ISSN: 0973-7510
E-ISSN: 2581-690X
Vanillin is a natural fragrance molecule, we used a simple screening approach based on pH changes caused by ferulic acid breakdown to identify strains. By using a solvent extraction technique, wheat bran was collected and processed to get ferulic acid. The five MSJM strains exhibited a significant result by change in the colour from blue to yellow on the assay plates. The five MSJM strains biotransformed ferulic acid into vanillic acid, resulting in high yield (Yp/s) and productivity (Pr). To create vanillin, Bacillus subtilis MSJM5 was employed to ferment wheat bran with 0.05 percent ferulic acid as an persuader. To create vanillin, Bacillus subtilis was employed to ferment wheat bran with 0.05 percent ferulic acid as an persuar. HPLC was used to determine the amount of vanillin in the medium after three days of fermentation. The peak in standard vanillin corresponds to a sample from fermented medium with a purity of 99 percent. This demonstrated that vanillin was present in the fermentation medium. The antimicrobial activity and minimum inhibitory concentration in all the samples were also assessed by well diffusion method.
Microorganisms, ferulic acid, vanillin and Tetracycline Thiobarbituric acid
Vanillin (Vanilla planifolia A.)1 is the most commonly world widely used natural flavour in industries such as food, beverage, pharmaceuticals, cosmetics, and tobacco. Vanilla’s main ingredient is vanillin (4–hydroxy–3–methoxy benzaldehyde).2 Vanilla beans are now grown in countries such as Madagascar (46 tonnes), China (1300 tonnes), Indonesia (2600 tonnes), Tonga (202 tonnes), Comores (66 tonnes).3 In 2018, 97,800 hectares of vanilla were harvested globally, with an average yield of 774 hg/ha, according to FAOSTAT.2 Every year, a total of 7500 tonnes are produced (FAOSTAT, 2018).4 The entire market for flavours and fragrances (F&F) expanded by more than 7% in USD from 2016 to 2017, according to the most recent projections from industry research.5 According to the IAL Consultants Market Research Institute, the modern market for is estimated to be valued more than $23 billion in 2017. Vanillin demand was over 18,600 tonnes in 2016, with a 6.2 % growth expected between 2017 and 2025. According to Grand View Research, Inc., the global vanillin market is estimated to reach 724.5 million USD by 2025. Vanilla planifolia, the most common source of vanillin, can only provide around 1% of the yearly market demand.6
Organic vanilla flavouring manufacturing, despite its high yields, has a number of downsides, including a significant ecological effect and a common final product,7 both of which have an influence on human health. Food safety has increased as a result. Chemically produced vanillin’s usage in the food and pharmaceutical sectors has been regulated by international regulatory agencies.8 The essential polymers generated from lignin create derivatives such as vanillin and ferulic acid,9 according to a recent assessment from 2020.10
In order to meet the above industrial need for vanillin production, the current research work aims to produce bio vanillin from microbial source
Sample Collections
The soil samples were collected from groundnut cultivated fields of Kadiri, Anantapur (Dist) at a depth of 10cm for soil sample.
Isolation of Soil Microorganism
The samples were immediately brought to the lab and air dried for 24 hours in a laminar air flow. For the isolation of bacteria, fungi, and actinomycetes, one gramme of dried soil was serially diluted and inoculated onto nutrient agar, potato dextrose agar, and starch casein agar at varied time intervals. Nutrient agar was incubated for 2 days, potato dextrose agar was incubated for 7 days, and starch casein agar was incubated for 5 days.
Preparation of Vanillin Production (VP) Medium11
Composition of the Medium (g/L)
KH2PO4 – 0.9, Na2 HPO4 – 10, Mg SO4.2H20 0.2, CaCl2 – 0.01, FeSO4. 2H20 – 0.1,MnSO4 – 0.0006, Na2MoSO4 – 0.0006, Agar -15.0 and Bromothymol blue -0 .25, PH – 7.2, Ferulic acid was filter sterilised and put to sterile modified VP medium at a concentration of 0.1%. Pure strains were cultivated and incubated at 30°C for two days.
In Growing Cultures, Ferulic Acid is Bio Transformed into Vanillic Acid and Vanillin
The strains that developed on VP agar plates were selected and tested in VP broth at 30°C and 120 rpm for two days for bio vanillin and vanillic acid production spectrophotometer was used to quantify bacterial growth at 600 nm every 2 hours throughout the fermentation process, and a pH metre was used to monitor pH changes. Before being quantitatively analysed, the culture supernatant was collected and filtered using Whatmann filter paper.
Estimation of Bio Vanillin using TBA Reagent12
In an equivalent volume of 70 mM phosphate buffer, the cell free supernatant was diluted (500 L, pH 7) A solution of 24 percent HCl (5 mL) and 1 % thiobarbituric acid was used to acidify the mixture (2 mL) Heat for 10 minutes in a water bath at 55°C, then cool for 20 minutes at room temperature. A spectrophotometer was used to detect the absorbance at 434 nm, and a vanillin standard was used to estimate the equivalent vanillin content.13
Bioconversion of Ferulic Acid to Vanillin14
Luria Bertani (LB) agar was used to maintain pure cultures, which were incubated at 37°C for 24 to 48 hours. Bacillus subtilis colonies were then transferred to 100ml of LB broth (Luria Bertani) and cultured for 24-48 hours at 37°C. 1 % inoculum was added to the fermentation broth, which contained 15g of wheat bran and 0.05 % percent ferulic acid as a biotransformation inducer (g/100ml). (pH: 5.6.) Under shaking conditions, the fermentation broth was incubated at 37°C for 48-72 hours at 180 rpm. HPLC analysis was used to determine vanillin in the fermentation medium after 72 hours of biotransformation.
HPLC Analysis of Vanillin Produced in the Fermentation Medium15
Once biotransformation, the fermented material was centrifuged to eliminate the bran particles. 6N HCl was used to acidify the supernatant to a pH of 2. Three times with equal quantities of ethyl acetate, phenolic components were extracted. The supernatant was then used to determine the amount of vanillin using HPLC. The HPLC equipment was used to examine the generation of vanillin in the fermentation broth. The separation was carried out on a C18 column with a diameter of 4.6 x 250mm (5), a mobile phase of 90:10 acetonitrile in water, and a flow rate of 1.0ml/min. The material was filtered via a 0.22m membrane, and 20l of it was put straight into the HPLC column, where the products were identified at 230nm. For conventional vanillin, the separation was done at the same time.
Evaluation of Antimicrobial Activity of Vanillin Compound16
The antimicrobial activity of bacterial pathogens (acquired from SVIMS-Tirupati) vanillin compound was determined by well diffusion method. The compound of vanillin is placed in a well that contains 50µl, 100µl, Std (vanillin150µl) and control (Tetracycline).15 The plates were incubated for 24 hours at 37°C. The antimicrobial activity were done against Shigella, Bacillus, Staphylococcus, Enterococcus, and Klebsiella.
Isolation of Soil Microorganism
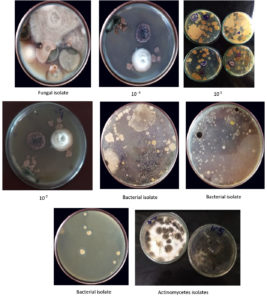
From the collected soil sample 20 numbers of bacterial strains on nutrient plate, 10 fungal strains on potato dextrose agar media and 2 Actinomycetes strains were isolated based on their cultural morphology and represented in Figure 1.
Screening
Rapid Colorimetric Screening to Identify Potential Ferulic Acid-vitiating Isolates
Several microbial cultures were identified that thrived on a medium containing solely ferulic acid as a carbon source. Five isolates were found to be able to digest ferulic acid and thrive on minimum VP agar plates after screening. This study outlines a procedure for isolating and choosing potential ferulic acid-vitiating isolates earlier fermenting them to convert ferulic acid to vanillic acid and vanillin. Microbial fermentation of ferulic acid produces vanillic acid, which lowers the pH to an acidic level. Bromothymol blue in VP screening plates becomes yellow instead of blue as a consequence and represented in Figure 2.
Growing Cultures Approach for Biotransformation of Ferulic Acid to Vanillic Acid and Vanillin
Using the ferulic acid calculation during the fermentation method, the pH was reduced to an acidic state after the positive strains were fully grown on VP medium. The strains had converted ferulic acid to vanillic acid, lowering the pH; however, when they were cultured on method with ferulic acid, the pH remained between 7.2 and 7.4 until the fermentation process was completed.
Estimation of Vanillin Concentration using TBA Reagent
The absorbance of the cell-free supernatant was measured at 434 nm, and the concentration of vanillin produced by isolates was calculated using the vanillin standard curve. As vanillin concentration rises, so does production. Thiobarbituric acid forms a yellow solution when it reacts with standard vanillin solutions. The presence of vanillin was confirmed by the presence of light yellow coloured solutions in the culture media samples. Spectrophotometric absorbance of standard vanillin solutions was measured over a calibration range of 0.1-1.0 mg/ml in Table.
Table:
Effect of Ferulic acid biotransformation yield of Vanillin.
| Substrate | Micro-organisms | Vanillin yield mg/ml | Vanillin yield % |
|---|---|---|---|
| Ferulic acid | MSJM1 | 0.123 | 12.3% |
| MSJM2 | 0.130 | 13.0% | |
| MSJM3 | 0.135 | 13.5% | |
| MSJM4 | 0.42.2 | 42.2% | |
| MSJM5 | 0.55 1 | 55 .1% |
HPLC Analysis of Vanillin Produced in the Fermentation Medium
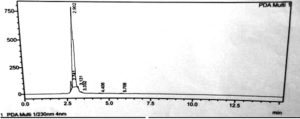
Among the five isolates MSJM5 was taken for estimation of the concentration of vanillin produced after 3 days of fermentation by HPLC. In the HPLC study, the supernatant obtained after centrifugation of the fermented mass was employed. A normal vanillin estimation was also carried out. A single peak with a retention period of 2.902 minutes and an area of 92.74 % was found in samples from the fermented medium. With a retention period of 2.877 minutes and an area of 100%, standard vanillin produced a single peak. The peak in the fermented sample with a retention time of 2.90 minutes matches the peak in standard vanillin with a retention time of 2.877 minutes and a purity of 99%. The amount of vanillin was produced to found to be 8gms/litre. This indicates the production of vanillin by the bioconversion activity of Bacillus subtilis MSJM5 using ferulic acid as inducer in the fermentation medium in Figure 3.
Evaluation of Antimicrobial Activity of Vanillin Compound
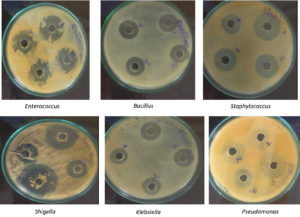
The plates were incubated for 24 h at 37°C and the results were recorded (Figure 4) the inhibitory activities of the samples were detected as a clear zone was done. Shigella, Bacillus, Staphylococcus, Enterococcus, and Klebsiella. The maximum inhibition of zone Staphylococcus.
When given ferulic acid as the only carbon source, these isolates cause bromothymol blue to change colour (from blue to yellow) on VP media. The change in colour of the medium should be proportional to the change in pH. According to the findings of this study, the pH of the medium has a significant impact on the bioconversion of ferulic acid to vanillin, vanillic acid, and vanillyl alcohol. This is not the case, according to. The stoichiometry of vanillin oxidation and reduction is pH dependent.
HPLC was used to determine the amount of vanillin produced in the fermentation medium, and it was compared to standard vanillin. The concentration of vanillin produced was calculated using the retention time and area peaks. The peak in the fermented sample with retention time 2.902 corresponds to the peak in standard vanillin with retention time 2.877. The amount of vanillin produced was 8 grammes per litre. These findings are in line with those reported by Vanillin generated by Bacillus subtilis MSJM5 has a retention duration of 2.76 minutes. The findings suggest that bran hydrolysate could be used as a growth substrate as well as a source of ferulic acid for the production of vanillin.
Vanillin is globally used as a flavouring in foods such as muffins, cakes, ice creams, and drinks. Since of its antibacterial and antioxidant properties. Due to its antibacterial and antioxidant properties, it has been suggested that it might be employed as a nutraceutical in further investigations.
The simple screening strategy enables the identification of a bio vanillin producer using ferulic acid as the only carbon source. The biotechnological production of natural vanillin as a viable alternative to traditional isolation from Vanilla pods is a hot topic, as demonstrated by the current study. Surprisingly, in the search for a novel practicable preparation process for natural vanillin, it was discovered that this can be obtained economically using bacteria in high yields and concentrations by converting ferulic acid from agro-industrial waste.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Achterholt S, Priefert H, Steinbuchel A. Identification of Amycolatopsis sp. strain HR167 genesinvolved in the bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol. 2000;54(6):799-807.
Crossref - Converti A, Aliakbarian B, Dominguez JM, Bustos G, Perego P. Microbial production of biovanillin. Br J Microbiol. 2010;41:519-530.
- Ander, Eriksson KE. Lignin degradation and utilization by microorganism. Prog Ind Microbiol. 1978;141-158.
Crossref - Bornscheuer UT, Altenbuchner J, Meyer HH. Directed evolution of an esterase: screening of enzymelibraries based on pH-indicators and growth assay. BioorgMed Chem. 1999;7(10):2169-2173.
Crossref - Civolani C, Barghini P, Roncetti AR, Ruzzi M, Schiesser A. Bioconversion of ferulic acid intovanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13. Appl EnvironMicrobiol. 2000;66(6):2311-2317.
Crossref - Mathew S, Abraham TE. Bioconversions of ferulic acid, a hydroxycinnamic acid. Crit Rev Microbiol. 2006;32(3):115-125.
Crossref - Overhage J, Steinbuchel A, Priefert H. Harnessing eugenol as a substrate for production of aromatic compounds with a recombinant strains of Amycolatopsis sp. HR167. J Biotechnol. 125,369-376.
Crossref - Karode B, Patil U, Jobanputra AJ. Biotransformation of low cost lignocellulosic substrates into vanillin by white rot fungus Phanerochaete chrysosporium. Indian J Biotech. 2013;12(2):281-283. URL : http://www.niscair.res.in/…/ijbt0.asp
- Muheim A, Lerch K. Towards a high yield bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol. 1999;51:456-461.
Crossref - Prince RC, Gunson DE. Just plain vanilla. Trends Biochem Sci. 1994;19(12):521.
Crossref - Priefert H, Rabenhorst J, Steinbuchel A. Biotechnological production of vanillin. Appl Microbiol Biotechnol. 2001;56(3-4):296-314.
Crossref - Cerruti P, Alzamora SM, Vidales SL. Vanillin as an antimicrobial for producing shelf-stable strawberry puree. J Food Sci. 1997;62(3):608-610.
Crossref - GPS Safety Summary Vanillin. 2011. http://www.cefic.org/Documents/IndustrySupport/RHODIA%20GPS%20Safety%20Summary.pdf. Accessed on May 29, 2013.
- Unno T, Kim SJ, Kanaly RA, Ahn JH, Kang SI, Hur HG. Metabolic Characterization of Newly Isolated Pseudomonas nitroreducens Jin1 Growing on Eugenol and Isoeugenol. J Agric Food Chem. 2007;55(21):8556-8561.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.