Fatemeh Siahmoshteh1, Zohreh Hamidi-Esfahani1* and Mehdi Razzaghi-Abyaneh2
1Department of Food Science and Technology, Faculty of Agriculture,
Tarbiat Modares University, Tehran, Iran.
2Department of Mycology, Pasteur Institute of Iran, Tehran – 13164, Iran.
Abstract
Aflatoxins, the most potent carcinogenic mycotoxins, are mainly produced by Aspergillus flavus and Aspergillus parasiticus. Different strategies have been used to control aflatoxin-producing fungi in field and storage conditions. In this study, a field isolate of Bacillus subtilis examined for its ability to inhibit the growth, spore germination and aflatoxin production by A. parasiticus NRRL 2999. Aflatoxin degradation and cell wall adsorption were also tested. Aflatoxins B1 and G1, the most potent carcinogens, were measured qualitatively by HPLC. According to the results, B. subtilis culture filtrate suppressed fungal growth and spore germination by 0-100% and 0-38% at different concentrations, respectively. The bacterium inhibited aflatoxins B1 and G1 more than 90% in 750 µl concentration. In addition to the aflatoxin production inhibition, the bacterial cells degraded the aflatoxins B1 and G1 in liquid medium up to 60% but there was no aflatoxin which could be adsorbed by the cell wall of the bacterium. The inhibitory potentials of this isolate could be extended to direct use of this strain in the market or as a biological control agent in the field to prolong the shelf life of commodities.
Keywords: Biodegradation, Bacillus subtilis, Aspergillus parasiticus, Aflatoxin, Antifungal activity.
Introduction
Aflatoxins (AFs) are a group of highly toxic and carcinogenic mycotoxins which contaminate food, in general and agricultural products, in particular. There are approximately 18 structurally similar aromatic compounds referred to as (AFs). AFB1 is the most toxic one which is followed by AFG1, AFB2, and AFG2. These toxins are mainly produced by fungi of the Aspergillus section Flavi, particularly A. flavus and A. parasiticus. While the former is more prevalent in the nature, each known strain of the latter has the capability of producing all four major kinds of AFs1, 2. Aflatoxigenic fungi can produce toxins on agricultural commodities in the field or even after the harvest. To control these fungi, different strategies have been proposed which can inactivate or degrade AFs3. Biological control with the use of antagonistic microorganisms, is one of the most effective and sustainable post-harvest strategies to control the pathogenic fungi4. A wide range of microorganisms are used for this propose of which biologically-active bacteria, especially those belonging to the genus Bacillus are well known for their antagonistic properties against different pathogenic fungi.
Bacillus subtilis is one of the bacterial strains which its use as a biocontrol agent is well documented5-8. It has been found that this species secretes different antifungal substances into the culture medium. These substances generally belong to lipopeptide compounds which are mainly classified as surfactin, iturin and fengycin families. These lipopeptides are synthesized by non-ribosomal peptide synthetases (NRPS) or hybrid polyketide synthases/NRPS in bacterial cells. Bacillus spp. could also produce extracellular enzymes capable of degrading chitin or cell walls of fungal mycelium like exo-chitinase, endo-chitinase, and β-1, 3-glucanase6, 9, 10.
In the field of detoxification, some researches have proven the toxin degradation capability of B. subtilis strains3, 11. However, there are a few published documents about other mechanisms of aflatoxin suppression by Bacillus genus. For aflatoxin degradation, the strains could produce some enzymes which would break the aromatic structure of the aflatoxins. Dehydrogenase, hydrolase and laccase are the main toxin degrading enzymes of Bacillus spp. which can break or cleave the lactone ring of AFs12.
In this study, a strain of B. subtilis which had been isolated from soil of pistachio orchards was grown in different culture conditions including different incubation times, temperatures and culture media. The effect of the bacteria supernatant which were harvested from these different conditions was studied on fungal growth and aflatoxin-production ability of A. parasiticus. Moreover, the ability of both the cells and the cell-free supernatant (CFS) of the bacterium to inhibit the spore germination was investigated. Beside the aflatoxin production inhibition, other mechanisms of aflatoxin suppression by the bacterium, including toxin degradation and cell wall adsorption were also tested.
Materials and methods
Microorganisms
Bacillus subtilis isolated from soil of pistachio orchard which was shown to have antifungal properties in the preliminary experiments was chosen for this study. The freeze-dried strain was cultured on BHI (Brain heart Infusion broth) medium for 24 h in 30 ºC. A. parasiticus NRRL 2999 spores were prepared by adding sterile distilled water contained 0.1% Tween 80 to the fungal culture on Sabouraud Dextrose Agar (E. Merck, Germany) slants. The number of conidia was adjusted using Neubauer chamber.
Effect of different doses of CFS on A. parasiticus Growth
A loop-full of the overnight culture was inoculated in 100 ml flasks containing TSB (Tryptic Soy Broth). After 3 days of incubation at 30 °C, the culture broth was centrifuged at 14,000 × g for 5 min. and the supernatant of each flask was separated, and then sterilized with 0.2 μm PTFE filter membrane. Different two-fold concentrations of CFS including 3000, 1500, 750, 375 ad 178 μl were added to the 6 well microplates (Orange Scientific, Belgium) containing Glucose Yeast extract Broth (GYB) as the medium and 50 μl of fungal spore suspension (107 spores/ml) was added to each well. CFS-free wells were used as control. The micro-plates were incubated at 28 ºC for four days in static conditions. To evaluate the growth inhibitory ability of B. subtilis, the mycelial biomass of each well was separated from the culture media by the cheese cloth and was placed in an oven at 80 ºC until reaching to a constant weight. The dried samples were weighed by a precise balance (accuracy= 0.0001 g). The remaining culture media of each well were gathered for extraction and aflatoxin analysis. The fungal growth inhibitory percentage of each well (i.e. each dose) of CFS was calculated by Equation 1:
Inhibitory percentage of each dose of supernatant = [(Net dry weight of ctrl sample – Net dry weight of mycelia of treatment samples)/ Net dry weight of mycelia in control well] × 100
Effect of different incubation conditions on antagonistic ability of harvested CFS
To investigate the effect of culture parameters on antagonistic ability of B. subtilis, a loop-full of the overnight culture was inoculated in 100 ml flasks containing two different media, namely TSB and LB (Luria Bertani broth). The flasks were incubated in a shaker incubator (150 rpm) at different temperatures of 25, 30, 37 ºC for different incubation periods of 24, 48, 72, 96 and 120 h. Three flasks (three replicates) were used for each treatment. After the incubation period, the culture broth was centrifuged at 14,000 × g for 5 minutes and the supernatant of each flask was separated, sterilized with 0.2 μm PTFE filter membrane and added to the 6 well plates as mentioned above. The growth inhibition percentage of the fungus was measured, according to Equation 1 mentioned above.
Spore germination assay
The effect of bacterial cells on spore germination of A. parasiticus was tested in GYB. Cells were harvested from 24 h culture medium by the centrifugation and the pellets were suspended in distilled water to reach the concentration of 107, 108 and 109 cfu/ml. Two hundreds microliter of the bacterial suspension and 200 µl of the fungus suspension with the concentration of 107 spores/ml were added to 6 ml of GYB. The effect of supernatant of the bacterium in different doses was tested on spore germination of A. parasiticus. Three days cultured bacterium was centrifuged (14,000g for 5 min) to harvest the CFS. Different volumes of CFS including 250, 500, 750 and 1000 µl were added to the GYB medium flasks containing 200 µl of the fungal spore suspension (concentration of 107 spores/ml). The final volume of each flask reached to 6 ml. The flasks were placed in shaker incubator at the speed of 130 rpm. The spore germination percentage of each sample was measured in different intervals by observing the germination of 100 spores under the optical microscope.
Aflatoxin production inhibition
The co-culture broth medium of each well from the 6 well plates experiment was analyzed for aflatoxin detection. The toxin content was measured qualitatively by HPLC. Briefly, after four days of incubation, the medium of each well was separated and filtered by 0.2 μm filter membrane to prepare for aflatoxin extraction. To extract aflatoxins from sterilized samples, 1 ml chloroform was added to 1 ml of sample. This process was repeated three times. The chloroform phase was then allowed to evaporate gently. The samples were subsequently dissolved in 1 ml of methanol (LiChroSolv, Merck) and were then ready to be analyzed for Aflatoxin. Aflatoxin content was quantified using HPLC, according to Razzaghi-Abyaneh et al. (2007)7. Ten microliters of each sample in addition to AFB1 and AFG1 standards (each, 1000ppm concentration) were injected at a flow rate of 1 ml/min. The mobile phase was acetonitrile: methanol: water (25:15:65, v/v/v). AFB1 was derived by a photochemical reactor (Waters, Milford, MA, USA) and measured by a fluorescence detector. The excitation and detection wavelengths were set at 350 and 450 nm, respectively.
Aflatoxin degradation or either cell wall adsorption in liquid medium
In addition to AF production inhibition, its reduction in liquid culture was also studied according to Farzaneh et al. with some modifications. Briefly, the strain was cultured in TSB medium for 24 h at 30 ºC in a shaker incubator. Two hundred microliters of the inoculum suspension with the concentration of 105 was added to containers with five ml LB medium which was contaminated with 200 ppb AFB1. Sterile LB and LB containing 200 ppb AFB1 were considered as control samples. The containers were incubated at 30 for 24, 48 and 72 h. Each sample was then centrifuged 10,000 rpm for 10 min. The supernatant was extracted for aflatoxin as described above. To confirm the degradation as the aflatoxin reduction mechanism, the bacterial cell walls were analyzed for aflatoxin adsorption. Briefly, the centrifuged cell pellets were washed and then sonicated with saline solution and then they were washed with the solution at 4000 rpm for 10 min. This step was repeated twice. The pellets were then extracted for aflatoxin analysis. The extracted samples were injected to HPLC under the conditions described previously13.
Statistical analysis
All experiments were done twice in three replicates. All data were analyzed by one way analysis of variance (ANOVA) and LSD test in the statistical software SPSS v. 18.0 for windows. Differences at P<0.05 were considered as significant.
Results
Effect of different doses of CFS on A. parasiticus growth
As shown in Table 1, five different concentrations of supernatant were used to examine the growth inhibitory ability of the CFS of B. subtilis. These CFSs were prepared by culturing bacteria in TSB medium at 30ᵒC for 48 hour. The inhibitory activity of each concentration was calculated by equation 1. According to the results, the two first concentrations (i.e. 3000 and 1500 ppm) showed considerable decreases in the fungal growth compared to the control. The third concentration (i.e. 750 ppm), reduced the fungal growth for approximately 50 %. Therefore, this concentration of supernatant can be considered as IC50 against A. parasiticus and was chosen for the aflatoxin assay. Two lower concentrations (i.e. 375, 187ppm) showed no inhibition activity while caused an increase in the dry weight of fungal mycelium compared to the control. The inhibitory percentage is shown in Table 1. Figure 1 also depicts the effect of the supernatant dose on the growth inhibition of A. parasiticus in a 6 well microplate.
Table 1. Growth inhibition of A. parasiticus NRRL 2999 by different concentrations of B. subtilis culture supernatant
Supernatant conc. (μl) |
Mycelium dry weight (mg) |
Inhibition (%) |
0 |
104b± 2 |
0 |
178 |
120.6a±4.5 |
0 |
375 |
106b±7 |
0 |
750 |
51c±3 |
51 |
1500 |
17d±1.5 |
83.7 |
3000 |
0e± 0.5 |
100 |
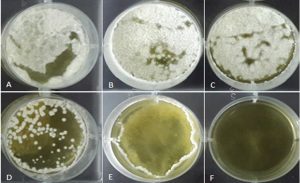 Fig. 1. The effect of different doses of superatat (ppm) on growth inhibition of A. parasiticus in a 6 well microplate: A) control; B) 178 µl; C) 375 µl; D) 750 µl; E) 1500 µl; F) 3000 µl
Fig. 1. The effect of different doses of superatat (ppm) on growth inhibition of A. parasiticus in a 6 well microplate: A) control; B) 178 µl; C) 375 µl; D) 750 µl; E) 1500 µl; F) 3000 µl
Effect of different incubation conditions on antagonistic ability of harvested CFS
The antagonistic effect of CFS harvested from different incubation conditions were tested but because of too much data, only the result from the first two concentrations of the supernatant (i.e. 3000, 1500 μl) were shown in Table 2. Among the 30 tested incubation conditions, the CFS harvested from 6 of them showed the highest antifungal activities. These optimum conditions are presented in Table 3.
Table 2. Inhibitory effect of B. subtilis culture supernatant harvested at different culture conditions on A. parasiticus growth
| Time (h) | Temp (ᵒC) | CFS (μl) | Media | |
| TSB | LB | |||
| 24 | 25 | 3000
1500 |
49.19
8.00 |
56.10
53.50 |
| 30 | 3000
1500 |
99.50a
99.50 |
47.42
26.87 |
|
| 37 | 3000
1500 |
61.94e
7.42e |
99.50a
98.72a |
|
| 48 | 25 | 3000
1500 |
75.75d
75d |
66.37e
46.68e |
| 30 | 3000
1500 |
90.40b,c
82.65 |
53.60
30.65b,c |
|
| 37 | 3000
1500 |
100a
82.5 |
99.27a
99.28a |
|
| 72 | 25 | 3000
1500 |
95.62a,b
63.7a |
98.45a,b
98.6a |
| 30 | 3000
1500 |
95.70a,b
86.70b |
93.67a,b
93.6b |
|
| 37 | 3000
1500 |
78.50c,d
55.92c |
98.52a,b
98.72d |
|
| 96 | 25 | 3000
1500 |
92.10b
76.72b |
62.40e
62.32b± |
| 30 | 3000
1500 |
97.10a,b
76.45b |
96.00a,b
97.75a,b |
|
| 37 | 3000
1500 |
85.00c
85.25a,b |
97.25a,b
99.5a |
|
| 120 | 25 | 3000
1500 |
75.65d
69.18b |
66.20e
54.58d |
| 30 | 3000
1500 |
99.75a
99.25a |
99.00a
38.62a |
|
| 37 | 3000
1500 |
99.75a
99.25a |
98.12a,b
97.1a |
|
Table 3. Optimal incubation conditions for antifungal activity of B. subtilis culture supernatant (CFS) against A. parasiticus
| Treatment | Culture conditions | CFS (μl) | Inhibition ratio (%) | ||
| Time (h) | Temp (ᵒC) | Media | |||
| 3000
1500 |
90.4
82.6 |
||||
| A | 48 | 30 | TSB | ||
| 3000
1500 |
100
82.5 |
||||
| B | 48 | 37 | TSB | ||
| 3000
1500 |
99.3
99.3 |
||||
| C | 48 | 37 | LB | ||
| 3000
1500 |
99.75
99.25 |
||||
| D | 120 | 30 | TSB | ||
| 3000
1500 |
99.75
99.25 |
||||
| E | 120 | 37 | TSB | ||
| 3000
1500 |
98.1
97.1 |
||||
| F | 120 | 37 | LB | ||
Spore germination assay
The effect of supernatant of the bacterium on spore germination was examined at different incubation intervals from time zero to 11 hours later. Up to 90% of the spores in the control sample were germinated after 11 hours of the incubation. However, this amount decreased dose-dependently for the samples containing supernatant of the bacterium. All treatments showed no spore germination during the first 3 hours. These results are presented in Table 4. The effect of co-culturing the bacterial cells and A. parasiticus on spore germination of the fungus is shown in Figure 2. According to these results, all three concentrations of the bacterium in co-cultured medium could efficiently inhibit conidial germination of the fungus during the 11 hours of the test period. However, it should be noted that the results were time and dose dependent. While higher concentrations of the bacterial cells showed more inhibition in all the test intervals, the percentage of the germination increased in all treatments by passing the time.
Table 4. Spore germination inhibition by cell free supernatant of B. subtilis with different concentration at different intervals
| CFS (µl) | Time (hour) | |||||
| 0 | 3 | 5 | 7 | 9 | 11 | |
| 250 | 0 | 0 | 8 | 10 | 8 | 7 |
| 500 | 0 | 0 | 9 | 11 | 16 | 17 |
| 750 | 0 | 0 | 20 | 25 | 20 | 22 |
| 1000 | 0 | 0 | 30 | 38 | 32 | 33 |
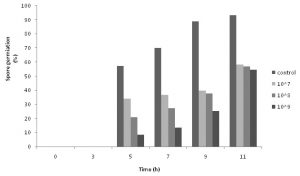 Fig. 2. Effect of bacterial cells with different concentration on spore germination of A. parasiticus at different intervals
Fig. 2. Effect of bacterial cells with different concentration on spore germination of A. parasiticus at different intervals
Aflatoxin production inhibition
As it is shown in Table 3, the supernatants produced in some culture conditions could suppress the fungal growth more than 99% even in lower concentrations (i.e. 1500 µl). In fact, no fungal growth was observed in the presence of 3000 ad 1500 µl of the supernatant. Hence, there was no fungal mycelium to produce aflatoxin. For this reason, aflatoxin contents of each well were assessed for the third well of the plates with CFS concentration of 750 µl which showed around 50% fungal inhibitory activity. The ability of the bacterial supernatant to suppress AFs production by A. parasiticus was confirmed by HPLC. The amount of AFB1, AFG1 and the suppression percentage of total aflatoxin (AFT) are shown in Table 5. In all tested conditions, CFS showed more than 90% toxin suppression and this amount reached to more than 98% (98.53±0.058) in condition C.
Table 5. Aflatoxin suppression by CFS at the dose of 750 µl in comparison to control in any culture conditions
| CFS harvesting CFS (µl) Aflatoxin (µg/mg dry weight) conditions |
||||
| B1 | G1 | B1+G1 | ||
| A | 0 (Control)
750 |
200
9.5 |
130
7 |
330
16.5 |
| B | 0
750 |
970
30 |
730
18 |
1600
48 |
| C | 0
750 |
991
14 |
655
11 |
1626
25 |
| D | 0
750 |
867
14.8 |
539
12.7 |
1406
27.5 |
| E | 0
750 |
500
13 |
466
13 |
966
26 |
| F | 0
750 |
151
13 |
122
10 |
273
23 |
Aflatoxin degradation or either cell wall adsorption by B. subtilis
To understand the mechanism of aflatoxin reduction by B. subtilis, we analyzed the cell walls of the bacterium to see any adsorption. The cell walls were lysed by sonication ad the solvents were added to the extracted aflatoxin. No aflatoxin was found in the cell walls so the bioadsorption by the cell walls was too low to report. It is concluded that the main mechanism of aflatoxin reduction by B. subtilis is toxin degradation. The results for aflatoxin degradation are shown in Figure 3. The percentage of degradation increased by the time and reached to 50% after three days of incubation.
 Fig. 3. Aflatoxins B1 and G1 degradation by B. subtilis in liquid medium
Fig. 3. Aflatoxins B1 and G1 degradation by B. subtilis in liquid medium
Discussion
subtilis is a well-known bacterial species with all the required characteristics for a so-called biocontrol agent and in some countries it is used as a commercial biocontrol agent in the fields and orchards. It is also used as a probiotic in human food as well as in animal feed14-16. In the present study, a field isolated strain of B. subtilis was studied for its inhibitory effect against a standard strain of Aspergillus parasiticus which produces all kinds of aflatoxins. The effect of different culture conditions (incubation time, temperature and medium) was studied on the fungal growth, spore germination and aflatoxin-production ability of A. parasiticus. Beside the aflatoxin production inhibition, other mechanisms of aflatoxin suppression by the bacterium, including toxin degradation and cell wall adsorption were also tested.
Our results on the growth inhibitory activity of B. subtilis CFS showed very high antifungal activity even in low doses of CFS. But sub-lethal doses of CFS induced fungal growth over that that of the control. In fact, low concentrations of the metabolites increased the fungal growth. It has been mentioned in some documents that very low doses of fungicidal compounds could stimulate the growth and also aflatoxin production of the fungi17, 18. In this research, CFS doses of lower than 375 µl were considered as the fungal growth stimulator doses.
Among thirty different incubation conditions tested, six of them showed better results which were chosen as the optimum conditions for the antifungal activity of CFS. The Optimal incubation conditions were occurred in 48 and 120 hours of the incubation period at higher temperatures in both TSB and LB media; however, the TSB results were more prominent. No significant difference was observed for temperatures of 30 and 37ᵒ C in any concentration. According to Lee and Kim (2012), among the different tested media, LB and TSB have shown better antifungal activities results compared to others media including NB and BHI19. Kumar et al. have studied the production of antifungal antibiotics of B. subtilis in different culture conditions. Among the four tested media, the maximum growth and antibiotic production were found in TSB medium at 37ᵒ C17, 21. Moita and colleagues have also shown that maximum antifungal activity of CFS could be obtained when the bacteria are cultured at 37 ºC and pH 87. In fact, higher temperatures, here 37 ºC, could also accelerate all the reactions, such as the enzymatic reactions and the metabolite productions. These results were completely in accord with our study where B. subtilis strains could produce different antifungal metabolites of different group of compounds, such as surfactins, fengycins and iturins. These components affect the membrane surface tension that leads to formation of pores on the cell wall, causing the leakage of K+ and other vital ions and consequently, the cell death22, 23. B. subtilis can also produce some cell wall degrading enzymes such as proteases, chitinase, and β-1, 3-glucanase7, 23. These enzymes and metabolites are synthesized at different phases of the bacterial growth cycle and the types and amounts of them are greatly influenced by the culturing conditions. For example, surfactins were mainly produced during the logarithmic growth phase while iturins and fengycins were produced during the stationary phase. It has also been reported that some Bacillus antifungal metabolites such as bacillomycin L, are synthesized at conditions where the nutrient depletion is favored9, 10.
There are some mechanisms describing biosuppression of aflatoxins by biological control agents. At first, the competition of the bacteria and the fungus in a co-culture medium may lead to the growth inhibition of the fungus and subsequently the aflatoxin production inhibition. Some bioactive compounds produced by biocontrol agents, inhibit AF production by inhibiting the synthesis of the enzymes that are active in AF production pathway. Therefore in these conditions, the fungus cannot convert the precursors to aflatoxin. These compounds are mainly secreted by lactic acid bacteria (LAB) and Streptomyces15, 24-26. Some antagonists like LAB can bind to AF with some components on their cell walls such as oligomannans15, 27 and 28 and finally, soil bacteria like the bacilli have a capability to degrade aflatoxins to nontoxic substances3, 29. In a culture medium, different factors can have an impact on the reduction of aflatoxin contents. These include changes in amount of different components of the media during the fungus growth and the production of different enzymes. Changes in the components of the medium such as nitrogen, during incubation can shift the fungal growth pathway to produce enzymes such as hydrolase and laccase which can degrade the produced aflatoxin by breaking the lactone ring12.
We showed that AFs synthesis by A. parasiticus could be suppressed significantly by the CFS of B. subtilis. The highest suppression of AFs production was recorded as 98% which was achieved when the aflatoxin-producing fungus was co-cultured with the CFS harvested from condition C (48h, 37ᵒC, LB). Also the bacterium degraded both AFB1 and AFG1, time-dependently but no adsorption of the toxins by the bacteria cell wall was evident.
Taken together, the results of this study showed that B. subtilis can be considered as a potent inhibitor of toxigenic A. parasiticus growth and aflatoxin production and the incubation conditions of the bacterium can affect this ability. This potential could be extended to direct use of this strain in the market or in the field to prolong the shelf life of commodities as a biological control agent. As an essential parameter, the safety of the antagonistic bacterium and its metabolites using a food model system is currently evaluated in our laboratory.
Acknowledgements
This work was supported financially by the Research Deputy of Tarbiat Modares University. Authors are grateful to Reza Tolouei for his kind technical assistance in aflatoxin analysis by HPLC.
References
- Razzaghi-Abyaneh, M., Yoshinary, T., Shams-Ghahfarokhi, M., Rezaee, M.B, Nagasawa, H., Sakuda, S. Dillapiol and apiol as specific inhibitors of the biosynthesis of aflatoxin G1 in Aspergillus parasiticus. Biosci. Biotechnol. Biochem. 2007; 71: 2329-2332.
- Sangare, L., Zhao, Y., Folly, Y.M., Chang, J., Li, J., Selvaraj, J.N., Liu, Y. Aflatoxin B1 Degradation by a Pseudomonas strain. Toxins. 204; 6: 3028-3040.
- Farzaneh, M., Shi, Z., Ghassempour, A., Sedaghat, N., Ahmadzadeh, M. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated frompistachio nuts of Iran . Food Cont. 2012; 23: 100-106.
- Patharajan. S., Spadaro, D., Lore, A., Gullino, L.M., Garibaldi, A. Efficacy of yeast antagonists on invitro biodegradation of Ochratoxin A. Food Cont. 2011; 25: 1-23.
- Akpa, E., Jaques, P., Wathelet, B., Paquot, M,. Influence of Culture Conditions on Lipopeptide Production by Bacillus subtilis. Appl Biochem Biotech. 2001; 91: 551-561.
- Obagwu, J., Korsten, L. Intregrated control of citrus green and blue molds using Bacillus subtilis in combination with sodium bicarbonate or hot water. Postharvest Biol Tec. 2003; 28: 187-194.
- Razzaghi-Abyaneh, M., Shams-Ghahfarokhi, M., Rezaee, M.B., Jaimand, K. Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Cont, 2009; 20: 1018–1024.
- Reddy, K.R, Raghavender, C.R., Reddy, B.N., Salleh, B. Biological control of Aspergillus flavus growth and subsequent aflatoxin B1 production in sorghum grains. African J Biotech. 2010; 9: 4247-4250.
- Razzaghi-Abyaneh, M. Aflatoxins – Recent Advances and Future Prospects. First ed. Rijeka: InTech; 2013.
- Toure, Y., Ongena, M., Jacques, P. Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J Appl Microbiol. 2004; 96: 1151–1160.
- Lee, H.A., Kim, J.H. Isolation of Bacillus amyloliquefaciens Strains with Antifungal Activities from Meju. Prev. Nutr. Food Sci. 2012; 17: 64-70.
- Eshelli, M., Harvey, L., Edrada-Ebel, R. Metabolomics of the bio-degradation process of aflatoxin B1 by actinomycetes at an initial pH of 6.0. Toxins. 2015; 7: 439-456.
- Spadaro, D., Zhang, D., Garibaldi, A., Gullino, M.L. The role of competition for iron and cell wall degrading enzymes in mechanism of action of postharvest biocontrol agents. Acta Hort. http://dx.doi.org/10.17660/ActaHortic. Accessed 2011.905.8.
- Duc, L.H, Hong, H.A., Barbosa, T.M., Henrique, A.O. Characterization of Bacillus Probiotics Available for Human Use. Appl Environ Microb. 2004; 70(4): 2161-2171.
- Fuchs, S., Sontag, G., Stidl, R., Ehrlich, V. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem Toxicol. 2008; 46: 1398-1407.
- Green, D.H, Wakeley, P.R., Page, A., Barnes, A. Characterization of Two Bacillus Probiotics. Appl Environ Microb. 1999; 65: 4288-4291.
- Badii, F., Moss, M. The effect of the fungicides tridemorph, fenpropimorph and fenarimol on growth and aflatoxin production by Aspergillus parasiticus Speare. Lett Appl Microbiol. 1988; 7: 37-39.
- Palumbo, J.D., Baker, J., Mahoney, N. Isolation of Bacterial Antagonists of Aspergillus flavus from Almonds. Microbial Ecol. 2006; 52: 45-52.
- Lee, H.A., Kim, J.H. Isolation of Bacillus amyloliquefaciens Strains with Antifungal Activities from Meju. Prev Nutr Food Sci. 2012; 17: 64-70.
- Moita, C., Savluchinske, F.S., Nunes, L. Optimisation of physical factors on the production of active metabolites by Bacillus subtilis 355 against wood surface contaminant fungi Int Biodeter Biodegr. 2005; 55: 261–269.
- Kumar, A., Saini, P., Shrivastava, J.N. Production of peptide antifungal antibiotic and biocontrol activity of Bacillus subtilis. Indian J Exp Biol. 2009; 47(1): 57-62.
- Gordillo, M.A., Navarro, A.R., Benitez, L,M., de Plaza, M.T. Preliminary Study and Improve the Production of Metabolites with Antifungal Activity by a Bacillus sp Strain IBA 33. Microbiology Insights. 2009; 2: 15-24.
- Thimon, L., Peypoux, F., Maget-Dana, R., Michel, G. Surfactive properties of antifungal lipopeptides produced by Bacillus subtilis. AOCS. 1992; 69: 92-93.
- Gourma, H., Bullerman, B.L. Inhibition of growth and aflatoxin production of Aspergillus flavus by Lactobacillus species. J Food Prot. 1995; 58: 1249–1256.
- Sakuda, S. Studies on mode of action of aflastatin A, an inhibitor of aflatoxin production. Mycotoxins. 2002; 52: 153-158.
- Yoshinari, T., Akiyama, T., Nakamura, K., Kondo, T. Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology. 2007; 153: 2774–2780.
- El-Nezami, H.S, Kankaanpaa, P., Salminen, S., Ahokas, J. Ability of Dairy Strains of Lactic Acid Bacteria to Bind a Common Food Carcinogen, Aflatoxin B1. Food Chem Toxicol. 1998; 36: 321-326.
- Haskard, C.A., El-Nezami, H.S., Kankaanpaa, P.E., Salminen, S. Surface Binding of Aflatoxin B1 by Lactic Acid Bacteria. Appl Environ Microbiol. 2001; 67: 3086–3091.
- Gao, X., Ma, Q., Zhao, L., Lei, Y. Isolation of Bacillus subtilis: screening for aflatoxins B1, M1, and G1 detoxification. Eur Food Res Technol. 2011; 232: 957-962.

