%) | 85 (41%) | ||
| Urine | 18 (69.2%) | 8 (30.7%) | ||
MSSA: Methicillin-sensitive Staphylococcus aureus, MRSA: Methicillin-resistant Staphylococcus aureus, Patient reg: Patient registration, OP: Outdoor patients, IN: Indoor patients HVS: High vaginal swab
Among cases occurring outdoors, 95 (36.7%) were reported as MRSA, whereas among indoor patients, there were 29 (39.2%) cases of MRSA. The frequency of MRSA infection has somewhat increased among hospitalized patients. However, there is no statistically significant link between the isolation of MRSA and patient hospitalization, as indicated by a p-value >0.05 (0.685), as shown in Table 1.
However, the presence of S. aureus exhibited a robust correlation with specimen type. A total of 207 cases were recorded from pus and wound cultures, with a statistically significant p-value <0.001. MRSA isolation was found to be significantly correlated with the specimen type. The maximum isolation was observed in pus and wounds (85 of 124 cases), with a p-value of 0.021 (<0.05), as shown in Table 1.
Table (2):
Antimicrobial susceptibility pattern of S. aureus (n = 333) to the tested antibiotics
| Antibiotics | Antibiogram | Count | Percentage |
|---|---|---|---|
| Penicillin | susceptible | 13 | 3.9% |
| resistant | 320 | 96.1% | |
| Oxacillin | susceptible | 209 | 62.8% |
| resistant | 124 | 37.2% | |
| *Erythromycin | susceptible | 203 | 66.1% |
| resistant | 104 | 33.8% | |
| *Clindamycin | susceptible | 265 | 86.3% |
| resistant | 42 | 13.6% | |
| Gentamicin | susceptible | 319 | 95.8% |
| resistant | 14 | 4.2% | |
| Ciprofloxacin | susceptible | 272 | 81.7% |
| resistant | 61 | 18.3% | |
| Levofloxacin | susceptible | 274 | 82.3% |
| resistant | 59 | 17.7% | |
| Tetracycline | susceptible | 316 | 94.9% |
| resistant | 17 | 5.1% | |
| Co-trimoxazole | susceptible | 281 | 84.4% |
| resistant | 52 | 15.6% | |
| Vancomycin | susceptible | 333 | 100.0% |
| resistant | 0 | 0.0% | |
| Linezolid | susceptible | 333 | 100.0% |
| resistant | 0 | 0.0% | |
| Fusidic acid | susceptible | 143 | 42.9% |
| resistant | 190 | 57.1% |
*Erythromycin and clindamycin were not tested for urinary isolates
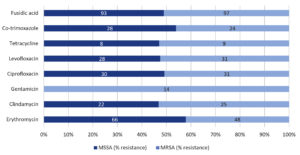
Table 2 presents the antibiograms of all the tested antibiotics. Vancomycin and linezolid demonstrated 100% effective against all S. aureus isolates, resulting in a 0% occurrence of VRSA or VISA isolation in our clinical settings. Tetracycline and gentamicin ranked second highest in efficacy against S. aureus infections, with susceptibility rates of 95.8% and 94.9%, respectively. Clindamycin, co-trimoxazole, levofloxacin, ciprofloxacin, erythromycin, and fusidic acid followed with susceptibility rates of 86.3%, 84.4%, 82.3%, 81.7%, 66.1%, and 42.9%, respectively. All gentamicin-resistant cases were MRSA isolates, whereas no resistance was observed against gentamicin in the MSSA isolates, as shown in Figure.
Figure. Comparison of antimicrobial percent resistance of MSSA and MRSA to the different antibiotics
Based on their multidrug-resistance patterns, 33.87% (n = 42/124) of MRSA isolates were classified as HA-MRSA, while the majority of isolates i.e., 66.12% (n = 82/124) were CA-MRSA. The D-test was used to assess 307 S. aureus samples for inducible clindamycin resistance. However, urinary isolates were not included in this test. A total of 28 isolates tested positive in the D-test, with 12 cases of MSSA and 16 cases of MRSA. A statistically significant correlation was observed between the S. aureus resistant phenotype and the D-test, with a p-value of 0.039 (Table 3).
Table (3):
Inducible clindamycin resistance (D-test) association with MSSA and MRSA phenotypes of S. aureus (n = 307)
| D-test | Total | ||
|---|---|---|---|
| Negative n (%) | Positive n (%) | ||
| MSSA | 179 (93.7%) | 12 (6.2%) | 191 |
| MRSA | 100 (86.2%) | 16 (13.7%) | 116 |
| Total | 279 (91.1%) | 28 (9.1%) | 307 |
P-value = 0.039
The study period involved the analysis of 11,837 culture specimens, of which 3795 (32%) tested positive for bacterial growth and susceptibility. The most common specimens yielding S. aureus were pus and wound specimens (62.2%). Our result is supported by another study, which reported 64.8% isolation of S. aureus from pus and wound cultures.19 Consistent with our findings, Naimi et al. found no statistically significant association between MRSA isolation age and patient sex.20
Several studies have examined the frequency of local MRSA infections. In our setting, MRSA accounted for 3.3% of all isolates. A literature survey showed that the prevalence rates vary by time and place. Studies have reported MRSA prevalence rates ranging from 4% in a hospitals in western Saudi Arabia21 to as high as 39.5% in the western region.22 Moreover, a meta-analysis found 38% MRSA prevalence in Saudi Arabia.23 Variations in prevalence rates were also evident within different cities, with MRSA prevalence ranging from 5.97% to 94% in Dhahran and Riyadh, respectively.24 Additionally, one study found that CA-MRSA infections increased from 9.9 per 10,000 admissions in 2001 to 67 in 2008.13
The prevalence of MRSA in Saudi Arabia was investigated in a specific healthcare setting. Similarly, a study conducted in Jeddah reported a prevalence of community-acquired MRSA at 25.2%.25 Similarly, a study from a tertiary hospital in Riyadh found the prevalence of MRSA to be 39%.15 These findings highlight the importance of understanding MRSA prevalence in various healthcare settings in Saudi Arabia. Notably, the prevalence of MRSA has varied over time. For instance, a study reported a significant increase in the prevalence of MRSA from 29.9% to 38% over a certain period.23 Similarly, another study found that the prevalence of MRSA in Saudi Arabia ranged from 7.5% to 33%.26 These variations underscore the dynamic nature of the MRSA prevalence and the need for ongoing surveillance and research. In conclusion, the prevalence of MRSA in Saudi Arabia varies across regions and healthcare settings. The reported prevalence rates range from 4% to 39.5%, with significant increases observed over time. These findings emphasize the importance of continued monitoring and research to better understand the epidemiology of MRSA in Saudi Arabia.
The presence of S. aureus showed a significant correlation with the type of specimen, particularly pus and wound cultures, indicating a strong association between the presence of S. aureus and these types of specimens.27 Moreover, MRSA isolation showed a statistically significant correlation with specimen type, with the highest number of isolates recovered from pus and wound samples.28 The study found a significant increase in MRSA in 2012 compared to 2004-2005.28 This study supports these findings by highlighting the isolation of S. aureus from various clinical specimens, with the highest isolation rate observed in pus samples.29 Methicillin-resistance in S. aureus can be traced to the acquisition of the exogenous gene mecA, which is part of the staphylococcal cassette chromosome mec (SCCmec). This mecA gene encodes an additional penicillin-binding protein, PBP2a. PBP2a is a peptidoglycan transpeptidase that can confer resistance to all β-lactam antibiotics, including penicillins, cephalosporins, and carbapenems. Other S. aureus isolates may exhibit a broader range of resistance owing to the presence of additional resistance genes within specific SCCmec types, such as types II and III. The presence of PBP2a or mecA is the basis for the identification of MRSA or oxacillin-resistant S. aureus (ORSA).30
Based on the provided references, it is evident that vancomycin and linezolid have consistently demonstrated complete susceptibility against S. aureus.31-34 This aligns with our findings that there was a 0% occurrence of vancomycin-resistant S. aureus (VRSA) or vancomycin-intermediate S. aureus (VISA) isolation in our clinical settings. Additionally, gentamicin and tetracycline have shown high efficacy in treating S. aureus infections, with susceptibility rates of 95.8% and 94.9%, respectively.
The majority of MRSA isolates were classified as CA-MRSA, while HA-MRSA accounted for 33.87%. This distribution is consistent with findings from another region of Saudi Arabia, where 80% of MRSA isolates were reported as CA-MRSA.35 Inducible clindamycin resistance has been found in many S. aureus isolates, with a statistically significant correlation between the resistance phenotype and D-test.33,36-38 The D-test remains a reliable method for detecting inducible clindamycin resistance in S. aureus.39 Among other antibiotics tested, clindamycin, co-trimoxazole, levofloxacin, ciprofloxacin, erythromycin, and fusidic acid showed varying susceptibility levels, with clindamycin demonstrating a susceptibility rate of 86.4%.36,40 These findings have important clinical implications, especially in light of the limited therapeutic options available for the treatment of MRSA infections and the known limitations of vancomycin.38
In conclusion, the introduction of methicillin in the 1960s marked a turning point in the battle against Staphylococcus aureus infections, but also lead to the emergence of MRSA strains that pose significant challenges to healthcare systems worldwide. The evolution of MRSA, from early detection to the development of multidrug-resistant strains, underscores the urgent need for comprehensive strategies to combat the spread of these bacteria. Understanding the epidemiology, transmission dynamics, and resistance mechanisms of MRSA is essential for guiding infection control practices, antimicrobial stewardship efforts, and for developing novel treatment options to address infections caused by resilient pathogens. By remaining informed and proactive in managing MRSA infections, healthcare professionals can help prevent the further spread of these antibiotic-resistant bacteria. Collaboration among healthcare facilities, researchers, and public health agencies is crucial to effectively address the challenges posed by MRSA. Through collective effort and focus on evidence-based practices, we can work to reduce the impact of MRSA on patient outcomes and healthcare systems worldwide.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
FK conceptualized and designed the study. FK, IA, AE and CP performed data collection. FK, AA and HA performed analysis and results interpretation. FK and OTK wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Research Committee of Dr. Sulaiman Al Habib Medical Group Research Center, Riyadh, Saudi Arabia, vide reference number RC24.05.13.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Al-Wahaibi L, Al-Sudairi R, Balkhair A, Al-Awaisi H, Mabruk M. Methicillin-Resistant Staphylococcus aureus Colonization Among Healthcare Workers in Oman. J Infect Dev Ctries. 2021;15(10):1426-1435.
Crossref - Harkins CP, Pichon B, Doumith M, et al. Methicillin-Resistant Staphylococcus aureus Emerged Long Before the Introduction of Methicillin Into Clinical Practice. Genome Biol. 2017;18(1):130.
Crossref - Pradhan S, Regmi SM, Shrestha N. Inducible Clindamycin Resistant Staphylococcus aureus Among Patients Attending Tertiary Care Centre: A Descriptive Cross-Sectional Study. JNMA Nepal Med Assoc. 2021;59(243):1111-1115.
Crossref - Dogramachy NS. Prevalence of Nasal Carriage Rate for Methicillin-Resistant Staphylococcus aureus and Its Antibiotic Susceptibility Profiles in Health Care Workers at Nanakaly Hospital, Erbil, Iraq. Zanco J Med Sci. 2018;22(3):411-419.
Crossref - Diep BA, Otto M. The Role of Virulence Determinants in Community-Associated MRSA Pathogenesis. Trends Microbiol. 2008;16(8):361-369.
Crossref - Baede VO, Tavakol M, Vos MC, Knight GM, van Wamel WJB, MACOTRA study group. Dehydration Tolerance in Epidemic Versus Nonepidemic MRSA Demonstrated by Isothermal Microcalorimetry. Microbiol Spectr. 2022;10(5):e0061522.
Crossref - Udeani TK, Onyebuchi CJ, Ikpenwa MC, Ezenwaka UR. Prevalence and Antibiotic Susceptibility Pattern of Methicillin Resistant Staphylococcus aureus In Burns and Pressure Ulcer Patients. Afr J Clin Exp Microbiol. 2015;17(2):130.
Crossref - Mater ME, Yamani AE, Aljuffri AA, Binladen SA. Epidemiology of burn-related infections in the largest burn unit in Saudi Arabia. Saudi Med J. 2020;41(7):726-732.
Crossref - Taha AE, Al-Ruwaili NM, El-Masry EA, Saad AE, Taher IA. MRSA as an indicator of infection control measures in Turaif General Hospital, Northern Area-Saudi Arabia. J Infect Dev Ctries. 2022;16(06):1037-1044.
Crossref - Al-Zahrani IA, Azhar EI, Jiman-Fatani AA, et al. Impact of mass migrations on the clonal variation of clinical Staphylococcus aureus strains isolated from the Western region of Saudi Arabia. J Infect Public Health. 2019;12(3):317-322.
Crossref - Senok A, Ehricht R, Monecke S, Al-Saedan R, Somily A. Molecular characterization of methicillin-resistant Staphylococcus aureus in nosocomial infections in a tertiary-care facility: emergence of new clonal complexes in Saudi Arabia. New Microbes New Infect. 2016;14:13-18.
Crossref - Senok A, Somily AM, Nassar R, et al. Emergence of novel methicillin-resistant Staphylococcus aureus strains in a tertiary care facility in Riyadh, Saudi Arabia. Infect Drug Resist. 2019;12:2739-2746.
Crossref - Alrabiah K, Al Alola S, Al Banyan E, Al Shaalan M, Al Johani S. Characteristics and risk factors of hospital acquired – Methicillin-resistant Staphylococcus aureus (HA-MRSA) infection of pediatric patients in a tertiary care hospital in Riyadh, Saudi Arabia. Int J Pediatr Adolesc Med. 2016;3(2):71-77.
Crossref - El-Kersh TA, Marie MA, Al-Sheikh YA, Al-Agamy MH, Al-Bloushy AA. Prevalence and risk factors of early fecal carriage of Enterococcus faecalis and Staphylococcus spp. and their antimicrobial resistant patterns among healthy neonates born in a hospital setting in central Saudi Arabia. Saudi Med J. 2016;37(3):280-287.
Crossref - Althaqafi AO, Matar MJ, Moghnieh R, et al. Burden of methicillin-resistant Staphylococcus aureus pneumonia among hospitalized patients in Lebanon and Saudi Arabia. Infect Drug Resist. 2017;10:49-55.
Crossref - Abo-Amer AE, El-Rab SMFG, Halawani EM, Niaz AM, Bamaga MS. Prevalence and Molecular Characterization of Methicillin-Resistant Staphylococcus aureus from Nasal Specimens: Overcoming MRSA with Silver Nanoparticles and Their Applications. J Microbiol Biotechnol. 2022;32(12):1537-1546.
Crossref - El-Saed A, Balkhy HH, Alshamrani MM, et al. High contribution and impact of resistant gram negative pathogens causing surgical site infections at a multi-hospital healthcare system in Saudi Arabia, 2007-2016. BMC Infect Dis. 2020;20(1):275.
Crossref - Al-Obeid S, Haddad Q, Cherkaoui A, Schrenzel J, Francois P. First Detection of an Invasive Staphylococcus aureus Strain (D958) with Reduced Susceptibility to Glycopeptides in Saudi Arabia. J Clin Microbiol. 2010;48(6):2199-2204.
Crossref - Senthamarai DS, Sivasankari DS, Anitha DC, et al. Prevalence of methicillin resistant of Staphylococcus aureus (MRSA) its antibiotic susceptibility pattern from various clinical specimens in a tertiary care hospital. Journal of Medical Science and Clinical Research. 2019;07(02):129-132.
- Naimi HM, Rahimi MH, Noori AZ. Prevalence and Antimicrobial Susceptibility Patterns of Staphylococcus aureus/methicillin- resistant Staphylococcus aureus Nasal Carriage among Kabul University Students. International Journal of Innovative Research and Scientific Studies. 2020;3(1):1-8.
Crossref - Al-Tawfiq JA. Incidence and Epidemiology of Methicillin-Resistant Staphylococcus aureus Infection in a Saudi Arabian Hospital, 1999-2003. Infect Control Hosp Epidemiol. 2006;27(10):1137-1139.
Crossref - Alaklobi F, Aljobair F, Alrashod A, et al. The prevalence of community-associated methicillin-resistant Staphylococcus aureus among outpatient children in a tertiary hospital: A prospective observational study in Riyadh, Saudi Arabia. Int J Pediatr Adolesc Med. 2015;2(3-4):136-140.
Crossref - Adam KM, Abomughaid MM. Prevalence of Methicillin-resistant Staphylococcus aureus in Saudi Arabia Revisited: A Meta-analysis. The Open Public Health Journal. 2018;11(1):584-591.
Crossref - Zaini RG, Ismaiil KA, Rezk HM, Dahlawi H. Pilot Study to Detect the Presence of MRSA among Healthcare Workers Who Practice Ablution. J Transm Dis Immun. 2017:1:1.
Crossref - Madani TA. Epidemiology and clinical features of methicillin-resistant Staphylococcus aureus in the University Hospital, Jeddah, Saudi Arabia. 2002;13(4):245-250.
Crossref - Asghar AH, Momenah AM. Methicillin Resistance among Staphylococcus aureus Isolates from Saudi Hospitals. Medical Principles and Practice. 2005;15(1):52-55.
Crossref - Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin Microbiol Rev. 2015;28(3):603–61
- Wang HK, Huang CY, Huang YC. Clinical features and molecular characteristics of childhood community-associated methicillin-resistant Staphylococcus aureus infection in a medical center in northern Taiwan, 2012. BMC Infect Dis. 2017;17(1):470.
Crossref - Khawaja A, Ejaz A, Abeer, et al. Frequency and Sensitivity Patterns of Staphylococcus aureus in a Tertiary Care Setting. J Pharm Res Int. 2021;33(38B):121-126.
Crossref - Garoy EY, Gebreab YB, Achila OO, et al. Methicillin-Resistant Staphylococcus aureus (MRSA): Prevalence and Antimicrobial Sensitivity Pattern among Patients-A Multicenter Study in Asmara, Eritrea. Can J Infect Dis Med Microbiol. 2019;2019(1):8321834.
Crossref - Ghosh S, Banerjee M. Methicillin resistance & inducible clindamycin resistance in Staphylococcus aureus. Indian J Med Res. 2016;143(3):362-364.
Crossref - Thapa D, Pyakurel S, Thapa S, et al. Staphylococcus aureus with inducible clindamycin resistance and methicillin resistance in a tertiary hospital in Nepal. Trop Med Health. 2021;49(1):99.
Crossref - Rajak KC, Poddar CK, Kumar R, Jha AK. Inducible clindamycin resistant Staphylococcus aureus isolates from tertiary care hospital, bettiah, India. J Evol Med Dent Sci. 2018;7(36):3984-3990.
Crossref - Chaudhary NK, Piya R. Macrolide-lincosamide-streptogramin b resistance among Staphylococcus aureus in chitwan medical college teaching hospital, NepaL. Asian J Pharm Clin Res. 2021;14(5)61-65.
Crossref - Alsolami A, ALGhasab NS, Alharbi MSM, et al. Community-Acquired Methicillin-Resistant Staphylococcus aureus in Hospitals: Age-Specificity and Potential Zoonotic-Zooanthroponotic Transmission Dynamics. Diagnostics. 2023;13(12):2089.
Crossref - Adhikari RP, Shrestha S, Barakoti A, Amatya R. Inducible clindamycin and methicillin resistant Staphylococcus aureus in a tertiary care hospital, Kathmandu, Nepal. BMC Infect Dis. 2017;17(1):483.
Crossref - Pappu RK, Poddar CK, Kumar S, Sinha RN, Shahi SK. Incidence of inducible clindamycin resistance in clinical isolates of Staphylococcus aureus isolates from tertiary care hospital; experience in Koshi area (Northern Bihar), India. Journal of Evidence Based Medicine and Healthcare. 2019;6(2):71-76.
Crossref - Shetty V, Bangera J, Parameshwaran S. Inducible clindamycin resistance in erythromycin-resistant Staphylococcus aureus. J Evol Med Dent Sci.. 2018;7(18):2202-2204.
Crossref - Banik A, Khyriem AB, Gurung J, Lyngdoh VW. Inducible and constitutive clindamycin resistance in Staphylococcus aureus in a northeastern Indian tertiary care hospital. J Infect Dev Ctries. 2015;9(07):725-731.
Crossref - Alshomrani MK, Alharbi AA, Alshehri AA, Arshad M, Dolgum S. Isolation of Staphylococcus aureus Urinary Tract Infections at a Community-Based Healthcare Center in Riyadh. Cureus. 2023;15(2):e35140.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.