ISSN: 0973-7510
E-ISSN: 2581-690X
Sesame seed dregs or residue refers to the byproduct that is left after sesame seeds have been processed to extract oil and can used in a variety of ways. The residues were subjected to spontaneous fermentation for 7 days at room temperature (23-27°C). The purpose of this research was to analyze antimicrobial and antioxidant activity of ethanolic extract obtained from fermented black sesame seed dregs. The diameter of inhibition zones of extracts against 16 pathogenic microorganisms ranged from 6.88 to 11.00 mm and the minimum inhibitory concentrations ranged from 6.25 to 50.00 mg/mL, with the extract being particularly effective against P. aeruginosa ATCC9027 and C. albicans ATCC10231. Minimum bactericidal/fungicidal concentrations were spread from 25.00 to >50.00 mg/mL. P. aeruginosa ATCC9027 and C. albicans ATCC10231 were completely killed in 2 h incubation time at 4 × MIC in a time-kill kinetics assay. In comparison to raw seeds and seed dregs extracts, the fermented seed dregs extract displayed a higher total phenolic content. In addition, the fermented extract had a lower IC50 concentration compared to raw seeds and seed dregs extracts. These results suggest that fermented black sesame seed dregs can be used as an alternative method to reduce the microorganism’s growth. This is crucial for ensuring food safety and minimizing the risk of foodborne poisoning.
Bioactivities, Extraction, Fermentation, Sesame Seeds
Due to claims that they are better for human health than white sesame seeds, black sesame seeds are more expensive. According to Want et al., because the detected components in their research were similar to those with specified features in other studies, black sesame seeds can be categorised as a healthy food.1 Nowadays, sesame oil is used more and more frequently for cosmetic, medicinal, and dietary purpose, particularly in Kore and Indonesia. Essential unsaturated fatty acids including oleic and linoleic acids, as well as vitamin E, are abundant in sesame oil.1 Sesame oil was extracted, resulting in a useful by-product known as sesame oil meal, cake, or dregs, which is high in protein. The protein in these sesame dregs is good for animal.2 According to Chitra, depending on how the seeds are extracted, sesame dregs can be used as fertilizer, feed for livestock, particularly ruminants and poultry, or as food grade (from dehulled sesame seeds).2 Surono also mentioned that by using a fermentation method, the remnants of items such as coconut, peanuts, and sesame seeds can be used as food or functional food.3
In Indonesia, it is interesting to point out that in Central Java and West Java, they are consumed sesame seed dregs after spontaneous fermentation and known as cabuk and dage, respectively. Dage and cabuk are fermented foods made from defatted sesame seed. Sesame seed dregs only ferment spontaneous in dage while combination of rice straw ash water with sesame seed dregs which is known as alkaline solid-state fermentation is produced a cabuk.4 In Sierra Leone, Nigeria, fermented sesame seeds, also known as ogiri, are a traditional delicacy that are utilised as inexpensive sources of protein by Nigerians, particularly by poor families, to replace fish during the rainy season when the cost of fish is too high.5 In order to replace fish during that season, some protein can be provided by fermented sesame seeds because it have a longer shelf life.
The utilization of fermentation which is the process that releases water molecules, which causes the complex substance to break down into simpler ones stands as a significant contributor to human progress, and it can be regarded as one of the earliest manifestations of biotechnology. The increasing global population has led to a heightened demand for food, prompting the widespread adoption of fermentation to meet these new nutritional needs on a larger scale. To assure the safety of food, it will expand the area of raw materials and indirectly generated edible food items while removing anti-nutritional elements.6 The use of natural solutions to remove microbial contamination of food or food-related materials has gained popularity recently. Extract from the black sesame seed dregs may include antibacterial substances that are effective against pathogens and germs that cause food spoiling. Secondary metabolites of plants, such as flavonoids and alkaloids are known as phytochemical components. These substances, including tannins and other aromatic chemicals, serve as a defence mechanism against predators.7 Nwodo et al. revealed that certain phytochemical components, such as flavonoids, alkaloids, tannins, cyanogenic glycosides, and anthraquinones, may have antibacterial properties that act as natural defences for the plants against microbial pathogens and insect predators.8
One of the vital elements present in plants, natural antioxidants are capable of scavenging free radicals within the biological system.9 Additionally, plant extract may contain antibacterial substances that can fight against germs that cause health issues and food spoilage. Sesamin and sesamolin, two lignans found in sesame seeds, aid to reduce rancidity.10 During the roasting process, the sesamolin will change into sesamol. Sesamol has anti-oxidative actions that cause cancer cells to stop growing and undergo apoptosis.11 Additionally, it contains phenolic and benzodioxide groups, which support antioxidant activity.
To the best of our knowledge, studies on the bioactivities of extracts from fermented black sesame seed dregs are lacking. Thus, the purpose of this research is to ascertain the antioxidant and antibacterial properties of fermented black sesame seed dregs. The study of the fermentation of sesame seeds dregs can be of significant importance, particularly in the context of waste reduction, sustainable agriculture, and the production of value-added products such as an alternatives method to reduce the microorganism’s growth for ensuring food safety and minimizing the risk of foodborne poisoning. Other than that, biotechnology advancements are one of the foreseeable future prospects, especially in the development of microbial strains and fermentation techniques that can be applied to other agro-industrial waste products.
Sample collection
A black sesame seed was bought in the Pasar Baru Herbal Market in Bandung, Indonesia. Prior to being used, the material was kept in the Laboratory of Natural Products at the Institute of Bioscience (IBS) of Universiti Putra Malaysia (UPM).
Preparation of fermented black sesame seed dregs
Black sesame seed dregs were obtained by pressing the seeds using hydraulic presser (Power Team No. 9517, USA) (550 psi, 1 min) to squeeze the oil from the seeds. Then, the seed dregs were steamed using steamer about 30 min. The dregs were then dried and allowed to cool for an hour at ambient temperature (23 to 27°C). Next, an amount of 50 g of dried dregs were weighed onto clean banana leaf and wrapped. Lastly, wrapped black sesame seed dregs were kept into tight lid container and left at room temperature (23 – 27°C) for 7 days for fermentation process.
Extraction of fermented black sesame seed dregs
The Rukayadi et al. method12 was used to extract the fermented black sesame seed dregs, with a slight modification. Fermented seed dregs went into drying about two hours at 50°C in an oven dryer. An amount of 50 g of died dried dregs on dry weight basis were ground using dry blender Panasonic MK-5087M (Panasonic Corporation, Osaka, Japan) and transferred into the graduated bottle with 200 ml of 99.8% ethanol (Chem AR, Malaysia). The water shaker (Protech, Tech-Lab Scientific Sdn. Bhd., Malaysia) was used to shake the graduated bottle at 40°C and 80 rpm for overnight. The mixture was filtered with Whatman filter paper No. 1 and an EYELA A-1000S aspirator pump which were connected to a flask and Buchner funnel. After that, the filtrate was concentrated using a Heidolphlaborota 4000 efficient rotary vacuum evaporator operating at 50°C and 150 rpm for 20 min. Then, to prepare for use, the crude extract was kept at 4°C. Raw black sesame seeds and black sesame seed dregs also had been extracted using the same method as above and used in total phenolic content analysis and DPPH radical scavenging assay.
Preparation of fermented black sesame seed dregs extract
Ethanolic fermented black sesame seed dregs extract was made by diluting 100 mg of the crude extract into 1.00 mL of dimethyl sulfoxide (DMSO) (R & M chemicals, Selangor, Malaysia) to obtain 100 mg/mL (10%).
Bacteria and fungi cultures
There were sixteen strains of bacteria that were frequently reported to cause food poisoning and food spoilage were obtained from American Type Culture Collection (ATTC) (Maryland, United States). The Mueller Hinton agar (MHA) (Difco, USA) for bacteria and Potato dextrose agar (PDA) (Difco, USA) for yeast subcultures were employed to maintain all the microbial strains utilised in this research.
Disc diffusion assay
Disc diffusion assay was conducted in determination of the antibacterial of sample extract against different bacteria and fungi species.13 Disc diffusion assay was carried out by referring to method as illustrated in CLSI 2020.13 Media were utilised for this test are MHA (for foodborne pathogen) and PDA (for Candida spp.). The 106 – 108 CFU/mL inoculums were swabbed onto MHA/PDA plates using the sterile cotton swab. Then, the sterile 6 mm diameter discs were aseptically placed onto the surface of MHA/PDA using sterile tweezers and 10 μl of 10% concentration of the sample extract was impregnated onto the disc. A 10% of dimethyl sulfoxide (DMSO) is used as negative control and 0.1% chlorhexidine (CHX) is used as positive control. Labelled plates were incubated for 12 to 24 h at 37°C (bacteria) and 24 to 48 h at 35°C (Candida spp.). Zones of inhibition (in mm) were observed and measured using HiAntibiotic ZoneScale (HiMedia Laboratories Limited, Mumbai, India).
Minimum inhibitory concentration (MIC)
MIC was carried out using two-fold standard broth microdilution methods in 96-well U-shaped microtiter plate by referring to method as illustrated in CLSI 2020.13 The inoculum concentration was approximately 106-108 CFU/mL (bacteria and Candida spp.) were placed 100 µL into each well number 2 until number.12 A concentration of 100 mg/mL of sample extract was mixed and diluted two-folds in the respective medium containing inoculum. Column 12 had the highest concentration of extract (50.00 mg/mL), while column 3 had the lowest concentration (0.19 mg/mL). Column 1 of the microtiter plate was contained negative control (only sterile Muller Hinton broth (MHB), no inoculum and sample extract), whereas positive growth control was at column 2 which contained bacteria inoculum without sample extract. The plate was incubated for 24 h at 37°C (for other microorganisms) and for 24–48 h at 35°C (for Candida spp.). The MIC was established as the lowest concentration of antimicrobial agent that resulted able to inhibit microorganism’s visible growth completely.14
Minimum bactericidal/fungicidal concentration (MBC/MFC)
MBC/MFC was carried out by referring to method as illustrated in CLSI 202013. MHA/PDA plates were given labels that ranged from 1 to 12 in a clockwise direction. 10 µl of MHB on well number 1 of microtitration plate was put into agar at label number 1. Then, 10 µl of the inoculum culture from well number 2 was put at label number 2. Next, 10 µl of inoculum culture dilution from microtitration plate was put follow the number which is from well number 12 into number 12 on microtitration plate, number 11 into number 11 and continue until number 3. The agar plates were incubated at 37°C for 24 h for other microorganisms and 35°C for 24 to 48 h for Candida spp. The colony present was observed. MBC was the number that appeared to be clean of all colonies, while MIC was the number that appeared to be beginning to appear in any colony.
Time-kill curve assay
Time-kill curve assay was performed on the Pseudomonas aeruginosa ATCC9027 and Candida albicans ATCC10231 by referring to method as illustrated in CLSI 202013, with slight modification. Time tested for the microorganism is 0, 0.5, 1, 2 and 4 h. 0×, 1×, 2× and 4× MIC concentrations of each extract were prepared by dissolving each extract with MHB containing inoculums as prepared previously. The total volume of the tube used was 1 ml. The tubes were then shaken at 150–175 rpm while being incubated at 37°C. At predominant time of 0, 0.5, 1, 2 and 4 h, 10 µl of each solution of every tube were transferred into 1% of phosphate buffer saline solution 3 times for making 10-2, 10-3 and 10-4 dilutions. Then 10 µl of solutions were spread on MHA/PDA with hockey stick. The agar plates were then be incubated at 37°C overnight and the colony count was done after 24 h. The experiment for each microorganism was conducted duplicated. The average values were obtained and standard deviations were also calculated manually. A graph was plotted and the error bars were added into the graph based on the standard deviations. Then, the analysis was done based on the curve pattern of the curve, and the concentration of the extracts and the time to completely kill the foodborne pathogens tested.
Total phenolic content
The total phenolic content of the sample extracts was determined using the method reported by Zhang et al., with slight modification.15 An amount of 1-5 mg of extract was weighed and 1 mL of methanol was added. The solution was agitated by vortex until it was completely dissolved. The solution was centrifuged at speed of 12 000 × g for 10 minutes. A 100 µL of supernatant was collected and the water concentration was altered to 1:10 depending on the final sample solution’s absorbance. Make 2–10 times more dilution if the absorbance is outside the standard curve range. As the sample solution, used this solution. A 96-well microplate was filled with 20 µL of each sample solution and the serial standard solution. A volume of 100 µL Folin-Ciocalteu reagent was added, mixed well and waited for 5 min. A volume of 80 µL of 7.5% sodium carbonate solution was added and mixed well. The plate was covered and left at room temperature in the dark for two hours. A spectrophotometric microplate reader was used to measure the absorbance at l 750 nm (auto mix was set for 60 s prior to reading). Methanol was employed as a blank, and the control sample had the same ingredients as the sample solution but was devoid of sodium carbonate and Folin-Ciocalteu. Gallic acid was used as a standard solution. A stock solution (200 µg/mL) were prepared by dissolving 4 mg of gallic acid into 20 mL distilled methanol. The serial dilutions were made from stock solution and the standard solution at the concentration 200, 100, 50, 25 and 12.5 µg/mL were obtained.
Antioxidant activity (2,2- diphenyl-1-picrylhydrazil (DPPH) radical scavenging assay)
The antioxidant activity was evaluated using DPPH radical scavenging assay to measure the capacity of sample to scavenge the free radicals as described by Prieto,16 with some modification. DPPH absorbance at 515 nm was used as a stable indicator of free radicals in antioxidant activity determination. The positive control of Trolox was prepared in few concentrations ranging from 31.25 ppm to 500 ppm. DPPH solution with 11.8 mg/100 mL was prepared. The solution was kept at room temperature and in the dark until further analysis. A total 500 µL of each sample was pipetted into 24-well microplate. Another 500 µL of DPPH solution was added into each sample. The mixture was left for 30 min to stand in dark at room temperature. The absorbance of the mixture was then measured by universal microplate reader (BioTek, USA) at 515 nm against blank sample. An amount of 500 µL methanol with 500 µL DPPH solutions was used as the blank sample. The antioxidant activity of a series of concentrations of Trolox (31.25 ppm – 500 ppm) was also determined using similar steps mentioned above. The percentage of scavenging activity was calculated using the formula as follow:
Scavenging activity (%) = [1-((Absorbance of sample) / (Absorbance of control)) × 100]
Statistical analysis
All experiments were performed in triplicate. The data were analysed using MINITAB application software for the analysis of variance (ANOVA). In addition, the Tukey’s test was employed throughout the research to establish the significance of differences (p < 0.05) between those samples from various fermentation days. The information was examined and expressed as a mean value ± standard.
Disc diffusion assay (DDA)
DDA is a preliminary test for identifying the antibacterial activity of particular plants against particular bacterial species. According to the DDA, the greater the zone of inhibition, the higher the antibacterial activity. Table 1 displayed the inhibition zones for ethanolic extract of fermented black sesame seed dregs, 0.1% of chlorhexidine (positive control) and 10% of DMSO (negative control) against sixteen strains that used in this research.
Table (1):
Inhibition zone of ethanolic extract of fermented black sesame seed dregs against foodborne pathogen
| Strains | Inhibition zone (mm) | ||
|---|---|---|---|
| *CHX | *DMSO | Fermented seed dregs | |
| Escherichia coli *ATCC43895 | 13.38 ± 0.48a | *N.A. | 7.13 ± 0.25bF |
| Enterobacter aerogenes ATCC13048 | 9.25 ± 0.50a | N.A. | 7.50 ± 1.00bDEF |
| Klebsiella pneumoniae ATCC13773 | 12.25 ± 0.50a | N.A. | 8.50 ± 1.47bBDEF |
| Listeria monocytogenes ATCC19112 | 12.63 ± 0.25a | N.A. | 8.88 ± 0.25bB |
| Pseudomonas aeruginosa ATCC9027 | 15. 13 ± 0.63a | N.A. | 6.88 ± 1.03bEF |
| Proteus mirabilis ATCC21100 | 13.50 ± 0.00a | N.A. | 7.25 ± 0.05bF |
| Salmonella Typhimurium ATCC14028 | 12.50 ± 0.00a | N.A. | 7.13 ± 0.85bEF |
| Staphylococcus aureus ATCC29737 | 14.88 ± 0.25a | N.A. | 7.38 ± 0.48bF |
| Bacillus cereus *ATCC33019 | 12.75 ± 0.29a | N.A. | 7.38 ± 0.48bF |
| Bacillus megaterium ATCC14581 | 11.88 ± 0.25a | N.A. | 9.00 ± 0.41bBC |
| Bacillus pumilus ATCC14884 | 14.75 ± 0.87a | N.A. | 11.00 ± 0.41bA |
| Bacillus subtilis ATCC6633 | 12.50 ± 0.00a | N.A. | 8.38 ± 0.48bDE |
| Candida albicans ATCC10231 | 18. 38 ± 0.48a | N.A. | 8.00 ± 0.00bD |
| Candida glabrata ATCC2001 | 16.25 ± 1.19a | N.A. | 9.75 ± 0.65bB |
| Candida krusei ATCC32196 | 14.00 ± 0.41a | N.A. | 9.00 ± 0.82bBC |
| Candida parapsilosis ATCC22019 | 14.75 ± 0.29a | N.A. | 8.13 ± 0.25bCDE |
*N.A. = No activity; CHX = Chlorhexidine (positive control; 0.1%); DMSO = Dimethyl sulphoxide (negative control; 10%); ATCC = American Type Culture Collection. Diameter of inhibition zone is in mm (including 6 mm disc). Results were expressed as mean ± standard deviation (SD) of replications (n = 4). Values with different superscript small letters within the same rows of inhibition zone are significantly different (p < 0.05). Values with different superscript capital letters within the same columns of inhibition zone are significantly different (p < 0.05).
The highest inhibition zone was showed on ethanolic extract of fermented seed dregs against B. pumilus ATCC14884 which is 11.00±0.41 mm where it is significantly different with other 15 bacteria species. Studied by Ruga had characterized the inhibition zone activity (mm) into >15.0 (excellent), 13.1-15.0 (very good), 10.1-13.0 (good), 8.1-10.0 (moderate), 6.1-8.0 (weak) and ≤6.0 (no activity).17 Based on the result in Table 1, fermented seed dregs extract was characterized in good inhibition zone against B. pumilus ATCC14884. In addition, most of inhibition zones by fermented seed dregs extract were characterized in moderate categories against pathogenic microbial. This showed that fermented seed dregs extract potentially has antimicrobial agents against foodborne pathogens.
Various bacteria have different susceptibility against sesame seeds dregs extract. In addition, there are few restrictions on extract, such as their ability to flow through pore discs and incapability of hydrophobic substances to permeate into the agar medium, which results in unreliable findings for disc diffusion tests because they are unable to perform their intended roles.18 Furthermore, due to their hydrophobic character, the majority of essential oils and plant extracts are able to hinder the constant diffusion of these compounds through the inoculated medium.19
Typically, the disc diffusion test is utilised as an initial screening process to ascertain that the plant extracts’ active components can pass through; this is done before making a further assessment. Hence, it cannot be accurately measuring the antibacterial activity of the extracts. Screening only served as a preliminary check for antibacterial activity as the diameters of the inhibition zone did not always linearly correlate to the MICs of the plant extracts.20
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration/minimum fungicidal concentration (MBC/MFC)
MIC was determined using standard two-fold dilution techniques. The definition of MIC is the lowest concentration required to prevent at least 99% of bacterial growth while MBC is the lowest concentration of extracts from plant needed to eliminate not less than 99% of the bacteria (bactericidal).12 The results of MIC and MBC/MFC of fermented black sesame seed dregs extracts against foodborne pathogen and Candida species are shown in Table 2. Based on Table 2, broad-spectrum activity is displayed by extracts against foodborne pathogens with MIC values ranging from 6.25 to 50.00 mg/mL. Among them, P. aeruginosa ATCC9027 and C. albicans ATCC10231 that treated by ethanolic extract of sample were discovered to be the pathogenic bacteria that were most vulnerable with the MIC value of 6.25 mg/mL. The MBC value may be equal to or greater than the MIC value, but it is impossible for it to be lower than the MIC value. From the table result, MBC was in ranged of 25.00 mg/mL to >50.00 mg/mL. L. monocytogenes ATCC19112, S. Typhimurium ATCC14028 and B. subtilis ATCC6633 gave the lower MBC values (25.00 mg/mL) compared to other strains.
Table (2):
Minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC) of ethanolic extracts of fermented black sesame seed dregs against foodborne pathogen
Strains |
MIC (mg/mL) |
MBC/MFC (mg/mL) |
|---|---|---|
Escherichia coli *ATCC43895 |
12.50 |
50.00 |
Enterobacter aerogenes ATCC13048 |
25.00 |
50.00 |
Klebsiella pneumoniae ATCC13773 |
12.50 |
>50.00 |
Listeria monocytogenes ATCC19112 |
12.50 |
25.00 |
Pseudomonas aeruginosa ATCC9027 |
6.25 |
>50.00 |
Proteus mirabilis ATCC21100 |
12.50 |
>50.00 |
Salmonella Typhimurium ATCC14028 |
12.50 |
25.00 |
Staphylococcus aureus ATCC29737 |
12.50 |
50.00 |
Bacillus cereus *ATCC33019 |
12.50 |
50.00 |
Bacillus megaterium ATCC14581 |
12.50 |
>50.00 |
Bacillus pumilus ATCC14884 |
12.50 |
50.00 |
Bacillus subtilis ATCC6633 |
12.50 |
25.00 |
Candida albicans ATCC10231 |
6.25 |
>50.00 |
Candida glabrata ATCC2001 |
50.00 |
50.00 |
Candida krusei ATCC32196 |
50.00 |
50.00 |
Candida parapsilosis ATCC22019 |
50.00 |
50.00 |
*ATCC = American Type Culture Collection.
The hydrophilic outer membrane of gram-negative bacteria contains a high concentration of lipopolysaccharide molecules, which serves as a vital protection that blocks the invasion of foreign macromolecules.21 However, apart from this acknowledged statement, Kotzekidou et al. stated that some researcher found that Gram distinction only had little bearing on the ability of some herbs to prevent the growth of both Gram-positive and Gram-negative bacteria.22 Besides, according to Zgurskaya et al., due to porin proteins in outer membrane layer of Gram-negative bacteria, phenolics compound with molecular mass below 600 Da able to penetrate into their plasma membrane.23 Therefore, both types of bacteria potentially have similar effect to arise by longer contact between active compounds in extract with tested bacteria.21
Previous studies indicated that sesame (Sesamum indicum L.) seeds consist of flavonoids and other phenolic compounds.10 Flavonoids have antibacterial properties because they have the ability to interact directly with the bacteria’s DNA. The growth process and metabolism of the bacteria product will be affected by the disruption of their DNA double helix by flavonoids compound as the structure of DNA is crucial in transcription and duplication process.24 Moreover, flavonoids can also produce transduction energy that will affect the cytoplasm of bacteria and slow its movement. It is known that the hydroxyl ion is present in flavonoids, which can chemically alter the organic compound and nutritional transport mechanisms, thus causing toxic effects to the bacterial cell.24 In addition, phenolic compounds capable to damage the cytoplasmic membrane, disturbing cell contents coagulation and combating proton motive force, electron flow and active transport.25
MIC is framework that usually used as the guidance of antimicrobial agent selection by foresee the effectiveness of the agent during treatment after 18-24 h incubation at a standard inoculum approximately 105 CFU/mL. However, there is inadequate information on kinetics action of antimicrobial by using MIC. Therefore, time-kill curve analysis was conducted as it is can show the correlation between the bactericidal activity rate with the incubation time and antimicrobial agent concentration.13
Time-kill curve assay
Time-kill curves were established for vegetative cells of tested bacterial strains to ascertain the relationship between MIC and bactericidal activity of ethanolic fermented black sesame seed dregs at pre-determined time (0, 0.5, 1, 2 and 4 h) and concentrations ranging from 0× MIC to 4× MIC. P. aeruginosa ATCC9027 and C. albicans ATCC10231 were analysed as both of them showed the lowest MIC which is 6.25 mg/ML.
Time-kill curve of Pseudomonas aeruginosa ATCC9027
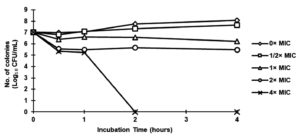
Massive use of antibiotics has resulted in the emergence of resistance against them, which is another problem affecting public health. Pseudomonas aeruginosa is one of the main bacteria with multidrug resistance. This has resulted in the strong demand of new antibiotics by consumers against pathogens and an interest has been developed by the scientific community for using herbal medicines with antimicrobial properties.19 Figure 1 shows P. aeruginosa ATCC9027 was completely killed at 4× MIC (25 mg/mL) in less than two hours after being exposed to ethanolic extract of fermented black sesame seed dregs. The sensitivity of P. aeruginosa to the high concentration of fermented black sesame seed dregs extract may be the reason why the killing impact rose dramatically at 25 mg/mL as early as 2 hours. Fick’s Law illustrated that the destruction of membrane cell can happened because of the rising of concentration of plant extract as it will increasing the extract diffusion into the cell.26 On the other hand, as reported by Ramli et al., the bactericidal effect on P. aeruginosa by extract of Syzygium polyanthum L. (Salam) leaves showed at 5.00 mg/mL at 0.5 h of incubation time.27 Hence, both of this plant extract might be use as new antibiotics against P. aeruginosa.
Figure 1. Time-kill curve for Pseudomonas aeruginosa ATCC9027 following exposure to ethanolic extract of fermented black sesame seed dregs at 0× MIC, 1/2× MIC, 1× MIC, 2× MIC and 4× MIC (0, 3.13, 6.25, 12.50 and 25.00 mg/mL, respectively)
Time-kill curve of Candida albicans ATCC10231
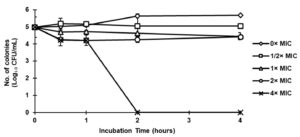
Figure 2 shows C. albicans ATCC10231 was totally destroyed at 4× MIC (25 mg/mL) in less than two hours after being exposed to ethanolic extract of fermented black sesame seed dregs. According to Jobim et al., bacteriostatic activity against C. albicans was achieved by A. aculeatum extract at a dosage of 186 mg/mL for 10 hours of incubation.28 Additionally, Yusoff et al. found that after 1 hour of incubation, C. caudatus K. extract was able to eradicate C. albicans ATCC10231 at 50 mg/mL.29 This means that as compared to A. aculeatum and C. caudatus K. extract, fermented black sesame seeds extract had greater antibacterial activity against C. albicans ATCC10231.
Figure 2. Time-kill curve for Candida albicans ATCC10231 following exposure to ethanolic extract of fermented black sesame seed dregs at 0× MIC, 1/2× MIC, 1× MIC, 2× MIC and 4× MIC (0, 3.13, 6.25, 12.50 and 25.00 mg/mL, respectively)
Total phenolic content
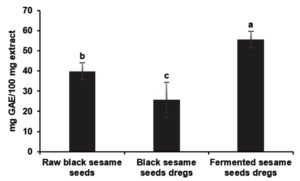
Phenolic compounds are essential component in the signalling and defence mechanism in plants and able to fight the stress from pathogenic microorganisms. Moreover, these compounds are able to prevent biological molecules oxidation due to its capability to donate a hydrogen atom or an electron for stable radical intermediates formation.25 These compounds have one or more unsaturated rings with one or more hydroxyl group. Previous studies showed that several oilseeds and their by-products have phenolic compounds as safe sources for natural antioxidant.30 Khoddami et al. reported that phenolic substances in food from plants such as anthocyanidins, flavones and flavanols have greater antioxidant efficacy than the vitamins C and E.31 In this research, the total phenolic contents were done on ethanolic extract of raw seeds, seed dregs and fermented seed dregs. The amount of total phenolic contents was calculated in gallic acid equivalent (GAE) as shown in Figure 3.
Figure 3. Total phenolic content of ethanolic extract of raw black sesame seed, seed dregs and fermented seed dregs. Value (n = 5) with different letters are significantly different (p < 0.05). *GAE = Gallic acid equivalent
Based on Figure 3, total phenolic content in ethanolic extract of fermented seed dregs extracts (55.64 ± 4.06 mg GAE/100 mg) showed significantly higher than raw seeds (39.92 ± 4.14 mg GAE/100 mg) and seed dregs (25.68 ± 8.79 mg GAE/100 mg). The bioactivities on foods that include chemical reactions during the fermentation process, since the fermentation has an impact on food qualities,32 may be the reason for the fermented seed dregs have more phenolic compounds rather than non-fermented dregs. Previous studied stated that the characteristics of the phenolic compounds in various type of plants affecting the total phenolic content.31 However, because other substances like ascorbic acid and anthocyanins can react with the reagent, the Folin-Ciocalteu method is not technically selective for measuring phenolic compounds.33,34
Antioxidant activity (2,2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging assay)
The antioxidant activity of ethanolic extract of fermented black sesame seed dregs, raw seeds and seed dregs were done by DPPH radical scavenging assay. The 2,2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging is an assay for scavenging activity against free radicals.16 The antioxidants in DPPH radical scavenging have the ability in reducing power which able to transfer electron or hydrogen atom to free radicals.35 The results of antioxidant activity for ethanolic extract of fermented sample, raw seeds and seed dregs by DPPH radical scavenging were presented in Table 3 in IC50 and expressed as mg/mL.
Table (3):
DPPH of ethanolic extract of raw black sesame seed, black sesame seed dregs and fermented black sesame seed dregs
Sample |
Percentage of inhibition at 12.5 mg/mL (%) |
*IC50 value (mg/mL) |
|---|---|---|
Raw black sesame seeds Black sesame |
72.72 ± 5.09c |
11.28 ± 1.54a |
seed dregs |
93.71 ± 0.89ab |
3.22 ± 0.50b |
Fermented sesame seed dregs |
88.56 ± 0.58b |
0.77 ± 0.003c |
Trolox (at 0.0313 mg/mL) |
96.46 ± 0.28a 0.012 ± 0.004c |
*DPPH = 2,2-diphenyl-1-picrylhydrazil radical scavenging assay; IC50 = 50% of inhibition concentration. Mean ± standard deviation with different letters are significantly different (p < 0.05).
The plotted inhibition percentages graph was used to calculate the extract concentration in 50% inhibition (IC50) against tested extracts. The IC50 for ethanolic extract of fermented sesame seed dregs showed the lowest concentration which was 0.77 mg/mL than raw seeds (11.28 mg/mL) and seed dregs (3.22 mg/mL). Particularly, the lower value of IC50 shows the lower concentration of extract for 50% inhibition, the higher the antioxidant activity occured.36 Generally, the IC50 for all extracts varied significantly with Trolox. Trolox is utilised in this study because, according to some academics, its chemical structure is similar to oil. Additionally, according to Abramovic et al., Trolox has a less effect on the quantity of electrons transferred than other antioxidants (such gallic acid, caffeic acid, and catechin), making it more suited as a standard molecule.37
Previous studies by Ibukun on sesame seed reported that the percentage of DPPH free radical scavenging on raw seeds had highest antioxidant activity than fermented seeds.38 However, the results also showed the DPPH free radical scavenging activity were increasing about 20% along the fermentation period (day 0 to day 7). The scavenging activity of extracts might be due to the presence of phenolic hydroxyl groups in phenolic compounds. In addition, Qadir et al. reported that the presence of sesamol compounds in sesame seeds affect the antioxidant activity.10 Besides, research by Huda-Faujan et al. reported that, the ethanol extract will be provided high antioxidant activity.39 This is due to the ability of ethanol that more solubility to DPPH radical in organic acid rather than other such as water solvent.35
Throughout this study, fermented black sesame seed dregs showed potentially high antimicrobial and antioxidant activity. It’s had antibacterial effect on P. aeruginosa ATCC9027 and C. albicans ATCC10231. It also had high total phenolic content and lower IC50. The results show that fermented black sesame seed dregs can be listed as one of the options for controlling the microorganism’s growth, which is crucial for ensuring food safety and lowering the incidence of foodborne illness.
ACKNOWLEDGMENTS
The authors are thankful to University Putra Malaysia for granting permission to conduct the study and experiments, and are also thankful to Geran Putra IPS for supporting Yaya Rukayadi through Project Number GP-IPS/2018/9620200.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Wang D, Zhang L, Huang X, et al. Identification of Nutritional Components in Black Sesame Determined by Widely Targeted Metabolomics and Traditional Chinese Medicines. Molecules. 2018;23(5):1180.
Crossref - Chitra P. Potential and utilization of by-products of oilseeds in animal feed industry. Biot Res Today. 2021;3(8):655-657.
- Surono IS. Ethnic fermented foods and beverages of Indonesia. In Tamang JP. (eds), Ethnic Fermented Foods and Alcoholic Beverages of Asia, Springer, New Delhi. 2016:341-382.
Crossref - Sarkar PK, Nout MR. Handbook of Indigenous Foods Involving Alkaline Fermentation. CRC Press. 2014.
Crossref - Wickrama PSSL, Koralagama DN, Sandika AL. Assesing Seasonal Price Behaviour of Selected Dried Fish Varieties in Sri Lanka. Trop Agric Res Ext. 2021;24(1):21-34.
Crossref - Steinkraus KH. Handbook of Indigenous Fermented Foods, revised and expanded. CRC Press. Food Science and Technology (USA). 2018;73:76-79.
Crossref - Sutrisno, Retnosari R, Marfu’ah S. Study of antibacterial activity of Tamarindus indica Linn seed oil and its fatty acids. IOP Conf Ser: Earth Environ. Sci. 2019;299:012004.
Crossref - Nwodo UU, Obiiyeke GE, Chigor VN, Okoh AI. Assessment of Tamarindus Indica Extracts for Antibacterial Activity. Int J Mol Sci. 2011;12(10):6385-6396.
Crossref - Nimse SB, Pal D. Free Radicals, Natural Antioxidants and Their Reaction Mechanisms. RSC Advances. 2015;5(35):27986-28006.
Crossref - Qadir A, Ali A, Arif M, Al-Rohaimi AH, Singh SP, Ahmad U, Kumar A. Solvent Extraction and GC-MS Analysis of Sesame Seeds for Determination of Bioactive Antioxidant Fatty Acid/Fatty Oil Components. Drug Res. 2018;68(06):344-348.
Crossref - Tunde-Akintunde TY, Oke MO, Akintunde BO. Sesame Seed. In Oilseeds. InTech. 2012. ISBN: 978-953-51-0665-4.
- Rukayadi Y, Lau KY, Zainin NS, Zakaria M, Abas F. Screening of antimicrobial activity of tropical edible medicinal plant extracts against five standard microorganisms for natural food preservatives. Int Food Res J. 2013;20(5):2905-2910.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. Approved standard M100, National Committee for Clinical Laboratory Standards, 30th Ed. Wayne, Penn. 2020.
- Zainin NS, Lau KY, Zakaria M, Son R, Abdull Razis AF, Rukayadi Y. Antibacterial activity of Boesenbergia rotunda (L.) Mansf. A. extract against Escherichia coli. Int Food Res J. 2013;20(6):3319-3323.
- Zhang Q, Zhang J, Shen J, Silva A, Dennis DA, Barrow CJ. A Simple 96-Well Microplate Method for Estimation of Total Polyphenol Content in Seaweeds. J Appl Phycol. 2006;18(3-5):445-450.
Crossref - Prieto JM. Procedure: Preparation of DPPH Radical, and antioxidant scavenging assay. DPPH Microplate Protocol 7-9. 2012.
- Ruga R. Antibacterial Agents from Dracaena cochinchinensis (Lour.) SC Chen and Eleutherine americana (Aubl.) Merr. Ex K. Heyne (Doctoral dissertation, Chulalongkorn University). 2017.
- Gonzalez-Pastor R, Carrera-Pacheco SE, Zuniga-Miranda J, et al. Current Landscape of Methods to Evaluate Antimicrobial Activity of Natural Extracts. Molecules. 2023;28(3):1068.
Crossref - Subramanian S, Shenoy PA, Pai V. Antimicrobial Activity of Some Essential Oils and Extracts from Natural Sources on Skin and Soft Tissue Infection Causing Microbes: An In-vitro Study. Res J Pharma Tech. 2021;14(7):3603-3609.
Crossref - Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71-79.
Crossref - Anna M, Dara K, Hickey I, Mercedes AG, Martin W. Evaluation of Antimicrobial Activities of Commercial Herb and Spice Extracts Against Selected Food-Borne Bacteria. J Food Res. 2013;2:431-437.
Crossref - Kotzekidou P, Giannakidis P, Boulamatsis A. Antimicrobial Activity of Some Plant Extracts and Essential Oils Against Foodborne Pathogens in Vitro and on the Fate of Inoculated Pathogens in Chocolate. Food Sci Tech. 2008;41(1):119-127.
Crossref - Zgurskaya HI, Lopez CA, Gnanakaran S. Permeability Barrier of Gram-Negative Cell Envelopes and Approaches to Bypass It. ACS Infect Dis. 2015;1(11):512-522
Crossref - Kocharyan GH, Minasyan SH, Tavadyan LA. Interaction of flavonoids: morin, quercetin and rutin, with DNA. Proceedings of the YSU B: Chemical and Biological Sciences. 2016;50(1(239)):49-54.
- Miklasinska-Majdanik M, Kepa M, Wojtyczka RD, Idzik D, Wasik TJ. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int J Environ Res Public Health. 2018;15(10):2321.
Crossref - Swamy TA, Obey J, Mutuku NC. Phytochemical analysis of Vernonia adoensis leaves and roots used as a traditional medicinal plant in Kenya. Int J Pharma Biol. 2013;3(3):46-52.
- Ramli S, Radu S, Shaari K, Rukayadi Y. Antibacterial Activity of Ethanolic Extract of Syzygium polyanthum L. (Salam) Leaves Against Foodborne Pathogens and Application as Food Sanitizer. Biomed Res Int 2017:9024246.
Crossref - Jobim ML, Santos RCV, Alves CFDS, et al. Antimicrobial Activity of Amazon Astrocaryum aculeatum Extracts and Its Association to Oxidative Metabolism. Microbiol Res. 2014;169(4):314-323.
Crossref - Yusoff NAH, Noor NF, Rukayadi Y. Effects of Cosmos caudatus Kunth. (Ulam raja) extract on microflora in raw chicken meat. Int J Current Microbiol Appl Sci. 2015;4(2):426-435.
- Zeb A, Muhammad B, Ullah F. Characterization of sesame (Sesamum indicum L.) seed oil from Pakistan for phenolic composition, quality characteristics and potential beneficial properties. J Food Meas Charact. 2017;11(3):1362-1369.
Crossref - Khoddami A, Wilkes MA, Roberts TH. Techniques for Analysis of Plant Phenolic Compounds. Molecules. 2013;18(2):2328-2375.
Crossref - Mehta BM, Kamal-Eldin A, Iwanski RZ. (Eds.). Fermentation: Effects on Food Properties (1st ed.); 2012. CRC Press.
Crossref - Asna AN, Noriham A. Antioxidant activity and bioactive components of oxalidaceae fruit extracts. Malaysian J Anal Sci. 2014;18(1):116-512.
- Stratil P, Kuban V, Fojtova J. Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometer methods. Czech J Food Sci. 2008;(4):242-253.
Crossref - Akter MJ, Khatun R, Khatune NA, Alam AK, Rahman MAA. In vitro antioxidant and free radical scavenging activity of the bark of Dillenia indica L. Bangladesh Pharma J. 2022;25(1):38-43.
Crossref - Proestos C, Lytoudi K, Mavromelanidou O, Zoumpoulakis P, Sinanoglou V. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants. 2013;2(1):11-22.
Crossref - Abramovic H, Grobin B, Poklar Ulrih N, Cigic B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin-Ciocalteu. J Chem. 2018;4608405.
Crossref - Ibukun EO. Fermentation attenuates the free radical scavenging and antioxidant activities of sesame seed (Sesanum indicum). Int J Pharma Appl. 2012;3(2):324-331.
- Huda-Faujan N, Rahim ZA, Rehan MM, Ahmad BHA. Comparative analysis of phenolic content and antioxidative activities of eight Malaysian traditional vegetables. Malaysian J Anal Sci. 2015;19(3):611-662.
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.