ISSN: 0973-7510
E-ISSN: 2581-690X
The objective of the study is to assess the sensitivity pattern of commonly isolated uropathogens towards the drugs Nitrofurontoin and Nalidixic acid. Widespread and irrational use of antibiotics has led to the development of highly resistant microorganisms. As the antibiotic sensitivity patterns of the microorganisms are frequently changing, this study was performed to assess the antibiotic sensitivity pattern of Nitrofurontoin and Nalidixic acid in urinary tract infections. This was a prospective, cross-sectional, observatory study conducted in the Department of Microbiology from January 2017 to July 2017 at RMMC, Chidambaram.300 culture positive urine samples were studied for the antibiotic susceptibility testing towards Nitrofurontoin and Nalidixic acid. The processing of the samples were done by standard microbiological methods. The antibiotic susceptibility was measured by disk diffusion test. CLSI guidelines were used for the antibiotic susceptibility evaluation [1]. Out of the 300 culture positive samples, the most common pathogen isolated in this study was E. coli (145), followed by Klebsiella (63), Staphylococcus aureus (35), Pseudomonas (36), Staphylococcus saprophyticus (10), Enterococcus (6), Sreptococcus (4), Proteus (1). The antibiotic susceptibility pattern for Nitrofurontoin: E. coli showed sensitivity (n=105) and resistance (40), Klebsiella showed sensitivity (47) and resistance (16), Staphylococcus aureus showed sensitivity (30) and resistance (5), Pseudomonas showed sensitivity (12) and resistance (24), Staphylococcus saprophyticus showed sensitivity (4) and resistance (2), Enterococcus showed sensitivity (5) and resistance (1), Streptococcus showed sensitivity (4) and resistance (0) and Proteus showed sensitivity (0) and resistance (1). The antibiotic susceptibility pattern for Nalidixic acid: E. coli showed sensitivity (n=23) and resistance (122), Klebsiella showed sensitivity (29) and resistance(43), Staphylococcus aureus showed sensitivity(9) and resistance (30), Pseudomonas showed sensitivity (4) and resistance (32), Staphylococcus saprophyticus showed sensitivity (2) and resistance (4), Enterococcus showed sensitivity (1) and resistance (5), Streptococcus showed sensitivity (0) and resistance(4) and Proteus showed sensitivity (0) and resistance (1). Urinary pathogens were more sensitive to Nitrofurontoin than Nalidixic acid. Klebsiella sp. was more sensitive towards Nitrofurontoin than E. coli. Hence Nitrofurontoin can be used as an effective drug in UTI.
E. coli, Enterococcus spp, Pseudomonas spp, Nitrofurantoin, Nalidixic acid, UTI.
Urinary tract infection (UTI) is one of the most common bacterial infection in the human population affecting all the age groups.1 It remains a major public health problem. UTI is defined by a combination of clinical feature and the presence of significant bacteria in urine. Significant bacteria are defined by the presence of more than 100,000 colony forming unit of single bacteria in culture in urine. The clinical feature of UTI may include both specific and nonspecific signs and symptoms. UTI is more common in women especially in the reproductive age group. Short female urethra, proximity of the female urethral meats to the vagina and rectal mucosa with their abundant microbial flora and sexual intercourse have been reported as influencing factors for the higher occurrence of UTI in women. Study showed, UTI is most common in the age group of 61-80 years among males. Elderly males had a higher incidence of UTI when compared with the elderly females. This finding is like study conducted by Shah et al and Sood et al3,4 This is probably because with advancing age, the incidence of UTI increases among males due to prostate enlargement, neurogenic bladder and requirement of catheterization in the form of intervention5,6.
This situation is further complicated by the fact that accurate diagnosis depends upon both the presence of symptoms and a positive urine culture, although in the most outpatient setting this the diagnosis is made without the benefit of culture. Catheter-associated UTI is the most common nosocomial infection, according for >1 million case in hospital and nursing home. Urinary tract infection is due to an inflammatory response of urothelium to the invading pathogenic organisms. Uropathogenic E. coli is responsible for >80% of community acquired UTIs. Other organisms causing UTI are Klebsiella pneumoniae, Pseudomonas sp, Staphylococcus aureus, Staphylococcus saprophyticus, Enterococcus sp, Streptococcus sp, and Proteus sp. E.coli causes most of the urinary infections in patients with or without catheters, in situations of urinary calculi and urological abnormalities. To treat UTIs, an updated knowledge of the organisms causing UTI and their antibiotic susceptibility pattern is required.
Periodic evaluation of antibacterial susceptibility pattern is needed to update the information .High prevalence of urinary tract infection, irrational use of antibiotics, over-the counter availability of antibiotics leads to the development of multidrug resistance. This study was conducted to know the sensitivity pattern of uropathogens towards Nitrofurontoin and Nalidixic acid7,8.
A prospective study was conducted from January 2017 to July 2017.
Inclusion criteria
Positive urine cultures showing pure growth of organism and their sensitivity pattern with the following criteria were included in the study9,10
From all departments
- Both sex
- Both inpatients and out patients
- Only bacterial isolate.
Exclusion criteria
Urine cultures showing mixed organisms
- Urine cultures showing organisms other than bacteria.
Study population and sample processing
Total 300 positive urine culture samples were taken for this study their antibiotic susceptibility pattern for Nitrofurontoin and Nalidixic acid were done by Kirby Bauer method. Majority of the samples were midstream clean catch urine and others included catheterized urine samples. Samples were collected in a sterile screw-capped wide-mouth container. The containers were labelled with a unique sample number, date and time of collection. The urine samples were processed within two hours after collection in the clinical microbiology laboratory. Using a calibrated loop (volume – 0.005ml) streaking was done on cysteine lactose electrolyte deficient medium11,12. Isolates were tested for antimicrobial susceptibility testing on Mueller-Hinton agar plates by the standard Kirby-Bauer disc diffusion method 12. Plates were incubated at 37oC for 18-24 hours, after that inhibition zones were measured and reported as per clinical and laboratory standards institute guidelines (CLSI). Nitrofurontoin disc containing concentration of 300 mcg and Nalidixic acid disc containing concentration of 30 mcg were used. The results were interpreted according to clinical and laboratory standards institute guidelines16,17 .Analyzation of data was carried out on focusing on the gender, age, isolation of bacteria, gram staining and Antimicrobial sensitivity. The quality control strains used were E. coli American type culture collection (ATCC) 25922, Pseudomonas aeruginosa ATCC 27853, Enterococcus fecalis ATCC 29212 and Staphylococcus aureus ATCC 25923 for antimicrobial discs.
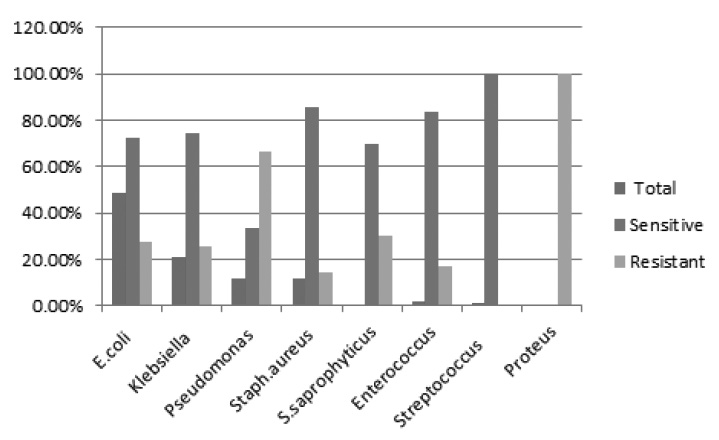
Fig. 1. Comparison of Antibiotic susceptibility pattern of urinary pathogens towards Nitrofurantoin
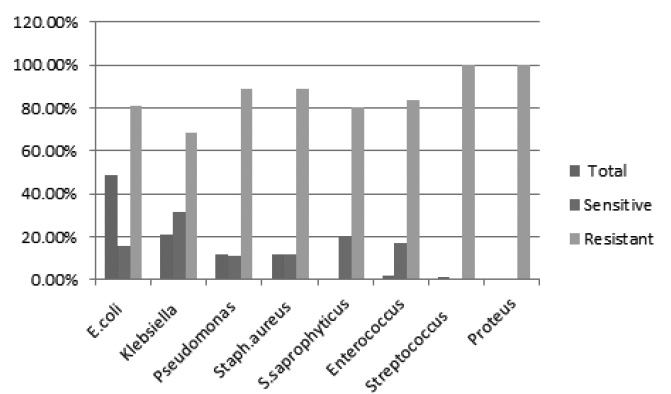
Fig. 2. Comparison of Antibiotic susceptibility pattern of uropathogens towards Nalidixic acid
- E. coli is still the most common urinary isolate irrespective of geographical area and this study confirmed it. There are several factors responsible for their attachment to uroepithelium. They colonize in the urogenital mucosa with adhesins, pili, fimbriae, and P-1 blood group phenotype receptor.21
- The data collected from other places around the world, also showed that E. coli and Klebsiella spp. are still the commonest uropathogens isolated.
- Nitrofurantoin, a chemotherapeutic compound of the Nitrofuran family, was introduced into clinical practice in 1952. Nitrofurantoin is a synthetic antimicrobial derived from furan by the addition of a nitro group and a side chain containing hydantoin. Nitrofurantoin is a weak acid and its solubility is affected by PH11. Nitrofurantoin spectrum of in vitro susceptibility includes the majority of Escherichia coli, Citrobacter species, group B Streptococci, Enterococci, Staphylococcus aureus, S. saprophyticus, Klebsiella pneumoniae and Enterobacter species12,13,14. Thus, its antibacterial spectrum is broad and is particularly effective against the main uropathogens, hence its use for the treatment of urinary tract infections (UTI)15.
Total 300 urine culture positive samples showing significant bacteriuria were analysed. Out of 300 (culture positive samples) 162 were females (54%) and 138 were males (46%). Female cases outnumbered male cases.
The urinary tract infections were found highest within 18-50 years of age group (50.82%) among the female patients. Whereas majority of the isolates of male patients were obtained from age group of 40-80 years (55%) .
The most common bacteria isolated was E. coli (n=145, 48.33%), followed by Klebsiella pneumoniae (n=63, 21%), Pseudomonas spp. (n=36, 12%) Staphylococcus aureus (n=35, 11.66%), Staphylococcus saprophyticus (n=10, 3.33% ) and Enterococcus spp. (n=6, 2%), in this study. The other less frequently isolated bacteria were and streptococcus sp (n=4,1.33%) Proteus spp. (n=1, 0.33%).
Table (1):
Antibiotic susceptibility pattern of uropathogens towards Nitrofurantoin.
Organism |
Total |
Sensitive |
Resistant |
|---|---|---|---|
E. coli |
145(48.33%) |
105(72.41%) |
40(27.59%) |
Klebsiella |
63(21%) |
47(74.60%) |
16(25.40%) |
Pseudomonas |
36(12%) |
12(33.33%) |
24(66.66%) |
Staph. aureus |
35(11.66%) |
30(85.71%) |
5(14.28%) |
S. saprophyticus |
10(3.33%) |
7(70%) |
3(30%) |
Enterococcus |
6(2%) |
5(83.33%) |
1(16.66%) |
Streptococcus |
4(1.33%) |
4(100%) |
_ |
Proteus |
1(0.33%) |
_ |
1(100%) |
E. coli was the common pathogen isolated (48.33%) in this study and it was 72.41% sensitive to Nitrofurantoin. Even though the percentage of isolation of Klebsiella was less 21%, it was more sensitive to Nitrofurantoin (74.60%). Pseudomonas isolation rate was 12% and it was 33.33% sensitive to nitrofurantoin and 66.66% resistant to it. Staphylococcus aureus showed high sensitivity 85.71% towards Nitrofurantoin. Staphylococcus saprophyticus showed sensitivity of 70% and resistance of 30% towards Nitrofurantoin. Enterococcus sp also showed high percentage of sensitivity towards Nitrofurantoin (83.33%) and resistance rate of 16.66%. Streptococcus showed 100% sensitivity toward Nitrofurantoin18,19.
Proteus spare intrinsically resistant to Nitrofurantoin and in this study also Proteus sp isolated were 100% resistant to it20,21.
Most of the uropathogens isolated were resistant to Nalidixic acid. The drug sensitivity rate was 31.75% for Klebsiella followed by 20% for Staphylococcus saprophyticus. Nalidixic acid resistant rate was high for Pseudomonas sp (88.89%) followed by Staphylococcus aureus (88.57%), E. coli (84.14%) and Enterococcus sp (83.33%). Streptococcus sp and Proteus sp were 100% resistant to Nalidixic acid22.
Table (2):
Antibiotic susceptibility pattern of uropathogens towards Nalidixic acid.
Organism |
Total |
Sensitive |
Resistant |
|---|---|---|---|
E. coli |
145(48.33%) |
23(15.86%) |
122(84.14%) |
Klebsiella |
63(21%) |
20(31.75%) |
43(68.25%) |
Pseudomonas |
36(12%) |
4(11.11%) |
32(88.89%) |
Staph. aureus |
35(11.66%) |
4(11.43%) |
31(88.57%) |
S. saprophyticus |
10(3.33%) |
2(20%) |
8(80%) |
Enterococcus |
6(2%) |
1(16.67%) |
5(83.33%) |
Streptococcus |
4(1.33%) |
_ |
4(100%) |
Proteus |
1(0.33%) |
_ |
1(100%) |
In this study most of the urinary pathogens isolated were more sensitive to Nitrofurontoin than to Nalidixic acid. Nitrofurantoin is an orally available drug and is active against most of the urinary pathogens. Thus, there is a need for making and following antibiotic usage policy that will guide the prescription and use of antibiotics through the regular surveillance of resistant organisms in the environments.
- Nigussie D, Amsalu A. Prevalence of uropathogen and their antibiotic resistance pattern among diabetic patients. Turk J Urol. 2017;43(1):85-92. Longo DL. Harrison’s principles of internal medicine. 18th ed. New York: McGraw-Hill; 2012: 2387-2395.
- Nalini R, Ramya J, Meenakshi B, Palniappan N, Poongodi S. Recent Sensitivity Pattern of Escherichia Coli in Urinary Tract Infection. RRJMB. 2014; 3(3):31-5.
- Dash M, Padhi S, Mohanty I, Panda P, Parida B. Antimicrobial resistance in pathogens causing urinary tract infections in a rural community of Odisha, India J Family Community Med. 2013; 20(1):20-6.
- Rock W, Colodner R, Chazan B, Elias M, Raz R. Ten years surveillance of antimicrobial susceptibility of community-acquired Escherichia coli and other uropathogens in northern Israel (1995-2005). Isr Med Assoc J. 2007; 9: 803-5
- Oladeinde BH, Omoregie R, Olley M, Anunibe JA. Urinary tract infection in a rural community of Nigeria. N Am J Med Sci. 2011; 3:75-7
- Sood S, Malhotra M, Das BK, Kapil A. Enterococcal infections and antimicrobial resistance. Indian J Med Res. 2008; 128:111-21.
- Badhan R, Singh DV, Badhan LR, Kaur A. Evaluation of bacteriological profile and antibiotic sensitivity patterns in children with urinary tract infection: A prospective study from a tertiary care center. Indian J Urol. 2016; 32(1): 50-6.
- Bano K, Khan J, Begum RH, Munir S, Akbar N, Ansari JA. Patterns of antibiotic sensitivity of bacterial pathogens among urinary tract infections (UTI) patients in a Pakistani population. Afr J Microbiol Res. 2012; 6:414-20.
- Bahadin J, Teo SS, Mathew S. Aetiology of community-acquired urinary tract infection and antimicrobial susceptibility patterns of uropathogens isolated. Singapore Med J. 2011; 52: 415-20.
- Das RN, Chandrashekhar TS, Joshi HS, Gurung M, Shrestha N, Shivananda PG. Frequency and susceptibility profile of pathogens causing urinary tract infections at a tertiary care hospital in western Nepal. Singapore Med J. 2006; 47:281-5.
- Shinde RS, Koppikar GV, Oommen S. Characterization and antimicrobial susceptibility pattern of clinical isolates of Enterococci at a tertiary care hospital in Mumbai, India. Ann Trop Med Public Health. 2012; 5:85-8.
- Ali S, Mirza IA, Yaqoob S, Hussain A, Khan I, Rafiq MY. Antimicrobial susceptibility pattern of Enterococcus species isolated from patients with urinary tract infection. Gomal J Med Sci. 2014; 12:11-4.
- Butt T, Leghari MJ, Mahmood A. In vitro activity of nitrofurantoin in enterococcus urinary tract infection. J Pak Med Assoc. 2004; 54: 466-9.
- Gupta V, Singla N, Chander J. Detection of ESBLs using third and fourth generation cephalosporins in double disc synergy test. Indian J Med Res. 2007; 126: 486-7.
- Shaifali I, Gupta U, Mahmood SE, Ahmed J. Antibiotic susceptibility patterns of urinary pathogens in female outpatients. N Am J Med Sci. 2012; 4: 163-9.
- Oladeinde BH, Omoregie R, Olley M, Anunibe JA. Urinary tract infection in a rural community of Nigeria. N Am J Med Sci. 2011; 3:75-7.
- Shah DA, Wasim S, Abdullah FE. Antibiotic resistance pattern of Pseudomonas aeruginosa isolated from urine samples of Urinary Tract Infections patients in Karachi, Pakistan. Pak J Med Sci. 2015; 31(2):341-5.
- Sood S, Malhotra M, Das BK, Kapil A. Enterococcal infections and antimicrobial resistance. Indian J Med Res. 2008; 128:111-21.
- Badhan R, Singh DV, Badhan LR, Kaur A. Evaluation of bacteriological profile and antibiotic sensitivity patterns in children with urinary tract infection: A prospective study from a tertiary care center. Indian J Urol. 2016; 32(1): 50-6.
- Bano K, Khan J, Begum RH, Munir S, Akbar N, Ansari JA. Patterns of antibiotic sensitivity of bacterial pathogens among urinary tract infections (UTI) patients in a Pakistani population. Afr J Microbiol Res. 2012; 6:414-20.
- Bahadin J, Teo SS, Mathew S. Aetiology of community-acquired urinary tract infection and antimicrobial susceptibility patterns of uropathogens isolated. Singapore Med J. 2011; 52:415-20.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


