ISSN: 0973-7510
E-ISSN: 2581-690X
The objective of the current work was to test the antimicrobial impact of Salvia officinalis and Mentha longifolia collected from Al-Madinah city in Saudi Arabia and extracted by hydrodistillation versus Escherichia coli (ATCC25922), Enterobacter cloacae (ATCC13047), Candida tropicalis (ATCC 13803), and Aspergillus fumigates (ATCC46645). M. longifolia essential oils showed higher inhibition zones versus tested microorganisms, especially against E. coli and C. tropicalis. Minimal inhibitory concentrations of M. longifolia essential oils were determined for M. longifolia, where 3.9 and 62.5 µg/ml were the resulted values of the essential oils versus E. coli and C. tropicalis, respectively. Antioxidant impact of essential oils from both plants was compared using a DPPH assay where, M. longifolia showed the most promising antioxidant action with an IC50 of 88.73 ± 2.59 µg/ml. Transmission electron microscopic examination was applied after treatment of E. coli and C. tropicalis using M. longifolia essential oils, which showed their impact to destroy tested bacterial and fungal microbes as standard drugs. M. longifolia essential oils were further tested versus colorectal cancer cells, showed their cytotoxic impact versus cancer cells with an IC50 of 97.61 ± 1.8 μg/ml and confirmed by flow cytometric analysis, which showed that treated cells by M. longifolia essential oils dramatically elevated their apoptotic rate (P< 0.05) compared to untreated Caco-2 cells. M. longifolia essential oils showed minimal cytopathic action versus Vero cells, which revealed their potency. These results illustrated the possible pleiotropic experimental roles of M. longifolia growing in Al-Madinah City to be applied in pharmaceutical applications after in vivo confirmation of results.
Salvia officinalis, Mentha longifolia, Antimicrobial Activity, Antioxidant Action, Antitumor Impact, MIC
Plants are a naturally occurring source of several products with a wide range of biological functions that can be used to cure a number of ailments.1-3 Natural Products (NP), especially those derived from medicinal plants or herbs, have been widely used to investigate the underlying mechanisms of both therapies and the ultimate natural sciences. These resources are a key research target for drug discovery due to the isolation of metabolites from them, their unique biological characteristics, lesser toxicities, and cost-effectiveness.4 There is an enormous need for the development of successive medical applications related to signal transduction pathways through screening novel chemical compounds from natural flora.5
Plant-derived molecules naturally fight off a variety of microbes that cause infections. Several industries place a high value on plant extracts because they contain significant amounts of volatile, aromatic, and bioactive substances.6 Plant essential oils contain a variety of active compounds, and depending on the active ingredient, the extraction protocol will vary.7
Salvia officinalis L. is an evergreen, rounded shrub of the Lamiaceae family. The major genus in this family, Salvia, with close to 900 species. S. officinalis is indigenous to the Mediterranean and Middle Eastern regions, although plants in this genus are found all over the world.8 Numerous investigations have been carried out in recent years to identify novel biological effects of S. officinalis and to document its traditional usage. Numerous therapeutic uses, such as anti-nociceptive, hypoglycemic, and hypolipidemic properties, have been identified by these investigations.9,10 Specific molecules discovered in various sage extracts that are being studied, usually in vitro or in animal research, have received a lot of interest. Investigators can obtain the appropriate composition with the highest prevalence for a special reason upon improvement of the extraction techniques. When a plant serves as the processing material, the collecting circumstances, geographic location, and plant itself all have an impact on the yield and ultimate bioactive component of the extract.11-13
Large populations of Mentha longifolia L. (family Lamiaceae) can be found all throughout the Mediterranean, in Europe, Asia, and North Africa.14 Various plant parts, such as the leaves, bark, and seeds, have also been used extensively in traditional folk medicine as carminatives, stimulants, and antispasmodics, as well as for the cosmetics industry.15 Since M. longifolia leaves have more elements, vitamins, and antioxidants than other plants, it has drawn a lot of interest from researchers.16 Mentha species’ freshly harvested and preserved plant materials can be made safer by cooking them before eating to minimize the amount of toxic substances, so it is used with some cautions.17
Jiang et al.18 reported that ethanol and acetone extracts of leaves and roots of S. officinalis reduced the proliferation of HepG2 cells and have promising antioxidant action. Furthermore, Elansary et al.,19 reported that the polyphnolic content of Mentha longifolia methanol leaf extract has antimicrobial action versus Pseudomonas aeruginosa and Aspergillus flavus, anticancer action versus breast adenocarcinoma (MCF-7), and cervical adenocarcinoma (HeLa) cell lines, as well as a minimal effect on HEK-293 (normal human cells). In the present work, the antimicrobial actions of S. officinalis and M. longifolia were tested, as well as other possible biomedical applications were characterized.
Collection of plants
M. longifolia and S. officinalis leaves were gathered in May 2022 from different locations in Al-Madinah City, in the north-west of Saudi Arabia. M. longifolia is a common plant in Al-Madinah, while S. officinalis was cultivated in Al-Madinah for better study.
Extraction process
Fresh leaves of both M. longifolia and S. officinalis weighing 500 g were first prewashed in tap water before being cleaned in sterile distilled water. Plant leaves that had been air dried were ground into a fine powder in a mill, then stored until use in sealed, dark vials. Leaf powders were hydrodistillated for 4 hours in the Clevenger-system (Glassco Laboratory Equipment Pvt., Mumbai, India).20
In vitro antimicrobial assay of essential oils
The Agar well diffusion technique was employed to assess the antimicrobial action of the M. longifolia and S. officinalis essential oils versus test microorganisms, with 100 μl of essential oils filling the pores. The zones of inhibition were determined at the end of the incubation time, and the groups were contrasted with the standard medicines.21
Detection of Minimal inhibitory concentrations (MIC)
For the test microorganisms, the MIC of M. longifolia and S. officinalis essential oils was calculated using the broth dilution method. Mueller Hinton Broth (Thermo Scientific™ Oxoid™, Germany) and Sabouraud Dextrose Broth (Thermo Scientific™ Oxoid™, Germany) were used to generate the dilutions of the bacterial and fungal test strains, respectively. The MIC was defined as the lowest extract concentration that prevented the tested microorganism from growing in any discernible way.22
Antioxidant testing
A freshly prepared (0.004%w/v) methanol solution of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was prepared and stored at 10°C in the dark. A methanol solution of the essential oils of M. longifolia and S. officinalis was prepared. A 40 uL aliquot of the methanol solution was added to 3 ml of DPPH solution. Absorbance measurements were recorded immediately with a UV-visible spectrophotometer (Milton Roy, Spectronic 1201). The decrease in absorbance at 515 nm was determined continuously, with data being recorded at 1 min intervals until the absorbance stabilized (16 min). The absorbance of the DPPH radical without antioxidant (control) and the reference compound ascorbic acid were also measured.23
Separation of compounds using GC-MS Spectrometry
Using a Shimadzu QP2010 Ultra Analytical Instrument (Shimadzu, Tokyo, Japan), the chemical composition of the volatile content of M. longifolia and S. officinalis were determined. On an Inc DB-5 60 m 0.25 mm/0.25 micron column, compounds were separated (Agilent Technologies, Santa Clara, CA, USA). The oven temperature program was started at 60°C and held there for three minutes. After that, it was increased at a rate of 10°C to 280°C min-1 and held there for fifteen minutes. In electron impact mode, the spectrophotometer was used. At 280°C for the injector, 280°C for the interface, and 240°C for the ion source, respectively. Split injection was performed using helium as the carrier gas and a split ratio of 1:20 with a sample volume diluted in n-hexane (1:1, v/v) and injected into a volume of 1 μL. Identification was done through a comparison of the sample’s components; proportional scores and mass spectra were identified using WIlEY NBS75K.L and NIST/EPA/NIH (2002 version software, USA).24
Ultrastructural examination
E. Coli and C. tropicalis untreated cultures and samples, which were treated by M. longifolia and standard drugs, were fixed for two hours with 2.5% glutaraldehyde. The specimens were next treated for two hours with 2% osmium tetroxide, and the components were dyed with 1% uranyl acetate before being dried with a graduated ethanol series. After that, resin was used to insert the specimens. Using an ultra-microtome (Leica, Wetzkar, Germany), the specimens were divided into slices. The slices were then examined under a transmission electron microscope (JOEL, Tokyo, Japan).25
Antitumor action and cytotoxicity
African green monkey cells (VERO) and Caco-2 (colorectal cancer cells) were examined for the cytotoxic effects of M. longifolia v essential oils. Cells were given essential oils at levels that ranged from 500 to 15.63 g/mL and incubated for 24 hours at 37°C. Cells were first allowed to connect for 24 hours. Following the addition of the new medium, 100 µl of MTT solution (5 mg/mL) was used after 4 hours at 35°C. The absorbance at 570 nm was determined with a plate scanner. Through the use of an inverted microscope, the plates were viewed, and a video CCD camera was used to take pictures (Zeiss, Berlin, Germany).26,27
Flow cytometry analysis
For this test, both untreated Caco-2 cells and treated cells with essential oils were employed. The Caco-2 cells were divided with trypsin in 0.25% pancreatin and washed with phosphate-buffered saline. The death rate was calculated using a propidium iodide and Annexin V-FITC coloring kit from B.D. Bioscience in the United States. Cells were incubated at 35°C for ten minutes while floating in a buffer containing Annexin V-FITC and/or P.I. standard solution. Analysis was done using flow cytometry.28,29
Statistic evaluation
For analysis of the findings of the experiments, GraphPad PRISM (V5) used the T-test, and all experiments were carried out in triplicate.
Antimicrobial impact
M. longifolia and S. officinalis essential oils were tested versus Escherichia coli (ATCC25922), Entrobacter cloacae (ATCC13047), Candida tropicalis (ATCC13803) and Aspergillus fumigates (ATCC46645), where Cephalosporin was used as a standard antibacterial drug while Fluconazole was used as a standard antifungal drug, as illustrated in (Table 1). It could be noticed that M. longifolia has the most promising inhibition zone 25.0±0.9mm versus Escherichia coli(ATCC25922). Furthermore, the inhibition zone of M. longifolia essential oils versus Candida tropicalis (ATCC 13803) was 14.2±1.2 mm. Thus, M. longifolia v essential oils has higher antimicrobial actions versus tested microorganisms compared to S. officinalis essential oils, as illustrated in (Table 1).
Table (1):
Testing antimicrobial action of M. longifolia and S. officinalis essential oils on various tested microorganisms.
| Tested Microbial strains |
Diameter inhibition Zone (DIZ) (mm) |
|||
|---|---|---|---|---|
| M. longifolia | S. officinalis | Cephalosporin | Fluconazole | |
| Escherichia coli(ATCC25922) | 25.0±0.9 | 16.6±0.5 | 27.0±0.9 | N/A |
| Enterobacter cloacae(ATCC13047) | 19.3±0.5 | 9.0±0.8 | 24.0±0.4 | N/A |
| Candida tropicalis (ATCC 13803) | 14.2±1.2 | 7.9± 1.8 | N/A | 17.2±0.7 |
| Aspergillus fumigates(ATCC46645) | 3.6±0.7 | 4.3±0.9 | N/A | 16.7±1.4 |
Testing of MIC
MIC of M. longifolia essential oils were recoded for test microorganisms, where the MIC of essential oils was 3.9 µg/ml versus E. coli. While, MIC of M. longifolia essential oils versus C. tropicalis was 62.5 µg/ml as shown in (Table 2).
Table (2):
MIC of M. longifolia essential oils versus tested microorganisms.
| Tested microbial strains | M. longifolia essential oils (µg/ml) | Standard (µg/ml) |
|---|---|---|
| MIC | Cephalosporin | |
| E. coli | 3.9 | 1.95 |
| E.cloacae | 6.9 | 31.25 |
| Fluconazole | ||
| C. tropicalis | 62.5 | 15.52 |
| A. fumigatus | 250 | 7.81 |
Antioxidant Impact
M. longifolia and S. officinalis essential oils were tested using DPPH assay, and the IC50 value for M. longifolia essential oils were 88.73± 2.59 µg/ml. While, S. officinalis essential oils had IC50 = 109.86± 4.12 µg/ml as depicted in (Figure 1). These results revealed that M. longifolia essential oils have higher antioxidant action relative to S. officinalis essential oils.
Figure 1. In vitro antioxidant assay at different levels of M. longifolia and S. officinalis essential oils relative to Ascorbic acid as reference drug
Bioactive compounds testing using GC-MS analysis
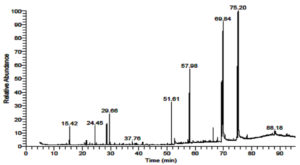
To examine the chemical difference in the essential oils of M. longifolia and S. officinalis: Both plants contained complex mixtures of volatile compounds, with a notable amounts of terpenes. GC-Mass spectrometry analysis of M. longifolia volatile oils revealed the presence of nine major compounds in the essential oils where 1H-Indene,1-hexadecyl-2,3-dihydro-(17%), 3 Hexadecanoic acid, 2,3,bis [(Trimethylsilyl) Oxy]Propyl ester (15%), Methyl stearate (10.64%), Hexadecanoic acid, methyl ester (5.32%), Piperitenone Oxide (4.21%), Cyclohexanone,5-methyl-2-(1-methylethylidene)- (2.7%) and Eucalyptol (2.41%), PALMITIC ACID,2-(TETRADECYLOXY)ETHYL ESTER (0.83%) and 1-(1-Chloro-2,3-dimethylcyclo propyl)-3,3 dimethyl-1 butyne (0.49%) as shown in ( Table 3, Figure 2).
Table (3):
Major identified molecules in M. longifolia essential oils using gas chromatographymass spectrometry.
RT |
Area % |
Compound Name |
Molecular Formula |
Molecular Weight |
|---|---|---|---|---|
15.42 |
2.41 |
Eucalyptol |
C10H18O |
154 |
24.44 |
2.7 |
Cyclohexanone,5-methyl-2-(1-methylethylidene)- |
C10H16O |
159 |
29.66 |
4.21 |
PIPERITENONE OXIDE |
C10H14O2 |
166 |
37.76 |
0.49 |
1-(1-Chloro-2,3-dimethylcyclo propyl)-3,3 dimethyl-1 butyne |
C9H12S2 |
184 |
51.61 |
5.32 |
Hexadecanoic acid, methyl ester |
C17H34O2 |
270 |
57.98 |
10.64 |
Methyl stearate |
C19H38O2 |
298 |
69.84 |
15.71 |
HEXADECANOIC ACID,2,3-BIS[(TRIMETHYLSILYL) OXY]PROPYL ESTER |
C22H46O3 |
386 |
75.21 |
17.37 |
1H-Indene,1-hexadecyl-2,3-dihydro- |
C25H42 |
342 |
88.18 |
0.83 |
PALMITIC ACID,2-(TETRADECYLOXY)ETHYL ESTER |
C32H64O3 |
497 |
Furthermore, investigation of S. officinalis volatile oils showed the existence of eleven major molecules in the essential oils, including a-Pinene (1.52%), Eucalyptol (26.28%), (+)-2-Bornanone (2.84%), Caryophyllene (3.77%) , Ledol (2.24%), Caryophylla-4(12),8(13)-dien-5a-ol (0.76%), 1 Naphthalenepropanol,
a-ethenyldecahydroa,5,5,8a-tetramethyl-2-methylene-,[1S-[1a(R*),4aa,8aa]]- (6.10%), Podocarpa-1,8,11,13-tetraen-3-one (2.09%), Podocarpa-1,8,11,13-tetraen-3-one, 14-isopropyl-1,13-dimethoxy- (5.62%), Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (4.95%) and 1H-Indene,1-hexadecyl-2,3-dihydro- (1.56%) as shown in ( Table 4, Figure 3).
Table (4):
Identified compounds in S. officinalis essential oils using gas chromatographymass spectrometry.
RT |
Area % |
Compound Name |
Molecular Formula |
Molecular Weight |
|---|---|---|---|---|
11.38 |
1.52 |
α -Pinene |
C10H16 |
136 |
15.46 |
26.28 |
Eucalyptol |
C10H18O |
154 |
20.02 |
2.84 |
(+)-2-Bornanone |
C10H16O |
152 |
33.21 |
3.77 |
Caryophyllene |
C15H24 |
204 |
39.62 |
2.24 |
Ledol |
C15H26O |
222 |
44.66 |
0.76 |
Caryophylla-4(12),8(13)-dien-5à-ol |
C15H24O |
220 |
55.22 |
6.10 |
1-Naphthalenepropanol,à-ethenyldecahydroà,5,5,8a-tetramethyl-2-methylene-,[1S-[1à(R*),4aá,8aà]]- |
C20H34O |
290 |
64.07 |
2.09 |
Podocarpa-1,8,11,13-tetraen-3-one |
C22H30O3 |
342 |
69.84 |
5.62 |
Podocarpa-1,8,11,13-tetraen-3-one, 14-isopropyl-1,13-dimethoxy- |
C22H30O3 |
346 |
75.21 |
4.95 |
Hexadecanoic acid,2-hydroxy-1-(hydroxymethyl)ethyl ester |
C19H38O4 |
330 |
88.46 |
1.56 |
1H-Indene,1-hexadecyl-2,3-dihydro- |
C25H42 |
346 |
Indeed, the study of the chemical composition of both plants revealed the difference of compounds in both of them where different compounds could be seen with minimal percentages in S. officinalis where Eucalyptol as monoterpenoid had the highest percentage(26.28%), while different molecules could be seen with considerable percentages: 1H-Indene,1-hexadecyl-2,3-dihydro-(17%), 3 Hexadecanoic acid, 2,3,bis [(Trimethylsilyl) Oxy]Propyl ester (15%), Methyl stearate (10.64%) while Eucalyptol (2.41%).
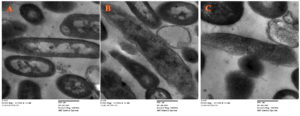
Transmission electron microscopic results
Antibacterial action of M. longifolia essential oils versus E. coli (ATCC25922) was confirmed by electron microscopic testing, as shown in (Figure 4). Untreated E. coli appeared as well-structured cells with smooth surface layers and clear internal organelles as shown as shown in (Figure 4A). While treatment of E. coli with M. longifolia essential oils formed holes in the bacterial surface and caused lysis of cellular organelles, as shown in (Figure 4B) with a similar impact to that has been produced by standard drug as shown in (Figure 4C).
Figure 4. Electon mictographs of (A) Untreated E.coli; (b) Treated E.coli by M. longifolia essential oils; (C) Treated E.coli by cephalosporin (Magnification, 5000x)
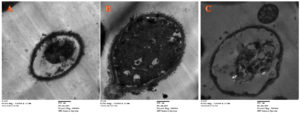
While, the antifungal role of M. longifolia essential oils versus C. tropicalis (ATCC 13803) was examined by transmission electron microscopy as shown in (Figure 5). Normal C. tropicalis (ATCC 13803) could be seen with a rounded structure and thick surface, as well as an intense nucleus and other organelles (Figure 5A). Applying M. longifolia essential oils versus C. tropicalis led to rupture of the fungal spore surface and disintegration of internal organelles as shown in (Figure 5B) relative to treatment using fluconazole, which had an aptotic impact on C. tropicalis cells as shown in (Figure 5 C).
Figure 5. TEM mictographs of (A) Untreated C. tropicalis; (B) C. tropicalis treated by M. longifolia essential oils; (C) C. tropicalis treated by fluconazole (Magnification, 5000x)
Anticancer and cellular toxicity assay
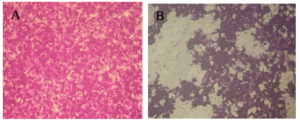
It was shown that the M. longifolia essential oils has a promising anticancer impact versus Caco-2 cells with an IC50 = 97.61 ± 1.8 μg/ml as depicted in (Figure 6). Furthermore, testing M. longifolia essential oils on Vero cells reveled to assure its biosafety with CC50 = 312.87 ± 0.7 μg/ml reveled its potency and possibility for use in different applications as depicted in (Figure 7).
Figure 6. (1 x 106 cells) Caco-2 cells examined using inverted microscope(A) Untreated Caco-2 cells; (B) Treated Caco-2 cells by M. longifolia essential oils (Magnification, 40x), IC50 = 97.61 ± 1.8 μg/ml
Figure 7. Examination of cytoxtoxity upon using (1 x 106 cells) Vero Cells where (A) Untreated Vero cells ; (B) Treated Vero cells by M. longifolia essential oils (Magnification, 40x); CC50 = 312.87 ± 0.7 μg/ml
Apoptotic assay
To confirm the role of M. longifolia essential oils versus Caco-2 cells by flow cytometry analysis, there was a dramatic elevation (P<0.05) of the apoptotic rate of Caco-2 cells treated by essential oils relative to untreated cells, as shown in (Figure 8).
For thousands of years, nature has been a resource for medications, and an astounding amount of contemporary medications have been separated from biological compounds, many of them founded on their conventional medical applications. More than two thirds of the worldwide population, or over 7,000 herbal treatments used in the European pharmacopoeia, are thought to rely on plant-derived medications.30 Plants have long been utilized in folk therapy to treat a variety of illnesses31 and they are still widely employed in rural parts of many nations worldwide.32,33 According to the World Health Organization (WHO), 60% of the worldwide population still depends only on traditional medicine, while almost 80% of people still get their medical assistance from plants.34 In most conventional approaches to medicine, plants serve as the primary active elements, and they have served as the motivation for some well-known pharmaceutical products.34,35
In the present work, S. officinalis and M. longifolia were extracted using a hydrodistillation protocol, and these essential oils were tested against some bacterial and fungal pathogens. It could be noticed that M. longifolia essential oils had higher inhibition zones than S. officinalis essential oils, with the highest zones of 25.0±0.9 and 14.2±1.2 mm versus E. coli (ATCC25922) and C. tropicalis (ATCC 13803), respectively. However, the S. officinalis cultivated in south Brazil and Tunisia showed lower action versus E. coli36 where E. coli act as commensals and are a natural component of the gut microbiota of many animals and humans. Additionally, there are pathogenic variants, classified as extraintestinal and diarrheagenic pathogens.35 In the present study, S. officinalis and M. longifolia essential oils have been tested against C. tropicalis (ATCC 13803) for the first time, where M. longifolia essential oils showed the highest antifungal action. One of the most significant species of Candida is now known as C. tropicalis. It is commonly acknowledged to be a very potent biofilm generator, outperforming C. albicans in the majority of studies.37,38
S. officinalis and M. longifolia essential oils contained a mixture of fatty acids, alkaloids, and terpenes which appeared to be the cause of the antioxidant activities from both minor and major components.39,40 In this study, GC-Mass spectrometry revealed that M. longifolia had major fatty acid percentages compared to S. officinali which may give interpretation to its antimicrobial and antioxidant properties. Furthermore, the findings of this investigation unequivocally suggest that essential oil from S. officinalis and M. longifolia growing in El- Medina, in Saudi Arabia, displayed a considerable degree of chemical diversity when compared to those extracted from other nations. This variance is most likely a result of the various growing environments. Indeed, the genetic makeup, environmental conditions, plant development stage, and extraction technique all have a significant impact on the oil composition.41,42 Studies conducted in the past have shown that the composition of plant essential oils varies greatly with respect to light intensity,43 soil mineral fertilization,44 weather patterns, growing location, and season.45 In the current work, the antimicrobial and antitumor activity of M. longifolia essential oils, which damaged cell structures and triggered an apoptotic process, was examined using both transmission and inverted microscopes. However, other research groups have concentrated on creating powerful tools to fight cancer and microbes as well as employing plant molecules in medical applications.46, 47 This has so far answered the requirement for more creative approaches to be used with other complementary therapies.
Wide-ranging plant compounds with substantial reducing power demonstrated their ability to function as electron donors and lower the oxidized precursor of oxidative stress, acting as antioxidants and suggesting potential biological applications.48-49 M. longifolia essential oils prepared from the common plant in Al-Madinah city (Saudi Arabia) have successive antimicrobial, antioxidant, and antitumor properties to be applied in large scale in the pharmaceutical industry.
ACKNOWLEDGMENTS
None.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Armendariz-Barragan B, Zafar N, Badri W, et al. Plant extracts: from encapsulation to application. Expert Opin Drug Deliv. 2016;13(8):1165-1175.
Crossref - Albalawi AE. Antileishmanial activity of Ziziphusspina-christi leaves extract and its possible cellular mechanisms. Microorganisms. 2021;9(10):2113.
Crossref - Al-Kaabi HKJ, Hmood BA, Jebur LS, Abdullah SM, AL-Qraghi SAA. Determination of Ziziphusspina-christi leaves extracts antibacterial activity against some pathogenic bacteria. Al-Kufa University Journal for Biology. 2021;13(1):14-20.
- Bhuwan BM, Vinod KT. Natural products: An evolving role in future drug discovery. Eur J Med Chem. 2011;46(10):104769-4807.
Crossref - Zhang L, Song J, Kong L, et al. The strategies and techniques of drug discovery from natural products. Pharmacol Ther. 2020;216:107686.
Crossref - Samadi S, Lajayer BA, Moghiseh E, Rodriguez-Couto S. Effect of carbon nanomaterials on cell toxicity, biomass production, nutritional and active compound accumulation in plants. Environ Technol Innov. 2021;21:101323.
Crossref - Bolouri P, Salami R, Kouhi S, et al. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules. 2022;27(24):8999. doi: 10.3390/molecules27248999
- Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med. 2017;7(4):433-440.
Crossref - Garcia CSC, Menti C, Lambert APF. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): antioxidant, and antitumor in mammalian cells. An Acad Bras Cienc. 2016;88(1):281-292.
Crossref - Hasanein P, Felehgari Z, Emamjomeh A. Preventive effects of Salvia officinalis L. against learning and memory deficit induced by diabetes in rats: possible hypoglycaemic and antioxidant mechanisms. Neurosci Lett. 2016;622:72-77.
Crossref - Jokic S, Molnar M, Jakovljevic M, Aladic K, Jerkovic I. Optimization of supercritical CO2 extraction of Salvia officinalis L. leaves targeted on Oxygenated monoterpenes, a-humulene, viridiflorol and manool. J Supercrit Fluids. 2018;133(Part 1):253-262.
Crossref - Zhang Q-W, Lin L-G, Ye W-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin Med. 2018;17:13-20.
Crossref - Bubalo M,C VidoviC S, Redovnikovic I, Jokic S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod Process. 2018;109:52-73.
Crossref - Mikaili P, Mojaverrostami S, Moloudizargari M, Aghajanshakeri S. Pharmacological and therapeutic effects of MenthaLongifolia L. and its main constituent, menthol. AncSci Life. 2013;33(2):131-138.
Crossref - Stanisavljevic DM, Stojicevic SS, Dorcevic SM, et al. Antioxidant activity, the content of total phenols and flavonoids in the ethanol extracts of Menthalongifolia (l.) Hudson dried by the use of different techniques. Chem Ind Chem Eng Q. 2012;18(3):411-420.
Crossref - Al-Taisan WA, Alabdallah NM, Almuqadam L. Moringa leaf extract and green algae improve the growth and physiological attributes of Mentha species under salt stress. Sci Rep. 2022;12(1):14205.
Crossref - Tafrihi M, Imran M, Tufail T, et al. The Wonderful Activities of the Genus Mentha: Not Only Antioxidant Properties. Molecules. 2021;26(4):1118.
Crossref - Jiang J, Li Z, Rupasinghe VHP. Antiproliferative effects of extracts from Salvia officinalis L. and Saliva miltiorrhiza Bunge on hepatocellular carcinoma cells. Biomed Pharmacother. 2017;85:57-67.
Crossref - Elansary HO, Szopa A, Kubica P, et al. Polyphenol Profile and Antimicrobial and Cytotoxic Activities of Natural Mentha × piperita and Menthalongi folia Populations in Northern Saudi Arabia. Processes. 2020;8(4):479.
Crossref - Perino S, Chemat-Djenni Z, Petitcolas E, Ginies C, Chemat F. Downscaling of industrial turbo-distillation to laboratory turbo-clevenger for extraction of essential oils. Application of concepts of green analytical chemistry. Molecules. 2019;24(15):2734.
Crossref - Yosri M, Basma H, Nermine N, Amal S, Sayed M, Nagwa M. Identification of novel bioactive compound derived from Rheum officinalis against Campylobacter jejuni NCTC11168. Sci Wor J. 2020:3591276.
Crossref - Sayed R, Safwat NA, Amin BH, Yosri M. Study of the dual biological impacts of aqueous extracts of normal and gamma-irradiated Galleria mellonella larvae. J Taibah Univ Med Sci. 2022;17(5):765-773.
Crossref - Elbatrawy EN, Ghonimy EA, Alassar MM, Wu FS. Medicinal Mushroom Extracts Possess Differential Antioxidant Activity and Cytotoxicity to Cancer Cells. Int J Med Mushrooms. 2015;17(5):471-479.
Crossref - Elshafie HS, Mancini E, Sakr S, et al. Antifungal activity of some constituents of Origanum vulgare L. essential oil against postharvest disease of peach fruit. J Med Food. 2015;18(8):929-934.
Crossref - Teodori L, Tagliaferri F, Stipa F, et al. Selection, establishment and characterization of cell lines derived from a chemically-induced rat mammary heterogeneous tumor, by flow cytometry, transmission electron microscopy, and immunohistochemistry. In Vitro Cell Dev Biol Anim. 2000;36(3):153-162.
Crossref - Cheng YL, Chang WL, Lee SC, et al. Acetone Extract of Bupleurum Scorzonerifolium Inhibits Proliferation of A549 Human Lung Cancer Cells via Inducing Apoptosis and Suppressing Telomerase Activity. Life Sci. 2003;73(18):2383-2394.
Crossref - Ahmed HY, Kareem SM, Atef A, et al. Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities. Antioxidants (Basel). 2022;11(10):1960.
Crossref - Yuan K, Mei J, Shao D, et al. Cerium oxide nanoparticles regulate osteoclast differentiation bidirectionally by modulating the cellular production of reactive oxygen species. Int J Nanomed. 2020;15:6355-6372.
Crossref - Yosri M, Elaasser MM, Abdel-Aziz MM, et al. Determination of Therapeutic and Safety Effects of Zygophyllum coccineum Extract in Induced Inflammation in Rats. Biomed Res Int. 2022;7513155.
Crossref - Tlili H, Hanen N, Ben AA, et al. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS ONE. 2019;14(9):e0213049.
Crossref - El-Seedi HR, Burman R, Mansour A, Turki Z, Boulos L, Gullbo, J. The traditional medical uses and cytotoxic activities of sixtyone Egyptian plants: discovery of an active cardiac glycoside from Urginea maritima. J Ethnopharmacol. 2013;145(3):746-757.
Crossref - Ouelbani R, Bensari S, Mouas TN, Khelifi D. Ethnobotanical investigations on plants used in folk medicine in the regions of Constantine and Mila (North-East of Algeria). J Ethnopharmacol. 2016;194:196-218.
Crossref - Cordell G. Changing strategies in natural products chemistry. Phytochemistry. 1995;40(6):1585-1612.
Crossref - Chevallier A. The encyclopedia of medicinal plants. Dorling Kindersley, London. 1996.
- Najjaa H, Arfa A, Ben Mathe A, Neffati M. Medicinal and Aromatic Plants of the World-Africa 2017;3.
- Delamare APL, Ivete TMP, Artico L, Atti-Serafini L, Echeverrrigary S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in south Brazil. Food Chem. 2007;100(2):603-608.
Crossref - Braz VS, Melchior K and Moreira CG. Escherichia coli as a Multifaceted Pathogenic and Versatile Bacterium. Front Cell Infect Microbiol. 2020;10:548492.
Crossref - Araujo D, Henriques M, Silva S. Portrait of Candida species biofilm regulatory network genes. Trends Microbiol. 2017;25(1):62-75.
Crossref - Zuza-Alves DL, Silva-Rocha WP, Chaves GM. An Update on Candida tropicalis Based on Basic and Clinical Approaches. Front Microbiol. 2017;8:1927.
Crossref - Wang W, Wu N, Zu YG, Fu YJ. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008;108(3):1019-1022.
Crossref - Tamil Selvi M, Thirugnanasampandan R, Sundarammal S. Antioxidant and cytotoxic activities of essential oil of Ocimum canum Sims. from India. J Saudi Chem Soc. 2015;19(1):97-100.
Crossref - Mader E, Lohwasser U, Borner A, Novak J. Population structures of genebank accessions of Salvia officinalis L. (Lamiaceae) revealed by high resolution melting analysis. Biochem Sys Ecol. 2010;38(2):178-86.
Crossref - Hadri A, del Rio MAG, Sanz J, et al. Cytotoxic activity of á-humulene and transcaryo-phyllene from Salvia officinalis in animal and human tumor cells. An R Acad Nac Farm. 2010;76(3):343-56.
- Li YL, Craker LE, Potter T. Effect of light level on essential oil production of sage (Salvia officinalis) and thyme (Thymus vulgaris). In: Proccedings Int. Symp. Medicinal and Aromatic Plants. Acta Hortic. 1996;426:419-26.
Crossref - Piccaglia R, Marotti M. Characterization of several aromatic plants grown in northern Italy. Flavour Frag J. 1993;8(2):115-122.
Crossref - Santos-Gomes PC, Fernandes-Ferreira M. Organ and season dependent variation in the essential oil composition of Salvia officinalis L. cultivated in two different sites. J Agric Food Chem. 2001;49(6):2908-2916.
Crossref - Bipransh K, Sony BI, Anoop K, Ranadhir C, Runu G. The in vitro cytotoxic activity of ethno-pharmacological important plants of Darjeeling district of West Bengal against different human cancer cell lines. BMC Complement Altern Med. 2015;15:22.
Crossref - Rosella F, Silvia M, Francesca P, Patrizia V, Aldo V. Plant-derived natural compounds in genetic vaccination and therapy for HPV-associated cancers. Cancers 2020;12(11):3101.
Crossref - Dhanani T, Shah S, Gajbhiye NA, Kumar S. Effect of Extraction Methods on Yield, Phytochemical Constituents and Antioxidant Activity of Withania somnifera. Arab J Chem. 2017;10(1):S1193-S1199.
Crossref - Petrelli R, Orsomando G, Sorci L, et al. Biological Activities of the Essential oil from Erigeron floribundus. Molecules. 2016;21(8):1065.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.