ISSN: 0973-7510
E-ISSN: 2581-690X
Importance of hospital environment in patient-care has been recognized widely in infection prevention and control. Inappropriate antibiotic use led to emergence of resistant strains that are difficult to treat with the available antibiotics. Progress in nanotechnology led to enhancement of nanoparticles with physicochemical characteristics and functionality that overcomes the constraints of common antimicrobials. Aim was to investigate effective antimicrobial role of Silver nanoparticle (Ag-NPs) against clinically important bacterial strains and observe effects of varying storage temperatures on Ag-NPs antimicrobial activity. Different concentrations of Ag-NPs were tested against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter baumannii using diffusion method. Zone of inhibition (ZOI) for each organism was directly proportional to concentration of Ag-NPs used. Mean ZOI values at different concentrations were significantly different for all organisms with p-value <0.001 for E. coli, S. aureus, P. aeruginosa and 0.004 for A. baumannii. Variation in storage temperature hardly showed any effects on the antimicrobial property of the Ag-NPs. Scanning electron microscopy (SEM) showed morphological and size variations in Ag-NPs exposed cells when compared to control strains, especially for S. aureus, E. coli and P. aeruginosa. Damaged cell membrane areas can be clearly distinguished in E. coli and P. aeruginosa thus suggesting bacterial membrane disruption. These finding can help design a larger study where Ag-NPs can be used in various medical instruments which are usually kept at room temperatures. Also, outcomes of this study may help in designing proper implants, prosthesis and equipment coated with minimum concentration of nanoparticles that might be considered safe for medical applications.
Silver Nanoparticle, Antimicrobial Agent, AFM, SEM, Bacteria
The significance of better healthcare in a hospital setting is commonly acknowledged by its Infection Prevention and Control (IPC) practices. The real incidence of Hospital Acquired Infections (HAIs) is likely to be underestimated as hospital stays may be shorter than the infected microorganism’s incubation period and symptoms may not appear until days after release from the hospital.1 A study from the Centre for Disease Control and Prevention (CDC) stated that between 12% and 84% of surgical site infections are identified after patients’ discharge from the hospital and mostly become apparent within 21 days of surgery.1 Globally, the incidence of HAI ranges from 3.6 to 19.1%. Out of these, high-income countries (HICs) account for 3.6–12%, whereas low- and middle-income countries (LMICs) account for 5.7 to 19.1%.2 A narrative review from India reported the incidence of HAI within the range of 4.4 to 83.09%.3 Advances in the therapy of bacterial infections with antibiotics have impressively reduced mortality from countless infectious diseases. Unfortunately, the emergence and distribution of bacterial resistance to antibiotics are currently posed as significant health issues that led to a large number of drugs being ineffective in therapy.4
Instead of treating established infections, preventing infection or biofilm formation would play a major role while combating antimicrobial resistance in the hospital setting. Hence, various newer propositions have emerged in this era of technology to combat antimicrobial resistant organisms. These include antimicrobial and antifouling coatings to prevent the adhesion of bacteria to medical devices or implants, creating vertically oriented graphene spikes to splice off bacterial cells5; further, plasma dispensers are some of the recent developments to combat resistant organisms. With the advent of nanotechnology, the use of nanoparticles having antimicrobial effects has paved a new path for research.
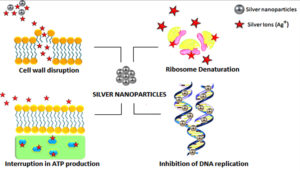
Progress in nanotechnology has led to the enhancement of nanoparticles with outstanding physicochemical characteristics and functionality that can overcome the constraints of common antimicrobials.5 On account of the distinctive physical and chemical properties, including electrical, heat, elevated electrical conductivity, and biological characteristics, silver nanoparticles (Ag-NPs) are increasingly used in multiple areas, including medical, food, and healthcare settings.6,7 The constant release of silver ions by Ag-NPs leads to cell death through various mechanisms which include, cell wall damage, inhibition of protein synthesis by denaturing ribosomes, obstructing deoxyribonucleic acid (DNA) replication, inhibiting adenosine triphosphate production, etc. in bacterial cells (Figure 1).8 It is likely that NPs can change their characteristics when stored under variable conditions and may evolve or regress in activity when exposed for long durations to suboptimal temperatures.9,10 Studies have been carried out observing the activity of various Ag-NPs concentrations which ranged from 10 µg/mL to various higher concentrations.7,11 Owing to the vast use of Ag-NPs, there have been reports on its toxicity to human health, leading to severe patient morbidities including kidney and liver damage.12 Thus, it’s important to find out the minimum concentration of Ag-NPs that helps in retaining their effective antimicrobial properties and also at the same time remains harmless to mammalian cells. This study aims to investigate the role of varying concentrations of Ag-NPs as an effective antimicrobial agent against different bacterial strains and observe the effects of different storage temperatures on Ag-NP antimicrobial activity. Clinically important bacteria like Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), and Acinetobacter baumannii (A. baumannii) were selected as the test organisms for this study as they belong to the organisms under the ‘Serious Threat’ category according to CDC reports.13
Inoculum and test culture plate preparation
American Type Culture Collection (ATCC) standard strains were used in this study viz. E. coli ATCC 25922, S. aureus ATCC 25923, P. aeruginosa ATCC 27853 and A. baumannii ATCC 19606. A saline suspension of each organism was prepared in sterile normal saline by inoculating isolated colonies from a 16-18 hr fresh culture plate. The inoculum was set to the density of 0.5 McFarland standard (1.5×108 CFU/mL). MHA plates were used as culture media for the study. Inoculation of all test plates was carried out according to the standard antimicrobial susceptibility test protocol [CLSI M100 29th Edition]. After plate inoculation, 4 wells with 6 mm radius and 4 mm depth were made in each plate using a media plate puncture for adding the desired Ag-NPs concentrations. Each plate possessed 4 wells for 4 different concentrations of Ag-NPs.
Ag-NPs Characterization using Atomic Force Microscopy (AFM) imaging
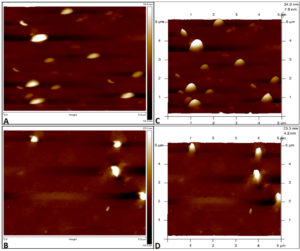
A commercially available Ag-NPs solution with purity of 99.9% and concentration of 1 mg/ml was obtained from Nano Research Lab, India. Tapping mode-based AFM imaging was carried out by using Innova SPM atomic force microscope to confirm the size of the obtained Ag-NPs. The specimen for AFM imaging was made by preparing a 1:1 ratio of Ag-NPs using distilled water as a solvent and centrifuged at 5000 rpm for 5 mins. The supernatant was discarded and the pellet was vortexed and washed using distilled water. 10 μl of this solution was placed on a glass slide, air dried, and used for AFM imaging, where 2D and 3D topography images of Ag-NPs were captured. The obtained image showed monodispersed, spherical particles with an average size ranging from 20-35 nm, as shown in Figure 2.
Figure 2. Atomic force Microscopy images of silver nanoparticles on glass slide with 2D topography (A, B) and 3D topography (C, D) images. The y-axis of image represents the height of nanoparticles
Ag-NPs concentration preparation
A stock solution of 400 μg/mL of Ag-NPs was prepared from the obtained commercially available Ag-NPs. From this stock solution, two sets of Ag-NPs solutions were prepared containing concentrations of 50, 100, 200, and 300 μg/mL (Table). One set of Ag-NPs was stored at 4°C and the other set was stored at room temperature. 50 μL of each concentration of Ag-NPs was dispensed into the wells of each test plate. This procedure was carried out on Ag-NPs stored at room temperature which ranged between 25-30°C and 4°C for 3 months each. The plates were then incubated at 37°C for 24 hrs. The zone of inhibition around the wells was observed and measured after incubation. Each experiment was performed in triplicates.
Table:
Preparation of desired concentration of Ag-NPs from a stock solution.
No. |
Ag NPs (µg/mL) |
Stock solution (mL) |
Distilled water (mL) |
|---|---|---|---|
1. |
300 |
0.6 |
1.4 |
2. |
200 |
0.4 |
1.6 |
3. |
100 |
0.2 |
1.8 |
4. |
50 |
0.1 |
1.9 |
Scanning Electron Microscopy (SEM) analysis
SEM imaging was carried out to observe the morphological changes of the targeted organism after exposure to Ag-NPs. Zeiss Evo MA-18 with Oxford (X-act) was used for SEM imaging with magnification ranging from 1X to 100000X, depth of focus at a magnification of 1000X, an aperture size of 100 microns, and a working distance of 10 nm. Sample slides were prepared as previously described.14,15 Sample slides were prepared separately for each study organism (ATCC strains) by exposing 0.5 McFarland standards of each organism to 50 µg/mL of Ag-NPs for 4-6 hours. The bacterial broth solution containing Ag-NPs was then centrifuged for 3 mins at 1000 rpm. The supernatant was discarded and the pellet was washed using normal saline or phosphate-buffered saline (this was repeated 2-3 times). After the final wash, 25 µL were pipetted onto a clean glass slide and air-dried. Control slides were prepared using each ATCC strain without exposure to Ag-NPs, to compare with the SEM images of Ag-NPs treated organisms. The prepared sample was later sputter coated with Au for 15-30 seconds and the slides were observed at 10.00 to 30.00
K X magnifications.
Statistical analysis
The obtained data were entered in the IBM® SPSS® version 16 statistical software platform. Descriptive statistical analysis was carried out with the observed ZOI to obtain the significant inhibitory effect of the Ag-NPs. Furthermore, Pearson’s correlation was conducted to measure the strength of the linear relationship between the ZOI and concentration of Ag-NPs.
Antimicrobial activity demonstrated by Ag-NPs
It was observed that an increase in the concentration of Ag-NPs against the microorganisms like E. coli (Pearson’s correlation = 0.837; p<0.001), S. aureus (Pearson’s correlation = 0.817; p<0.001), P. aeruginosa (Pearson’s correlation = 0.874; p<0.001) and A. baumanii (Pearson’s correlation = 0.818; p<0.001) resulted in the increased zone of inhibition (ZOI), as shown in Figure 3. Thus, the ZOI for each organism was directly proportional to the concentration of Ag-NPs used. Mean ZOI at different concentrations were significantly different for all organisms with p-value < 0.001 for E. coli, S. aureus, P. aeruginosa and 0.004 for A. baumannii. The mean ZOI at 300 µg/mL for E. coli and S. aureus were 14.8mm (+1.29) and 14mm (+1.81), respectively, thus showing a slight increase in the ZOI of E. coli compared to S. aureus, but no significant difference was observed in statistical analysis.
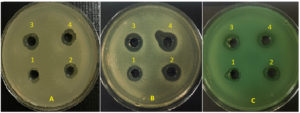
Figure 3. Zone of inhibition after exposure to Ag-NPs in ATCC strains of Staphylococcus aureus (A), Escherichia coli (B), and Pseudomonas aeruginosa (C), respectively.
A1: 50 µg/mL Ag-NPs, Zone= 9mm; A2: 100 µg/mL Ag-NPs, Zone= 13mm; A3: 200 µg/mL Ag-NPs, Zone= 14mm; A4: 300 µg/mL Ag-NPs, Zone= 15mm
B1: 50 µg/mL Ag-NPs, Zone= 13mm; B2: 100 µg/mL Ag-NPs, Zone= 15mm; B3: 200 µg/mL Ag-NPs, Zone= 16mm; B4: 300 µg/mL Ag-NPs, Zone= 17mm
C1: 50 µg/mL Ag-NPs, Zone= 11mm; C2: 100 µg/mL Ag-NPs, Zone= 13mm; C3: 200 µg/mL Ag-NPs, Zone= 14mm; C4: 300 µg/mL Ag-NPs, Zone= 15mm
Effect of Ag-NPs storage temperature
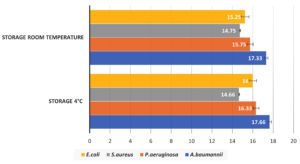
To observe the effect of storage temperature on the antimicrobial activity of Ag-NPs, the NPs were stored at 4°C and 25-30°C for 3 months. There was no significant difference in ZOI for all organisms when tested against Ag-NPs stored at 4°C and room temperature (Figure 4). Hence, the storage temperature of Ag-NPs had no significant impact on their anti-microbial activity.
SEM imaging analysis
Each organism was exposed to 50 µg/mL of Ag-NPs for 4-6 hrs and slides were prepared for SEM imaging. The SEM imaging showed morphological and size variations in Ag-NPs exposed cells when compared to control strains, especially for S. aureus, E. coli, and P. aeruginosa (Figure 5). In S. aureus, major morphological differences were not observed between the control slide and the Ag-NPs treated bacterial slide, but slight diameter variations were observed in the cells. Visual expansion of the Ag-NPs treated cells was observed when compared to the control S. aureus SEM images. Ag-NPs treated E. coli and P. aeruginosa showed aberrations in their cell wall and alteration in the bacilli length when compared to the control slides of E. coli and P. aeruginosa, respectively. The damaged cell wall areas can be clearly distinguished in E. coli and P. aeruginosa thus suggesting bacterial cell wall disruption.
Figure 5. SEM images of E. coli (A), P. aeruginosa (B) and S. aureus (C); A1, B1, C1- Control/ Ag-NPs untreated control strains; A2, B2- Cell wall aberrations and alteration in length of Ag-NPs treated E. coli and P. aeruginosa, respectively; C2- Ag-NPs treated S. aureus, with increased cocci diameter in a few cells
The difference in the action of Ag-NPs on various microorganisms may be due to differences in the cell shape and structure of microorganisms.16,17 S. aureus is a Gram-positive organism while the other three microorganisms – E. coli, P. aeruginosa and A. baumannii are Gram-negative. Some studies show Gram-positive bacteria are more resistant towards the action of nanoparticles.18-23 This difference was not observed significantly in our study as Ag-NPs equally inhibited the growth of both Gram-positive and Gram-negative organisms. This can further be substantiated with an increased sample size. Gram-negative bacteria have a thick layer of lipopolysaccharides of 1-3 µm thick and peptidoglycans of 5-10 nm thickness. This arrangement assists in the penetration of ions released from NPs into bacterial cells. Whereas, Gram-positive bacterial cells have a peptidoglycan layer much thicker than Gram-negative organisms; it is more than 80 nm with covalently attached teichoic and teichuronic acids. Hence, the extra thick protective layer of peptidoglycan found in Gram-positive bacteria acts as a shield against cell wall destruction that occurs from the physical interaction with the nanoparticles. The Ag-NPs’ positive surface charge is crucial for adhesion.24 The positive charge provides electrostatic attraction between Ag-NPs and the microorganism’s negatively charged cell wall, thus facilitating the attachment of Ag-NPs to cell wall and subsequently altering the structure of the cell membrane.25
According to the results obtained, E. coli had a comparatively higher mean ZOI compared to S. aureus. A potential reason for this could be the fact that E. coli is more negatively charged and rigid than S. aureus. This has been stated in studies of electrophoretic mobility and mathematical calculations.23,26 Studies have shown that acidic conditions favour the building of NPs in the bacterial cell wall via electrostatic interactions.27 However, a pH of 7.4 was maintained throughout this study as bacteria grow best at this level. The physical and chemical properties of NPs are different from that of its bulk material. Ag-NPs increase the production of reactive oxygen species, which causes consecutive damage and inactivation of essential biomolecules including DNA, proteins, and lipids.28 Ag-NPs interacting with the bacterial cell wall provided a concentrated source of ions leading to higher toxicity and penetration of the bacterial cell.29 Similar SEM images were also observed in other studies with E. coli and P. aeruginosa. These studies showed that Ag-NPs treated cells had different surface morphology compared to untreated controls. Disrupted membranes with intracellular components pooling around the bacterial cells were observed in treated cells. This is mainly due to membrane leakage. Studies have also stated that Ag-NPs break through the cell permeability of the outer membrane, and these have been termed “pits”.30 Previous studies mentioned the elongation of these treated bacterial cells mostly due to stress conditions arresting cell division; such observations have also been obtained in our study.11 Prominent morphological changes in S. aureus were not observed after exposure to Ag-NPs which can be due to the rigidity of the cell wall compared to Gram-negative bacteria. Increased concentrations of Ag-NPs or time of exposure to Ag-NPs may help in demonstrating more distinct morphological changes in the bacterial cell in S. aureus. Our study has also shown that storage temperature did not hamper the antimicrobial property of the Ag-NPs as demonstrated by other research articles.31,32
Stewart et al. has well-defined the four antimicrobial activities of Ag-NPs describing it as one of the best bactericidal agents.33 Future studies can be conducted carrying forward this study by testing the varying concentrations of Ag-NPs on clinically isolated and multidrug-resistant organisms. Studies can be conducted to shed light on the mode of action of the particles against Gram-positive and Gram-negative organisms. Work can be carried out to obtain the optimum minimum inhibitory concentration of Ag-NPs against commonly isolated clinical organisms.
This study indicates that the antimicrobial property of Ag-NPs increases with increased concentrations. Also, Ag-NPs retain their antimicrobial property irrespective of storage temperatures. Thus, demonstrating the NPs antimicrobial nature against common infection causing organisms like E. coli, S. aureus, P. aeruginosa and A. baumannii. These organisms belong to the ‘Serious Threat’ category according to the CDC reports, amongst which A. baumannii is one of the major nosocomial infection-causing organisms. Thus, these findings can help us to design a larger study where Ag-NPs can be used in various medical instruments which are usually kept at room temperature. But this can be one of the limitations as in our study, the antimicrobial property retention of Ag-NPs was carried out for only two storage temperatures which included 25-30°C and 4°C, while other varying temperatures were not tested. Also, further work needs to be carried out to elucidate the mode of action of the Ag-NPs against these bacteria. Certain studies have indicated reactive oxygen species (ROS) production by Ag-NPs leading to cell death which has not been explored in this study.34,35
It is also observed that exposure to the NPs leads to morphological variations in the organisms mainly due to disruption in the cell wall, indicating one of the modes of action of Ag-NPs against the bacteria. Thus, Ag-NPs may be used in various biomedical devices to provide a long-lasting antimicrobial effect. Along with this, Ag-NPs can be incorporated into cleaning agents used in operation theatres and intensive care units in hospitals to prolong and increase the bactericidal capacity of the cleaning agents. Results obtained from this study may enable the designing of implants, prostheses, and equipment coated with a minimum concentration of nanoparticles considered safe for medical applications.
ACKNOWLEDGMENTS
The authors would like to thank Central Instrumentation Facility (CIF) of Manipal Academy of Higher Education (MAHE) for their help and services to access AFM and SEM imaging.
K. Thripthi Ananda thank the Manipal Academy of Higher Education, Manipal, Karnataka, India, for the T.M.A. Pai Ph.D. scholarship.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
CM helped in study conceptualization and reviewing. VM and TA contributed in designing the work, material procurement and supervision. TA and AM conducted the experiments and prepared first draft of the manuscript. CM and VM revised the manuscript. All authors read and approved the manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Collins AS. Preventing health care-associated infections. Patient safety and quality: an evidence-based handbook for nurses. Chapter 41. Rockville (MD): Agency for Healthcare Research and Quality (US). 2008.
- Ananda T, Modi A, Chakraborty I, Managuli V, Mukhopadhyay C, Mazumder N. Nosocomial Infections and Role of Nanotechnology. Bioengineering (Basel). 2022;9(2):51.
Crossref - Ramasubramanian V, Iyer V, Sewlikar S, Desai A. Epidemiology of healthcare acquired infection-An Indian perspective on surgical site infection and catheter related blood stream infection. IJBAMR. 2014;3(4):46-63.
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417-33.
Crossref - Pandit S, Cao Z, Mokkapati VR, et al. Vertically aligned graphene coating is bactericidal and prevents the formation of bacterial biofilms. Adv Mater Interfaces. 2018;5(7):1701331.
Crossref - Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 2014;9(1):373.
Crossref - Li WR, Xie XB, Shi QS, Zeng HY, You-Sheng OY, Chen YB. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol. Biotechnol. 2010;85(4):1115-1122.
Crossref - Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, Chu CH. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J of Nanomed. 2020;15:2555.
Crossref - Gorham JM, Rohlfing AB, Lippa KA, MacCuspie RI, Hemmati A, Holbrook RD. Storage Wars: how citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions. J Nanoparticle Res. 2014;16(4):1-4.
Crossref - Tejamaya M, Römer I, Merrifield RC, Lead JR. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol. 2012;46(13):7011-7017.
Crossref - Lok CN, Ho CM, Chen R, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5(4):916-924.
Crossref - Ferdous Z, Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci. 2020;21(7):2375.
Crossref - Soltani J, Versporten A, Goossens H. Antibiotic resistance, A global concern; Current Situation and Action Plans. Erciyes Med. J. 2019;41(2):125-127.
Crossref - Fischer ER, Hansen BT, Nair V, Hoyt FH, Dorward DW. Scanning Electron Microscopy. Curr Protoc Microbiol. 2012;25(1):2B.2.1-2B.2.47.
Crossref - Piroeva I, Atanassova-Vladimirova S, Dimowa L, et al. A simple and rapid scanning electron microscope preparative technique for observation of biological samples: application on bacteria and DNA samples. Bulg Chem Commun. 2013;45(4):510-515.
- Duran N, Duran M, De Jesus MB, Seabra AB, Favaro WJ, Nakazato G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine. 2016;12(3):789-799.
Crossref - Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7:1831.
Crossref - Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662-668.
https://doi.org/10.1002/1097-4636(20001215)52:4<662::AID-JBM10>3.0.CO;2-3 - Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res. Part A. 2012;100(4):1033-1043.
Crossref - Mukha IP, Eremenko AM, Smirnova NP, et al. Antimicrobial activity of stable silver nanoparticles of a certain size. Prikl Biokhim Mikrobiol. 2013;49(2):215-23.
Crossref - Cavassin ED, de Figueiredo LF, Otoch JP, et al. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J Nanobiotechnol. 2015;13(1):1-6.
Crossref - Dorobantu LS, Fallone C, Noble AJ, et al. Toxicity of silver nanoparticles against bacteria, yeast, and algae. J Nanoparticle Res. 2015;17(4):1-3.
Crossref - Slavin YN, Asnis J, Hafeli UO, Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol. 2017;15(1):1-20.
Crossref - Abbaszadegan A, Ghahramani Y, Gholami A, et al. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015;16(1):53.
Crossref - More PR, Pandit S, Filippis AD, Franci G, Mijakovic I, Galdiero M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms. 2023;11(2):369.
Crossref - Sonohara R, Muramatsu N, Ohshima H, Kondo T. Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys Chem. 1995;55(3):273-277.
Crossref - Mu H, Tang J, Liu Q, Sun C, Wang T, Duan J. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci Rep. 2016;6(1):18877.
Crossref - Karakoti AS, Hench LL, Seal S. The potential toxicity of nanomaterials-the role of surfaces. Jom. 2006;58(7):77-82.
Crossref - McQuillan JS, Groenaga Infante H, Stokes E, Shaw AM. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology. 2012;6(8):857-866.
Crossref - Mirzajani F, Ghassempour A, Aliahmadi A, Esmaeili MA. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res Microbiol. 2011;162(5):542-549.
Crossref - Izak-Nau E, Huk A, Reidy B, et al. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. Rsc Advances. 2015;5(102):84172-84185.
Crossref - Velgosova O, Cizmarova E, Malek J, Kavulicova J. Effect of storage conditions on long-term stability of Ag nanoparticles formed via green synthesis. Int J Miner Metall Mater. 2017;24(10):1177-1182.
Crossref - Kim J, Pitts B, Stewart PS, Camper A, Yoon J. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrob Agents Chemother. 2008;52(4):1446-1453.
Crossref - Mazur P, Skiba-Kurek I, Mrowiec P, Karczewska E, Drozdz R. Synergistic ROS-associated antimicrobial activity of silver nanoparticles and gentamicin against staphylococcus epidermidis. Int J Nanomed. 2020:3551-3562.
Crossref - Lee B, Lee MJ, Yun SJ, Kim K, Choi IH, Park S. Silver nanoparticles induce reactive oxygen species-mediated cell cycle delay and synergistic cytotoxicity with 3-bromopyruvate in Candida albicans, but not in Saccharomyces cerevisiae. Int J Nanomed. 2019;4801-4816.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.