ISSN: 0973-7510
E-ISSN: 2581-690X
The study deals with the synthesis and evaluation of some novel triazoles. Amine linked triazoles were prepared through multistep syntheses. 4-Iodobenzoic acid was taken as initial reactant material, which was converted into methyl ester, then to hydrazide and finally into triazole via dithiocarbazate salt. The triazole was treated with chloroacetyl chloride and further with secondary amines to get the title compounds. Structures of the synthesized compounds were elucidated by Fourier transform infrared spectroscopy (FTIR), 13C and 1H nuclear magnetic resonance (NMR), mass spectrometry and elemental analysis. The synthesized compounds were screened for their antimicrobial activity. The study was carried out against four bacteria and three fungi by disc diffusion method using ciprofloxacin and fluconazole as reference drug for antibacterial and antifungal activities respectively. All the synthesized compounds exhibited zones of inhibition against Candida albicans, Aspergillus niger and Fusarium oxysporum, but the inhibition was more prominent as shown by compound T83. Similarly antibacterial activity was studied against Staphylococcus aureus, Basillus subtilis, Psedomonas aeruginosa and Escherichia coli. Data revealed that compounds T81 and T83 were effective against the bacterial strains. Minimum inhibitory concentration test (MIC) was also carried out to ascertain the bacteriostatic property of the test compounds.
Triazoles; Iodobenzoic acid; Secondary amines; Antifungal; Antibacterial; Zone of inhibition
Drug discovery requires biological study of the existing molecule or synthesis of a new chemical compound having some medicinal worth. Syntheses of medicinals have an ever changing role in modern drug discovery and development process. Synthetic compounds are increasingly involved in medicinal innovations. The most striking progress in medicinal chemistry have taken place in the last two decades, where the heterocyclic compounds have been recognized as important pharmacophore eliciting numerous biological activities1. The presence of three nitrogen in a five membered ring system creates an interesting class of compound, known as triazole. It has two types, namely 1,2,3-triazole and 1,2,4-triazole. The chemistry of 1,2,4-triazoles has achieved considerable attention in the last few years. Triazoles possess various biological activities amongst which antifungal2, anticancer3, anti-inflammatory4, antidepressant5 and hypoglycemic6 are more common. In addition to those biological applications, marcapto-1,2,4-triazoles have also notable utility in preparative chemistry7. The importance of triazole nucleus lies in the field of medicinal synthesis due to their significant chemotherapeutic values. Drugs such as Fluconazole, Itraconazole and Variconazole have become very popular because of their novel therapeutic properties. They all are antimycotic agents.
During the past two decades, the prevalence of fungal infection has expanded dramatically. The most common reason is drug resistance and immunity disorders8. Mycotic infections are continuously spreading all over the world. Up to 25% of total superficial infections are caused by mycotic flora9. The increasing frequency of microbial infections and development of drug resistance highlight the need of synthesis of new chemical entity having good therapeutic index.
Despite the recent introduction of newer antimycotic drugs, invasive fungal infections, ranking alongside bacterial infection, have become a major cause of morbidity and mortality10. This situation highlights the need for advent of new and effective antimicrobial agents. Interest in the field of antimicrobial compounds is growing up day by day due to the failure and drug resistance of therapeutic agents. Hence there is an interminable need of new antimicrobial agents with significant effectiveness.
It is evident from the existing literatures that compounds bearing Iodo- group have numerous biological applications11. They have high antimicrobial properties. In recent years, iodine compounds have also acquired significant place in medicinal scaffolds, viz. Diatrizoic acid12, Amiodarone13 etc. They have many catalytic applications also14. Taking these features into consideration, it was thought worthwhile to synthesize the iodo-triazoles for obtaining better therapeutic activity.
In view of the aforestated facts, the present study was designed and carried out in our laboratory. In this study, synthesis of a new series of 1,2,4 – triazoles have been carried out and screened for their antimicrobial activity.
Synthesis
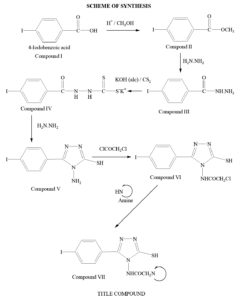
All required chemicals and reagents were procured from Spectrochem, Merck and Sigma-Aldrich. Synthesis of the triazole compounds accomplished in six steps. It involved synthesis of compound V (the basic triazole nucleus) via conversion of carboxylic acid into ester (Compound II), hydrazide (Compound III) and dithiocarbazate salt (Compound IV). The product was then cyclized using hydrazine. Finally the title compounds were obtained from compound V using chloroacetyl chloride and corresponding amines. Melting points were estimated using open capillary method. The route of synthesis is outlined in Figure-1 & 2.
Steps of synthesis
Step 1: Synthesis of methyl ester
The ester was synthesized using iodobenzoic acid15. Required amount of 4-iodo benzoic acid (0.1 mol.) was taken in methanol (100 mL) and conc. sulphuric acid (5.7mL) was added to it. The reactant mixture refluxed for 6 hours. Excess of methanol was distilled off at reduced pressure. Ice cold distilled water was then added. The product which was separated out was taken in sufficient carbon tetrachloride. The organic layers was washed several times using sodium bicarbonate (5% w/v) solution to get rid of unreacted acid if any. Finally the carbon tetrachloride was distilled off under reduced pressure to get the ester. The obtained ester was dried over anhydrous magnesium sulphate. It was then crystallized with absolute alcohol. Percentage yield and melting point were determined. Yield 92%, m.p. 114-117°C.
Step 2: Synthesis of hydrazide
Effective conversion of ester to hydrazide was achieved by the use of hydrazine hydrate16. In order to convert ester into hydrazide, 5.7 mL of hydrazine hydrate (0.15 mol.) was taken in round bottom flask and the synthesized ester (0.1 mol.) dissolved in ethanol was added drop wise with gentle stirring. The mixture was refluxed for 4 hours. After completion, the solvent was distilled off under reduced pressure. The obtained hydrazide was re-crystallized from absolute ethanol. Yield 94%, m.p. 168-171°C.
Step 3: Synthesis of dithiocarbazate salt
The reaction of dithiocarbazate salt with hydrazide was carried out by mixing alcoholic potassium hydroxide (8.4%) and carbon disulfide (0.15 mol., 9 mL) with compound II at cold condition17. Alcoholic potassium hydroxide was prepared by mixing potassium hydroxide (0.15 mol, 8.4 g) with ethanol (100 mL). The reaction mixture was stirred for 16 hours. Whole content was then filtered out using whatman filter paper and washed with diethyl ether.
Step 4: Synthesis of 4-amino-3-mercapto-5-(4-iodo) phenyl-1,2,4-triazole
The prepared salt was utilized for cyclization by adding 99% hydrazine hydrate (0.2 mol) and 6 mL of water18. The reactant mixture was refluxed for 3 hours. Progress of reaction was monitored by evolution of hydrogen sulfide gas. Finally the colour of reaction mixture changed to green which confirmed completion of reaction. After cooling the content was acidified and the precipitated product was obtained. Concentrated hydrochloric acid was utilized for acidification. The precipitated solid was filtered out and recrystallized from aqueous ethanol (50% v/v). Yield 61.3%, m.p. 210-213°C.
Step 5: Synthesis of 4-(chloro-acetyl amino)-3-mercapto-5-(4-iodo) phenyl-1,2,4-triazole
The synthesis was carried out using chloroacetyl chloride in dioxane medium19. The synthesized compound of previous step (0.1 mol.) was taken in 50 mL of dioxane and mixed with chloroacetyl chloride (0.11 mol.) drop wise. The reaction mixture was allowed to reflux for 2-3 hours. The completion of reaction was monitored by TLC. On completion, the product was cooled at room temperature and then poured on crushed ice. The acyl product precipitated out and washed with cold distilled water. Yield 61.5%, m.p. 205-207°C.
Step 6: Synthesis of the title compound
The synthesis was carried out using secondary amines20. The acyl product (0.025 mol.) was dissolved in a little amount of dimethyl sulfoxide and then diluted with benzene (100 mL) in a round bottom flask. Respective amine (0.025 mol.) was mixed to the flask. Equal mole of triethyl amine was also added. The content was allowed to reflux for 4 hours. Progress of reaction was monitored by TLC. On completion, the excess of solvent was distilled off under reduced pressure. The product was transferred to ice cold distilled water and left there for precipitation. The precipitated product was again washed with distilled water and recrystallized using suitable solvent.
Antimicrobial screening
Antibacterial activity
One loop full of bacterial inoculum was taken into 10 mL of Nutrient-broth. Broth was incubated for 24 hours at 37°C. It was then centrifuged at 3000 rpm for 10 minutes. Precipitate was repeatedly washed with normal saline and finally the obtained bacterial pellet was suspended in 10 mL of phosphate buffer saline (PBS). Different dilutions of bacterial suspension were prepared in PBS and matched with standardized 0.5 Mc Farland’s nephelometer in order to get approximately 108 CFU/mL concentration of bacterial cells.
Antibacterial property of the test compounds was determined by disc diffusion method21,22. Bacterial cells [approximately 108 cells/mL, equivalent to Nephelometer standard no. 0.5] volume 0.1 mL was spread on the growth media of petri discs using a sterile swab. Then sterile whatman paper discs (diameter 5 mm) were placed on the agar media of petri plate and impregnated with 30 µL of compound (dissolved in DMSO) at 6 mg/mL, 3 mg/mL and 1.5 mg/mL concentrations. The plates were incubated at 35±1°C for 24 hours. After the incubation period, the zone of inhibition around the paper discs was measured.
Non sensitive (-) : Inhibition zone less than 6 mm
Sensitive (+) : Inhibition zone6- 10 mm
Very sensitive (++) : Inhibition zone 11- 19 mm
Extremely sensitive (+++) : Inhibition zone larger than 20 mm
In order to find out the lowest inhibitory concentration of the synthesized compounds, determination of minimum inhibitory concentration (MIC) was carried out23,24. Broth dilution method was used to determine the MIC. In to 2 mL of fresh broth 1 mL of test drug (1.5 mg/mL prepared in DMSO) was mixed and labeled as test tube A. A series of dilution was prepared in 2:1 ratio. The preparation was carried out by adding 1 mL content of test tube A to another 1 mL of broth (test tube B) and further 1 mL of B in another 1 mL of broth (test tube C) and so on up to 10 test tubes. Then 0.1 mL of microbial culture was added in each test tube and incubated at 37°C for 18 hours. After incubation, the growth of bacteria was investigated through UV-Visible Spectrophotometer at 660nm. The obtained values of minimum inhibitory concentrations are given in Table 3.
Table (1):
List of the synthesized compounds with Nitrogen (%) and Rf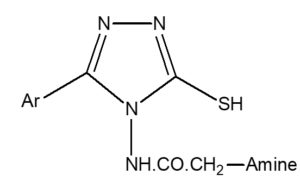
General Structure
Compound [CODE] |
-Ar |
Amine |
% Nitrogen Found (Calculate) |
Rf |
|---|---|---|---|---|
T81 |
18.51 (18.34) |
0.68 |
||
T82 |
15.14 (15.31) |
0.88 |
||
T83 |
15.56 (15.73) |
0.89 |
||
T84 |
15.01 (15.31) |
0.84 |
||
T85 |
16.11 (16.31) |
0.80 |
Solvent system used for the determination of Rf value : n-Hexane : Ethyl acetate (6:4, v/v)
Table (2):
Antibacterial activity of synthesized compounds [zone of inhibition]
| Compounds | Zone of Inhibition (mm) | ||||
|---|---|---|---|---|---|
| Code | Concentration (mg/mL) | S. aureus | B. subtilis | P. aeruginosa | E. coli |
| T81 | 6.0 | 19.67±0.21 | 14.67±0.21 | 10.33±0.21 | 11.00±0.36 |
| 3.0 | 18.33±0.56 | 13.33±0.21 | 9.67±0.21 | 10.33±0.21 | |
| 1.5 | 17.67±0.21 | 12.67±0.56 | 9.33±0.21 | 9.67±0.56 | |
| T82 | 6.0 | 13.67±0.56 | 11.66±0.21 | 11.00±0.36 | 12.33±0.21 |
| 3.0 | 12.33±0.42 | 10.00±0.36 | 10.67±0.21 | 11.00±0.36 | |
| 1.5 | 11.67±0.56 | 16.33±0.21 | 9.00±0.36 | 10.67±0.21 | |
| T83 | 6.0 | 19.00±0.36 | 12.67±0.21 | 14.00±0.36 | 13.33±0.21 |
| 3.0 | 17.67±0.21 | 11.33±0.56 | 13.33±0.21 | 12.00±0.36 | |
| 1.5 | 16.33±0.21 | 10.67±0.21 | 12.33±0.21 | 11.33±0.21 | |
| T84 | 6.0 | 11.67±0.21 | 11.33±0.42 | 9.33±0.42 | 10.67±0.21 |
| 3.0 | 10.33±0.56 | 9.33±0.56 | 7.33±0.21 | 8.33±0.42 | |
| 1.5 | 8.67±0.21 | 8.33±0.56 | 6.67±0.21 | 7.67±0.42 | |
| T85 | 6.0 | 10.33±0.21 | 10.16±0.40 | 8.00±0.36 | 10.00±0.36 |
| 3.0 | 9.16±0.17 | 8.00±0.36 | 6.33±0.21 | 9.67±0.21 | |
| 1.5 | 7.33±0.21 | 6.67±0.21 | 5.67±0.21 | 8.00±0.36 | |
| Control | DMSO, 30µL | —– | —– | —– | —– |
| Ciprofloxacin | 1.5 | 18.67±0.21 | 21.33±0.21 | 23.67±0.56 | 12.67±0.21 |
All the values are in Mean ± SEM. Microbes: Staphylococcus aureus [MTCC NO: 3160], Bacillus subtilis [MTCC NO: 10619], Escherichia coli [MTCC NO: 443], Pseudomonas aeruginosa [MTCC NO: 424]
Antifungal activity
Antifungal activity of the synthesized compounds was performed by disc diffusion method25. The test compounds were dissolved in DMSO to prepare a stock solution of 6mg/mL. The activity was carried out quantitatively in their respective media. For Candida albicans and Aspergillus nigar, Sabouraud Dextrose Agar media was taken, where as Fusarium oxysporum was grown on Potato Sucrose Agar media. Hundred micro liter of fungal suspension containing approximately 108 cell/mL was placed over agar in Petri plate and dispersed. Then, the sterile paper disc impregnated with the test drug (30µg) were placed on agar plate. The plates were incubated at 25°C for 72 hours. Antifungal potency of the test compounds was assessed by the zone of inhibition (Table 4).
Table (3):
MIC of synthesized compounds referring antibacterial activity
| Compounds | MIC, µg/mL | |||
|---|---|---|---|---|
| S. aureus | B. subtilis | P. aeruginosa | E. coli | |
| T81 | 31.25 | 125 | 250 | 250 |
| T82 | 250 | 125 | 250 | 250 |
| T83 | 31.25 | 250 | 125 | 250 |
| T84 | 250 | 250 | 500 | 500 |
| T85 | 250 | 250 | ˃500 | 500 |
| Control | —– | —– | —– | —– |
| Ciprofloxacin | 15.62 | 15.62 | 15.62 | 31.25 |
Staphylococcus aureus [MTCC NO: 3160], Bacillus subtilis [MTCC NO: 10619],
Escherichia coli [MTCC NO: 443], Pseudomonas aeruginosa [MTCC NO: 424].
Broth dilution method was followed for the assessment of minimum inhibitory concentration26 of the test compound. Hundred microliters of inoculum [approximately 108 CFU/mL; 0.5 Mc Farland] was in a series of test tubes having different dilutions of the test drugs. The test tubes were incubated at 25°C for 72 hours. The MIC is considered as the lowest concentration of the test compound that inhibits the growth of the test organisms. Vehicle DMSO was also tested as negative control.
Table (4):
Antifungal activity of synthesized compounds [zone of inhibition]
| Compounds | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Code | Concentration (mg/mL) | Candida albicans | Aspergillus niger | Fusarium oxysporum |
| T81 | 6.0 | 18.67±0.21 | 19.67±0.21 | 18.33±0.42 |
| 3.0 | 17.83±0.30 | 18.17±0.17 | 17.50±0.34 | |
| 1.5 | 16.67±0.21 | 17.17±0.31 | 16.33±0.21 | |
| T82 | 6.0 | 12.67±0.21 | 13.33±0.21 | 13.50±0.22 |
| 3.0 | 11.00±0.26 | 12.67±0.42 | 12.50±0.22 | |
| 1.5 | 10.67±0.33 | 11.33±0.21 | 11.67±0.21 | |
| T83 | 6.0 | 20.83±0.17 | 17.33±0.21 | 19.33±0.21 |
| 3.0 | 19.33±0.21 | 16.67±0.17 | 18.67±0.33 | |
| 1.5 | 18.67±0.21 | 15.17±0.31 | 17.33±0.21 | |
| T84 | 6.0 | 11.33±0.33 | 10.67±0.21 | 9.67±0.21 |
| 3.0 | 9.50±0.22 | 9.17±0.17 | 8.67±0.21 | |
| 1.5 | 8.00±0.25 | 7.50±0.22 | 7.00±0.36 | |
| T85 | 6.0 | 11.67±0.21 | 12.33±0.21 | 11.83±0.17 |
| 3.0 | 10.83±0.30 | 11.67±0.21 | 10.83±0.31 | |
| 1.5 | 9.33±0.21 | 10.67±0.21 | 9.33±0.33 | |
| Control | DMSO, 30µL | —— | —— | —— |
| Fluconazole | 1.5 | 24.33±0.21 | 23.33±0.42 | 19.67±0.21 |
All the values are in Mean ± SEM; Microbes: Candida albicans [MTCC NO: 1637], Aspergillus niger [MTCC NO: 9687], Fusarium oxysporum [MTCC NO: 2087]
Table (5):
MIC of synthesized compounds referring antifungal activity
| Compounds | MIC, µg/mL | ||
|---|---|---|---|
| Candida albicans | Aspergillus niger | Fusarium oxysporum | |
| T81 | 62.50 | 31.25 | 125 |
| T82 | 125 | 125 | 125 |
| T83 | 31.25 | 62.50 | 62.50 |
| T84 | 250 | 250 | 250 |
| T85 | 250 | 125 | 250 |
| Control (DMSO 30µL) | —- | —- | —- |
| Fluconazole | 7.81 | 7.81 | 15.61 |
Candida albicans [MTCC NO: 1637], Aspergillus niger [MTCC NO: 9687], Fusarium oxysporum [MTCC NO: 2087]
Characterization of the synthesized Triazoles
The title compounds were prepared by a series of reactions displayed in Scheme 1. Melting points of the synthesized compounds were determined by open capillary method and are uncorrected. Determination of nitrogen percentage was done using Thermo Scientific (FLASH 2000) Elemental Analyzer. Subsequently the physical and spectral data were analyzed for structure elucidation.
All the synthesized compounds were characterized on the basis of FTIR, GC-MS and NMR (13C and 1HIR spectra in KBr phase were taken by Shimadzu IR Affinity-1 FTIR spectrophotometer. 13C and 1H NMR spectra were recorded in dimethyl sulfoxide by Bruker Avance II 400 NMR spectrometer. The mass spectra were recorded using GC-MS (direct inlet probe on Thermo Scientific TSQ 8000 Gas Chromatograph – Mass Spectrometer). Subsequently the physical and spectral data were analyzed for structure elucidation. The spectral data are as follows.
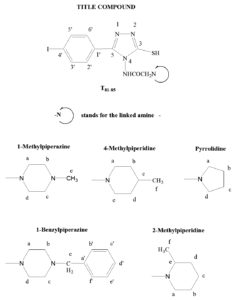
4-(4’-Methyl-piperazin-1’-yl ethanoyl) amino-3-mercapto-5-(4’-iodo) phenyl-1,2,4-triazole (T81)
Yield: 8.35 g (73%); mp 211-213ºC; FTIR (KBr, cm-1) nmax: 3271 (N-H), 3113 (C-H Ar.), 2981 (C-H Methyl), 2596 (S-H), 1627 (C=O), 1537 (C=C), 1510 (C=N), 1494 (C-H bend.), 1160 (C-N); 1H NMR (DMSO-d6, 400 MHz): d = 2.40 (s, 3H, CH3), 2.50-2.55 (m, 8H, CH2-piperazine), 3.43 (s, 2H, COCH2), 7.75-7.77 (d, J=9.1, 2x1H, Ar-H), 8.17-8.20 (d, J=9.1, 2x1H, Ar-H), 9.83 (s, 1H, CONH); 13C NMR (DMSO-d6, 100MHz): ä = 177.1 (NHCO), 167.0 (C5), 148.7 (C3), 137.3 (C3’ & C5’), 129.6 (C2’ & C6’), 125.1 (C1’), 98.7 (C4’), 58.2 (COCH2), 54.9 (Cb & Cc), 52.8 (Ca & Cd), 46.3 (Ce); GCMS m/z 458 [M+]; Anal. Calcd for C15H19IN6OS: C, 39.31; H, 4.18; N, 18.34; Found: C, 39.58; H, 4.34; N, 18.51.
4-(4’-Methyl-piperidin-1’-yl ethanoyl) amino-3-mercapto-5-(4’-iodo) phenyl-1,2,4-triazole (T82)
Yield: 8.91 g (78%); mp 201-203ºC; FTIR (KBr, cm-1) nmax: 3296 (N-H), 3126 (C-H Ar.), 2971 (C-H Methyl), 2565 (S-H), 1621 (C=O), 1519 (C=C), 1498 (C=N), 1472 (C-H bend.), 1176 (C-N); 1H NMR (DMSO-d6, 400 MHz): d = 1.19 (s, 3H, CH3-piper.), 1.82-1.95 (m, 9H, CH, CH2), 3.44 (s, 2H, COCH2), 7.76-7.78 (d, J=9.2, 2H, Ar-H), 8.78-8.81 (d, J=9.1, 2H, Ar-H), 9.84 (s, 1H, CONH), 14.02 (s, 1H, SH); 13C NMR (DMSO-d6, 100MHz): d = 176.1 (NHCO), 167.2 (C5), 149.1 (C3), 137.5 (C3’ & C5’), 128.8 (C2’ & C6’), 125.2 (C1’), 97.7 (C4’), 59.8 (COCH2), 52.5 (Ca & Ce), 34.1 (Cb & Cd), 30.8 (Cc), 22.6 (Cf); GCMS m/z 457 [M+]; Anal. Calcd for C16H20IN5OS: C, 42.02; H, 4.41; N, 15.31; Found: C, 41.76; H, 4.70; N, 15.14.
4-(4’-Benzyl-piperazin-1’-yl ethanoyl) amino-3-mercapto-5-(4’-iodo) phenyl-1,2,4-triazole (T83)
Yield: 9.74 g (73%); mp 217-220ºC; FTIR (KBr, cm-1) nmax: 3303 (N-H), 3092 (C-H Ar.), 2958 (C-H Methylene), 2581 (S-H), 1623 (C=O), 1540 (C=C), 1519 (C=N), 1498 (C-H bend.), 1212 (C-N); 1H NMR (DMSO-d6, 400 MHz): d = 2.44-2.48 (m, 8H, CH2-piperazine), 3.43 (s, 2H, COCH2), 3.78 (s, 2H, CH2-Ar), 7.49-7.49 (m, 5H, Ar-H), 7.60-7.62 (d, J=9.1, 2H, Ar-H), 8.23-8.25 (d, J=9.1, 2H, Ar-H), 9.83 (s, 1H, CONH), 13.87 (s, 1H, SH); 13C NMR (DMSO-d6, 100MHz): ä =175.2 (NHCO), 167.3 (C5), 148.9 (C3), 137.9 (Ca’), 129.2 (Cb’ & Cf’), 128.4 (C3’ & C5’), 128.2 (Cc’ & Ce’), 128.1 (C2’ & C6’), 127.3 (Cd’), 125.4 (C1’), 98.1 (C4’), 62.8 (Ce), 58.4 (COCH2), 52.8 (Ca & Cd), 52.7 (Cb & Cc); GCMS m/z 534 [M+]; Anal. Calcd for C21H23IN6OS: C, 47.20; H, 4.34; N, 15.73; Found: C, 47.02; H, 4.13; N, 15.56.
4-(2’-Methyl-piperidin-1’-yl ethanoyl) amino-3-mercapto-5-(4’-iodo) phenyl-1,2,4-triazole (T84)
Yield: 6.96 g (61%); mp 216-218ºC; FTIR (KBr, cm-1) nmax: 3298 (N-H), 3123 (C-H Ar.), 2974 (C-H Methyl), 2586 (S-H), 1649 (C=O), 1532 (C=C), 1494 (C=N), 1469 (C-H bend.), 1233 (C-N); 1H NMR (DMSO-d6, 400 MHz): ä = 1.16 (s, 3H, CH3-piper.), 1.92-1.96 (m, 9H, CH, CH2), 3.42 (s, 2H, COCH2), 7.70-7.72 (d, J=9.0, 2H, Ar-H), 8.15-8.18 (d, J=9.1, 2H, Ar-H), 9.83 (s, 1H, CONH), 13.86 (s, 1H, SH); 13C NMR (DMSO-d6, 100MHz): d = 176.18 (NHCO), 167.38 (C5), 148.74 (C3), 136.35 (C3’ & C5’), 129.67 (C2’ & C6’), 125.18 (C1’), 97.70 (C4’), 59.22 (COCH2), 56.35 (Ce), 50.18 (Ca), 34.45 (Cd), 24.38 (Cc), 24.16 (Cb), 18.83 (Cf); GCMS m/z 457 [M+]; Anal. Calcd for C16H20IN5OS: C, 42.02; H, 4.41; N, 15.31; Found: C, 41.77; H, 4.12; N, 15.01.
4-(Pyrrolidin-1’-yl ethanoyl) amino-3-mercapto-5-(4’-iodo) phenyl-1,2,4-triazole (T85)
Yield: 6.75 g (63%); mp 204-206ºC; FTIR (KBr, cm-1) nmax: 3288 (N-H), 3142 (C-H Ar.), 2975 (C-H Methylene), 2583 (S-H), 1651 (C=O), 1562 (C=C), 1489 (C=N), 1458 (C-H bend.), 1247 (C-N); 1H NMR (DMSO-d6, 400 MHz): d = 1.74-1.75 (m, 2x2H, CH2-pyrr.), 2.54-2.58 (t, 2x2H, CH2-pyrr.), 3.38 (s, 2H, COCH2), 7.59-7.62 (d, J=9.0, 2H, Ar-H), 8.10-8.12 (d, J=9.1, 2H, Ar-H), 9.84 (s, 1H, CONH), 13.85 (s, 1H, SH); 13C NMR (DMSO-d6, 100MHz): ä = 175.6 (NHCO), 167.3 (C5), 148.2 (C3), 136.3 (C3’ & C5’), 129.6 (C2’ & C6’), 126.6 (C1’), 98.1 (C4’), 59.2 (COCH2), 54.3 (Ca & Cd), 26.1 (Cb & Cc); GCMS m/z 429 [M+]; Anal. Calcd for C14H16 IN5OS: C, 39.17; H, 3.76; N, 16.31; Found: C, 38.90; H, 3.45; N, 16.11.
Antibacterial zones of inhibition and minimum inhibitory concentrations
The synthesized triazoles were tested for their in-vitro antibacterial activities against four bacterial strains, i.e., Staphylococcus aureus [MTCC NO: 3160], Bacillus subtilis [MTCC NO: 10619], Escherichia coli [MTCC NO: 443] and Pseudomonas aeruginosa [MTCC NO: 424] following disc-diffusion method, where Ciprofloxacin was used as standard drug. Interestingly, all the synthesized triazoles exhibited bacteriostatic potential against the taken strains with zones of inhibition from 7.33 mm to 19.67 mm diameter at various concentrations. The triazole (T81 and T83) showed greatest activities. The zones of inhibition along with the minimum inhibitory concentration are shown in Table 2 and Table 3.
Antifungal zones of inhibition and minimum inhibitory concentrations
In-vitro antifungal activities of the synthesized compounds were determined by measuring zones of inhibition and minimum inhibitory concentration (MIC) against Candida albicans [MTCC NO: 1637], Aspergillus niger [MTCC NO: 9687] and Fusarium oxysporum [MTCC NO: 2087]. The triazoles exhibited zones of inhibition ranging from 8.00 mm to 20.83 mm at various concentrations. The zones of inhibition along with the minimum inhibitory concentration are shown in Table 4 and Table 5.
The antimicrobial activity of the synthesized compound showed a differential activity against various bacterial and fungal strains. It can be clearly seen that the group ‘secondary amines’ attached to acylated compounds play an important role in inhibiting the microbial growth. The synthesized compounds T81 and T83 produced good antimicrobial action where as best antifungal effect was exhibited by compound T83. In general, all the synthesized compounds show moderate to strong activity against the pathogens but the most promising results are observed for T83. Ciprofloxacin and Fluconazole were used as reference drugs for antibacterial and antifungal activities.
Triazoles are known for their best antifungal activity among heterocyclic compounds. Recent researches revealed that it is an emerging pharmacophore in the world of medicinal synthesis. Its analogues and derivatives have variety of biological action. In the present study an attempt was made to synthesize some triazoles having antimicrobial activities. The scheduled synthesis was achieved as per the given scheme. The synthesized compounds were characterized through various spectral tools, viz. 1H NMR, 13C NMR, FTIR, GC-MS and elemental analysis. In 1H NMR spectra singlet of SH and CONH were observed in the range of d 13.85-14.02 ppm and d 9.83-9.84. The spectra of 13C NMR for C3 and C5 were seen in the range of d 148.2-149.1 and 167.0-167.3 ppm. FTIR spectra displayed characteristic bands for S-H, C=O and C=N in the range of 2565-2596 cm-1, 1621-1651 cm-1and 1489-1519 cm-1 respectively. All the synthesized compounds were evaluated for antimicrobial activities. The investigation was carried out against seven microbial strains. By comparing the activities, it was observed that the compounds are more persuasive against gram positive bacteria. It is believed that the sufficient lipophilicity of the molecule plays an important role in producing promising antimicrobial effect. Further, the compounds were also screened for antifungal activity. Based on the results of studies made so far, almost all the compounds have antifungal property while compound T83 produced the best result. As per the existing literatures, antifungal drugs have been classified into three classes of natural products (griseofulvin, polyenes and echinocandins) and four classes of synthetic drugs (azoles, allylamines, phenylmorpholines and flucytosine) having clinical value against mycotic infections. The azole classes of antifungal drugs are further divided into imidazoles and triazoles having asymmetric carbon atom as their functional pharmacophore. This functional pharmacophore works by blocking the active site of the enzyme known as lanosterol 14 a – demethylase or cytochrome P450.
In this study, the syntheses and antimicrobial evaluations of some amine linked 1,2,4-triazoles were carried out. The structures of synthesized compounds were elucidated through physical and spectral data. In-vitro antimicrobial studies were conducted against seven microbes. The compounds showed variant antimicrobial properties. Compounds T81 and T83 exhibited appreciable inhibition against S. aureus, B. subtilis, E. coli and P. aeruginosa. The synthesized triazoles were also tested against fungal strains. All the test compounds showed antifungal activity against Candida albicans, Aspergillus niger and Fusarium oxysporum but among the all compounds the most promising effect was shown by T83. However, these compounds require comprehensive evaluation against more microbes to ascertain their broad spectrum action. This study may lead to open an interesting and potential prospect for future researches.
ACKNOWLEDGMENTS
Authors are thankful to GLA University for providing required research facilities for the syntheses and evaluations. We also extend our thanks to SAIF-CIL Panjab University, Chandigarh, India for spectral studies.
- Arshad, M. An insight to the synthetically obtained triazole possessing numerous biological activities. Int. J. Pharm. Pharm. Sci. 2014; 6, 16–23.
- Shalini, K., Kumar, N., Drabu, S. & Sharma, P. K. Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J. Org. Chem. 2011; 7, 668–677.
- Kaur, R., Dwivedi, A. R., Kumar, B. & Kumar, V. Recent Developments on 1,2,4-Triazole Nucleus in Anticancer Compounds: A Review. Anticancer. Agents Med. Chem. 2016; 16, 465–89.
- Ahmadi, F. et al. Synthesis and evaluation of anti-inflammatory and analgesic activities of new 1,2,4-triazole derivatives. Med. Chem. (Shariqah (United Arab Emirates)) 2014; 11, 69–76.
- Kane, J. M., Dudley, M. W., Sorensen, S. M. & Miller, F. P. 2,4-Dihydro-3H-1,2,4-triazole-3-thiones as potential antidepressant agents. J. Med. Chem. 1988; 31, 1253–8.
- Blank, B., Nichols, D. M. & Vaidya, P. D. Synthesis of 1,2,4-triazoles as potential hypoglycemic agents. J. Med. Chem. 1972; 15, 694–696.
- Shaker, R. M. The chemistry of mercapto-and thione-substituted 1,2,4-triazoles and their utility in heterocyclic synthesis. Gen. Pap. Ark., 2006; 59–112.
- Vandeputte, P. et al. Antifungal Resistance and New Strategies to Control Fungal Infections. Int. J. Microbiol. 2012, 1–26 (2012).
- Lakshmanan, A., Ganeshkumar, P., Mohan, S. R., Hemamalini, M. & Madhavan, R. Epidemiological and clinical pattern of dermatomycoses in rural India. Indian J. Med. Microbiol. 2015; 33 Suppl, 134–6.
- Bhatt, V. R., Viola, G. M. & Ferrajoli, A. Invasive fungal infections in acute leukemia. Ther. Adv. Hematol. 2011; 2, 231–47.
- Yusubov, M. S. & Zhdankin, V. V. Iodine catalysis: A green alternative to transition metals in organic chemistry and technology. Resour. Technol. 2015; 1, 49–67.
- Hoppe, J. O. The Evaluation of Iodinated Organic Compounds as Radiopaque Media. J. Am. Pharm. Assoc. (Scientific ed.) 1959; 48, 368–379.
- Roden, D. M. Antiarrhythmic drugs: from mechanisms to clinical practice. Heart, 2000; 84, 339–46.
- Kumar, A. et al. Nickel complexes of 1,2,4-triazole derived amido-functionalized N-heterocyclic carbene ligands: Synthesis, theoretical studies and catalytic application. J. Organomet. Chem. 2015; 786, 63–70.
- Hasan, A., Thomas, N. F. & Gapil, S. Synthesis, Characterization and Antifungal Evaluation of 5-Substituted-4-Amino-1,2,4-Triazole-3-Thioesters. Molecules, 2011; 16, 1297–1309.
- Refat, H. M. & Fadda, A. A. Synthesis and antimicrobial activity of some novel hydrazide, benzochromenone, dihydropyridine, pyrrole, thiazole and thiophene derivatives. Eur. J. Med. Chem. 2013; 70, 419–426.
- Upmanyu, N., Kumar, S., Porwal, P., Shah, K. & Mishra, P. Synthesis and evaluation of 4-(substituted)-acetylamino-3-mercapto-5-(4-substituted) phenyl-1,2,4-triazole derivatives as antimicrobial agents. Med. Chem. Res. 2012; 21, 1967–1976.
- Upmanyu, N., Gupta, J. K., Shah, K. & Mishra, P. Synthesis of new 1,2,4-triazoles as anti-inflammatory and anti-nociceptive agents. Pharm. Chem. J., 2011; 45.
- Upmanyu, N., Kumar, S., Shah, K. & Mishra, P. Synthesis and Antimicrobial Studies of Some 4-(Substituted)-Ethanoylamino-3-Mercapto-5- (4-Substituted) Phenyl-1,2,4-Triazoles. Dhaka Univ. J. Pharm. Sci. 2012; 11, 7–18.
- Upmanyu, N., Gupta, J. K., Shah, K. & Mishra, P. Anti-inflammatory and antinociceptive evaluation of newly synthesized 4-(substituted ethanoyl) amino-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazoles. J. Pharm. Bioallied Sci. 2011; 3, 259–65.
- Kaya, M., Basar, E. & Colak, F. Synthesis and antimicrobial activity of some bisoctahydroxanthene-1,8-dione derivatives. Med. Chem. Res. 2011; 20, 1214–1219.
- Oliveira, A. V. et al. Antibacterial activity of propolis extracts from the south of Portugal. Pak. J. Pharm. Sci 2017; 30, 1–9.
- Holla, G., Yeluri, R. & Munshi, A. K. Evaluation of minimum inhibitory and minimum bactericidal concentration of nano-silver base inorganic anti-microbial agent (Novaron(®)) against streptococcus mutans. Contemp. Clin. Dent. 2012; 3, 288–93.
- Imran, M., Abida & Khan, S. Synthesis and Antimicrobial Activity of Some 2-Amino-4-(7- Substituted/Unsubstituted Coumarin-3-yl)-6-(Chlorosubstitutedphenyl) Pyrimidines. Trop. J. Pharm. Res. 2015; 14, 1265.
- Balouiri, M., Sadiki, M. & Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016; 6, 71–79.
- Dhiman, A., Nanda, A., Ahmad, S. & Narasimhan, B. In vitro antimicrobial activity of methanolic leaf extract of Psidium guajava L. J. Pharm. Bioallied Sci. 2011; 3, 226–9.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.