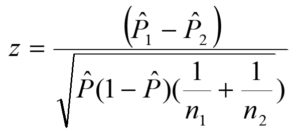ISSN: 0973-7510
E-ISSN: 2581-690X
Medicinal plants are widely used as part of home remedies to cure human diseases. The current research was conducted to explore the antibacterial effectiveness of Artemisia vulgaris, Nigella sativa, Origanum majorana, Moringa oleifera, Tetragonolobus purpureus, Camellia sinensis, Dolomiaea costus, Urtica dioica, and Ephedra viridis against human bacterial pathogens linked to gastrointestinal diseases. Qualitative and quantitative methods were applied to evaluate the effectiveness of the plant extracted material toward Escherichia coli, Proteus vulgaris, Bacillus subtilis, and Staphylococcus aureus. The analysis included agar diffusion method (ADM), Minimal Inhibitory Concentration (MIC), Broth Microdilution (BMD), and Phytochemical analysis of the extracts. The largest zones of ADM were obtained from U. dioica seeds against both B. subtilis and S. aureus. N. sativa was second in its ability to inhibit the same bacteria. M. oleifera showed apparent growth inhibition of B. subtilis. C. sinensis revealed moderate activity against S. aureus, E. coli, and P. vulgaris. The results of the BMD test showed that U. dioica, M. oleifera, N. sativa, and C. sinensis were effective against the tested bacteria. MIC50 ranged from 14.15-271.44 mg/ml against the tested bacteria. Phytochemical analysis showed that the test plants contained significant and variable antibacterial agents. The diversity of these agents explains their strong and different ability to inhibit bacterial growth. The research revealed the effectiveness of U. dioica, N. sativa, and M. oleifera seed extracts in combating bacteria that cause food poisoning while demonstrating the ineffectiveness of certain plants commonly prescribed to treat such diseases.
Medicinal Plants, Foodborne Pathogens, Antibacterial Activity, Phytochemical Analysis, Herbal Shops
Antibacterial drugs are primarily essential in minimizing the spread of emerging microbial infections globally. One important alternative to treat these diseases is using medicinal plants. These plants constitute valuable resources for traditional medicines, alongside chemical resources. However, the evolution of multidrug-resistant (MDR) strains has led to the emergence of a public health problem, limiting the choices for effective antimicrobials available to treat infections caused by pathogenic bacteria.1
The development of modern treatments that have evolved in the last few decades relies on plants. The World Health Organization approves including medicinal plants as alternatives to treat different diseases due to their availability to the general public, many of them have been used and proven effective and safe compared to modern synthetic treatments. The study of medicinal plants undergoes different preparatory stages, starting with pre-extraction and then extraction steps. These actions are necessary for deriving the bioactive components from these plants. Therefore, traditional methods such as maceration and Soxhlet extractions are frequently adopted when conducting restricted studies or during handling of small manufacturing enterprises.2
Modern extraction methods have been used in the processing of medicinal plants, including ultrasound-dependent extraction and supercritical fluid extraction techniques. Modifications have been adopted for these methods that aim to boost the output without increasing the expenses needed for extraction.3
The problem of antibiotic resistance in humans and animals is an ongoing issue; hence, it is likely to persist for a long time. As a result, there is an imperative necessity for developing alternative drugs to cure such infectious diseases. The plants can release diverse phytochemicals; some of these metabolites are vital for the plant’s growth and development, while others provide substances such as saponins, glycosides, alkaloids, terpenoids, flavonoids, steroids, quinones, coumarins, and tannins, which are primarily responsible for its medicinal properties.4-8 These natural molecules constitute the origin of Plant-Derived Antimicrobial Substances (PDAMs) effectively utilized in treating bacterial infections.9,10 The extraction and study of phytochemicals from these sources have been expanded over the years, providing the opportunity to identify numerous compounds with potential health benefits.
Medicinal plants have been a cornerstone of traditional medicine for centuries, with their therapeutic properties largely attributed to a diverse range of bioactive compounds known as phytochemicals. These natural products play a significant role in health and disease management, offering potential treatments for various ailments. This essay explores the categories, sources, mechanisms, and allusions of phytochemicals found in medicinal plants, with recent references underscoring their importance in modern research.11,12
The significance of phytochemicals extends beyond traditional use; their study has profound significance for modern medicine. Integrating phytochemicals into pharmaceutical development can lead to the discovery of new drugs and therapies. For example, the extraction and modification of plant-derived compounds have paved the way for numerous medications currently on the market.13
Furthermore, the rising interest in herbal medicine has led to a greater emphasis on the scientific advocacy of traditional practices. Clinical studies are increasingly focused on understanding the efficacy and safety of these compounds, aiming to provide evidence-based recommendations for their use.14
In Palestine, as in many other countries, bacterial food poisoning is a disease that affects individuals who eat contaminated foods. Most cases of food poisoning in the country resolve within days; however, food poisoning pathogens may cause persistent or recurrent infections from single or multiple bacterial infections, in addition to poor food storage, preparation, and hygiene that encourage the growth and spread of bacteria. According to some health reports, cases in Palestine indicate high levels of food poisoning, especially in summer, due to high temperatures, which increase the risk of food spoilage. Some cases may also occur due to the spread of pathogens because of poor sanitation in some areas.15-18
Food poisoning is one of the most serious and potentially life-threatening health problems for people of all ages. These diseases are usually caused by bacteria, including E. coli, S. aureus, P. vulgaris, and B. subtilis, which have a remarkable ability to contaminate various foods.
E. coli spreads and contaminates foods such as meat and meat products, as well as drinking water, causing severe diarrhea, abdominal pain, and cramps, while S. aureus causes severe food poisoning when consuming unsanitary food, causing severe intestinal pain, vomiting, and nausea. In addition, P. vulgaris is usually associated with animal products such as meat and dairy products, causing fever, nausea, and diarrhea accompanied by severe abdominal pain and discomfort, while B. subtilis is not limited to a specific type of food but usually contaminates all foods, and its toxins cause nausea, vomiting, and mild diarrhea.19,20
Palestinians often use medicinal plants based on recipes from folk medicine to treat food poisoning diseases without relying on scientific bases, as this is expected to cause substantial damage to their health. This study aimed to investigate nine different plants sold and traded in herbal shops for treating food poisoning diseases and to evaluate the antibacterial effect of their extracts against four species of bacteria considered causative agents of food poisoning in humans and known for their emerging drug-resistant characteristics (Table 1).19,20 These bacteria are widely spread in the Palestinian regions due to weak health facilities and insufficient public health perceptions, making them targets for several research works in the Palestinian territories.21,22 In this research, both qualitative and quantitative analyses of the phytochemicals were also performed.
Table (1):
Diseases caused by the studied bacterial species
Bacteria |
Disease |
Ref. |
|---|---|---|
Proteus vulgaris (P. vulgaris) |
Food poisoning, urinary tract, wound and soft tissue infections |
[20,23] |
Escherichia coli (E. coli) |
Urinary tract infection, and some strains cause traveler’s diarrhea and serious foodborne illness |
[19] |
Staphylococcus aureus (S. aureus) |
Infection of surgical wounds and toxic shock syndrome as well as food poisoning |
[19] |
Bacillus subtilis (B. subtilis) |
Food poisoning, Infection of central nerve system and pyogenic meningitis |
[24,25] |
Studying local plant extracts in the Palestinian environment is the core motivation for this research. Even though many of these plants have been researched worldwide, the unique traits and potential differences of these plants in the Palestinian context necessitate a region-specific investigation. Furthermore, the research integrates the gap between global studies and local knowledge, providing deep insights into Palestinian plant phytochemical content. Targeting the distinct environmental and cultural background will give the chance to reveal precious protectives and aid in local and global scientific progress.
Sample Collection
Several plant samples, including leaves, seeds, and stems, were collected from traditional medicine shops in the popular markets of the city of Jenin (32.48333°N 35.3°E) (Table 2). The samples were identified as medicinal plants according to Jamous,26 Shahzadi et al.27, Ahmad et al.28 and Saleem et al.29
Table (2):
Medicinal plants tested for their antibacterial activity in the study
Plant extract |
Common name |
Family |
Parts used |
|---|---|---|---|
Artemisia vulgaris |
Mug wort |
Asteraceae |
Leaves |
Nigella sativa |
Black seed |
Ranunculaceae |
Seeds |
Origanum majorana |
Marjoram |
Lamiaceae |
Leaves |
Moringa oleifera |
Moringa |
Moringaceae |
Leaves |
Tetragonolobus purpureus |
Sparagus pea |
Fabaceae |
Seeds & stem |
Camellia sinensis |
Green tea |
Theaceae |
Leaves |
Dolomiaea costus |
Costus |
Asteraceae |
Stem |
Urtica dioica |
Stinging nettle |
Urticaceae |
Seeds & leaves |
Ephedra viridis |
Mormon tea |
Ephedraceae |
Stem |
Sap processing
Plant juice was prepared in the Microbiology and Biochemistry laboratories/ Faculty of Science, Department of Biology and Biotechnology/The Arab American University of Palestine. The test plant specimens were allowed to dry in a hot air oven at 45 °C for 3 hours, then crushed using an automated grinder to get a fine powder. After that, they are soaked in a container with sterile distilled water 1:20 (w/v) for 2 hours. Afterward, the suspension was poured over a fine wire mesh for filtration, the filtrate was centrifuged for 4 minutes at 2000 g0. Next, aliquots of 100 mL of each plant supernatant were transferred into labeled Falcon conical tubes and frozen at -20 °C. Subsequently, the aliquots were dried at 45 °C using a hot air oven, then weighed using a scale. Finally, the final concentration was adjusted to 1000 mg/mL using sterile normal saline (0.9% NaCl).
Preparation of bacterial inoculum
The antibacterial activity of the test plants was evaluated using four bacterial strains obtained from the American Type Culture Collection (ATCC): B. subtilis (ATCC 6633), E. coli (ATCC 25922), P. vulgaris (ATCC 8427), and S. aureus (ATCC 25923). The bacterial inoculum was prepared by selecting colonies from 24-hour cultures and incubating them at 37 °C in sterile Mueller-Hinton broth. Thereafter, colonies were homogenized using sterile normal saline solution (0.9% NaCl) and mixed for 15 seconds using a vortex. The suspension of bacterial cells was fixed by comparing with 0.5 McFarland to get a cell count equal to 1-5 × 108 colony forming units per milliliter (CFU/mL).30
Seeding of petri plates
A spread plate technique was used to inoculate Mueller-Hinton media with 0.1 mL of bacterial suspension, spreading was performed using sterile L-shaped glass rods. Seeding was done separately in Petri dishes according to Strohl et al.31 The plates were covered and incubated for 24 hr at 37 °C.
Antibacterial screening activity
The agar diffusion method (ADM) was used to evaluate the antibacterial activity of plant extracts as reported by Chandrasekaran et al.32 Paper discs immersed with the nine selected plant extracts were placed in the center of the seed dishes. Each plant sample was tested in triplicate, then the radius of the inhibition zone around the discs was measured after incubating the Petri dishes at 37 °C for 24 hours.19,33
Quantitative antibacterial assays
Quantitative antibacterial effect of plants was determined using Minimal Inhibitory Concentration (MIC) and Broth Microdilution (BMD) procedures. These methods were performed only for plants that showed antibacterial activity according to the ADM results, namely C. sinensis, M. oleifera, N. sativa, and U. dioica, while the other plants were neglected from this analysis due to their limited or no antibacterial effect. Antibacterial activity assays were performed using 96-well flat-bottom microtiter plates according to Hulankova.34 Bacterial cell suspensions equivalent to 0.5 McFarland standard were prepared in Mueller-Hinton broth. Wells with only broth media were used as negative controls, while a mixture of broth and bacterial cell suspension was added as a positive control.
Various concentrations of plant extracts between 100-12.5 mg/ml were prepared using a two-fold serial dilution method. After that, volumes between 20-2.5 µl of plant extract along with a fixed volume of 20 µl of bacterial suspension were added to each well, then the total volume was adjusted to 200 µl using Muller-Hinton broth. Following incubation for 24 hours at 37 °C, all plates were evaluated for visible bacterial growth using positive and negative controls for comparison. Eventually, the optical density for each well was evaluated by measuring the absorbance through a plate reader at 570 nm, and the MIC value was calculated using the following formula:
Inhibition ratio = 1 – (test optical density (OD) / positive control OD) × 100.35,36 MIC-50 values were determined by using linear equations of standard MIC curves versus the concentration of plant extract.37
Phytochemical analysis
Phytochemical analysis was performed only for plants that revealed antibacterial activity according to MIC and BMD results, namely M. oleifera, U. dioica, and N. sativa. However, C. sinensis was excluded from this analysis due to its limited effect on the bacteria used in our study. Based on our results, high concentrations of this plant extract, ranging from 116-170 mg/mL, were needed to exert an antibacterial effect against the tested bacterial species.
Sample preparation for phytochemical analysis
Fresh viable plant seeds indicated above were sampled and stored for 24 hours inside the oven at 52 °C. After fine grinding, a concentration of the powder equivalent to 1:5 (w/v) was prepared by mixing it with distilled water and centrifuging for minutes at 2000 g0. The supernatant was separated in labeled tubes, then kept at 4 °C for subsequent tests.
Qualitative phytochemical analysis
The phytochemical content of the plant extracted products was determined according to the subsequent procedures:
Saponins foam test
A tube of crude extract and a 5 mL distilled water mixture was mixed thoroughly using a vortex-mixer. The generation of stable foam was considered an indication of the glycoside content of saponins. The thickness of the foam in the tube was measured in millimeters to express the approximate amount of this compound in the plant extract.
Flavonoids assessment test
A crude plant extract, mixed with a slight amount of magnesium ribbon, was subjected to concentrated HCL, which was added gradually. A reddish color formation a few minutes later indicated the presence of flavonoids. The flavonoid content of the plant extract was expressed by the degree of color change, expressed using (+): slight color change, (++): moderate color change, (+++): Intense color change.
Phenols and tannins evaluation tests
The presence of phenols and tannins was determined by adding a 2% FeCl3 solution to a tube with the plant extract. The generation of Blue-green or black color revealed the presence of phenols and tannins. The amount of these compounds is indicated by (+) for slight dark green, (++) for intense dark green, and (-) for orange (negative result).
Test for glycosides
2 mL of chloroform chemical reagent and a similar volume of concentrated H2SO4 were poured into a tube containing the crude extract, followed by gentle mixing. The appearance of intense red-brown coloration revealed the presence of glycoside content. The results were indicated as (+): dark brown, (++): reddish brown.
Quantitative evaluation of phytochemicals
Quantitative measurements of the active organic content of the seed concentrates were performed using the following procedures:
Determination of total phenolic content
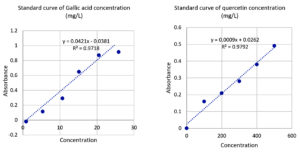
The phenol composition of the plant extracts was determined using the Folin-Ciocalteu procedure.17,21 A previously prepared mixture of 2 mL 2% Na2CO3 and 2.5 mL of 10% Folin-Ciocalteu reagent was added to 1 mL of plant concentrate. Then the optical density was measured at a wavelength of 765 nm after incubating the mixture for 15 minutes at ambient temperature. The absorbance of each reading is measured twice. Depending on Gallic acid, a standard curve was prepared to determine the phenolic content of the extract. The values were expressed as gallic acid equivalent 1 mg (GAE)/g dry matter.
Total tannins content
The Folin-Ciocalteu assay was used to determine the tannin content according to Mohammed and Abd Manan.38 In compliance with their procedure, 0.5 mL of the Folin-Ciocalteu reagent and 1 mL of 37 % Na2CO3 were mixed with 0.1 mL of the aliquot extract prepared in 0.75 mL of distilled water. After half an hour of incubation, the optical density was measured at 725 nm using a spectrophotometer. Blank and standard curves using gallic acid solutions were prepared as pointed out above. GAE/g dry matter was used to express the total tannins content as calculated from the standard curve with 0-100 mg/GA.39
Total flavonoids content
Colorimetric analysis was used to measure flavonoid content according to Proestos et al.40 Therefore, 0.15 mL of NaNO2 (5 % w/v) was mixed with 0.2 mL of extract and incubated for 10 minutes at 25 °C. Then, 0.15 mL of AlCl3.6H2O (10% w/v) was added and incubated in the same way at the corresponding temperature. Next, 0.8 mL of (10% w/v) solution of NaOH was added and incubated similarly for 10 min. The absorbance of the color was measured by a spectrophotometer at 510 nm using distilled water as a blank. Quercetin at concentrations between 0-500 µg/mL dissolved in 80% ethanol was used to represent the standard curve. The total flavonoids were expressed as mg quercetin equivalent (QE)/g dry matter.
Statistical analysis
Two-Sample Tests of Proportions (TSTP) were used to compare treatments. The results were analyzed using a level of significance when α = 0.05. The calculation was done according to the following equations. 41,42 Comparison was made between elements of the treatment.
Two – Proportion Z-test, pooled for H0: P1 = P2
P ̂= (X1+ X2) / (n1 + n2)
α, the probability of Type I error (rejecting a null hypothesis when it is in fact true)
n = sample size, n1 = sample 1 size, n2 = sample 2 size
P ̂= (X /n) = sample proportion
P0 = hypothesized population proportion,
P1= proportion 1, P2= proportion 2
The present study investigated the antibacterial activity of nine plant extracts against the test bacteria in vitro, using ADM, MIC, and BMD. The results revealed alternating responses of the bacterial species when treated with different plant extracts, though most of the extracts showed valuable antibacterial properties.
Antibacterial testing
Based on ADM, the antimicrobial activities of plant extracts against test bacteria were evaluated by measuring the radius of the growth inhibition zone, as shown in Figure 1.
Figure 1. ADM shows the zones of bacterial growth inhibition. (A): U. dioicia/B. subtilis, (B): U. dioicia/S. aureus, (C): M. oleifera/B. subtilis, (D): N. sativa/B. subtilis
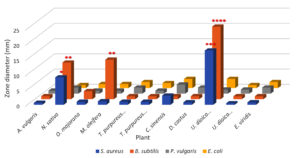
It is clear from Figure 2 that the largest bacterial inhibition zone was obtained from U. dioica seeds, which significantly affected both B. subtilis and S. aureus, rather than other species that exhibited less sensitivity. The greatest effect of extracts was seen against B. subtilis, showing an inhibitory effect reaching up to 23.83 mm. In addition, the same extract gave a strong inhibition zone of 17.83 mm against S. aureus. The findings of this study were consistent with another research, which revealed that the seed extracts of Urtica spp. have high antibacterial activity against food and plant pathogenic bacteria.43 Moreover, N. sativa ranked second in inhibiting the same bacterial species compared to other species. M. oleifera exhibited significant inhibition of B. subtilis growth, followed by C. sinensis, which exhibited a reasonable effect toward E. coli, S. aureus, and P. vulgaris.
N. sativa seed extracts possess particular significance in traditional treatment in Palestine, and some of their main constituents have been reported to be pharmacologically active.44 Moreover, leaf extracts of M. oleifera showed potential antibacterial effects against Gram-negative and Gram-positive bacteria, especially B. subtilis. This result was consistent with the results obtained by Rahman et al.45 who proved that the extract and juice from the leaves of this plant have a significant impact on various types of Gram-negative bacteria, including Shigella shinga, Pseudomonas aeruginosa, Shigella sonnei, and Pseudomonas spp., and six Gram-positive bacteria, including S. aureus, B. cereus, Streptococcus-B-haemolytica, B. subtilis, Sarcina lutea, and B. megaterium.
On the other hand, the results showed that A. vulgaris, D. costus, E. viridis, N. sativa, O. majorana, T. purpureus, and U. dioica leaves exhibited the least inhibitory effects against the test bacteria, as the radius of the inhibition zone ranged from 0.5 to 2.66 mm. This result suggests that the effect of these species was very minimal, regardless of the type of bacteria. The possibility of low bacterial activity can be attributed to the fact that the active substances in inhibiting bacterial growth are poorly stored in the leaves or seeds, or may be related to the normal saline extraction method, which extracts water-soluble components; therefore does not reflect the full potential for extracting the active compounds (Figure 2).
Furthermore, the results revealed that both S. aureus and B. subtilis were more affected by the antibacterial effects of most plant extracts, in contrast to the other bacteria, while the antibacterial effect on P. vulgaris and E. coli was significantly lower and was nearly identical across all tested plants (Figure 2).
Different numbers of asterisks indicate the significance of three independent experiments performed in triplicate. The more asterisks, the higher the level of significance.
Minimum inhibitory concentration
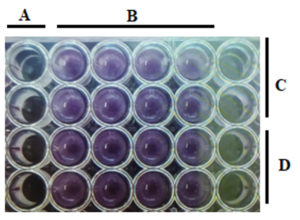
The MIC results of the present study provided essential information on the efficiency of the antibacterial activity of plant extracts, indicating how competent a given concentration of antibacterial agents is in inhibiting bacterial growth. Screening on the 96-well flat-bottom microtiter plates showed significant and clear discrimination between wells with positive samples compared to wells with negative samples that did not contain the antibacterial agent serving as a baseline (Figure 3).
Figure 3. MIC results of negative control (A) and positive control (B) and the effect of U. dioicia extracts on B. subtilis (C) and S. aureus (D)
Broth microdilution assay
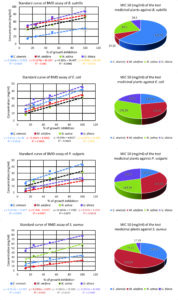
BMD test results showed that the plants, including U. dioica, N. sativa, and M. oleifera, had good activity against the tested bacteria, with some decrease in efficiency when C. sinensis was used. When MIC50 was calculated based on standard curves, these values ranged from 14.15-271.44 mg/mL for all extracts tested (Figure 4).
Furthermore, MIC50 values against Gram-positive bacteria ranged between 14.15 and 240.69 mg/mL, while values for the same assay for Gram-negative bacteria ranged between 110.1 and 271.44 mg/mL. The MIC results revealed that U. dioica extracts were generally effective against most types of bacteria, mainly B. subtilis and S. aureus. The MIC50 value of this plant extract against Gram-positive bacteria ranged from 14.5 to 17.19 mg/mL. Simultaneously, the MIC50 value in Gram-negative bacteria ranged between 110.1 and 230.9 mg/mL. The MIC50 for N. sativa against Gram-positive and Gram-negative bacteria was 43.1-75.31 and 164.59-176.29 mg/mL, respectively. Gram-positive and Gram-negative bacteria were also inhibited by C. sinensis extracts with MIC50 values ranging between 122.31-170.31 and 116.72-124.15 mg/mL, respectively. For M. oleifera, the MIC50 ranged from 14.15-240.69 and 154.91-271.44 mg/mL for Gram-positive and Gram-negative bacteria, respectively (Figure 4).
The results of MIC50 can be attributed to the fact that these plants contain valuable and varying amounts of phenols, tannins, and flavonoids, in addition to a reasonable and varying amount of saponins and glycosides that are believed to have a strong effect against the growth and division of bacteria. Both U. dioica and N. sativa were characterized by containing substantial quantities of glycosides and moderate amounts of saponins, while M. oleifera contained a significant amount of saponins and reasonable quantities of glycosides.
Phytochemical analysis
Qualitative phytochemical analysis
The following qualitative tests were used to identify and analyze the presence of specific antibacterial substances based on their distinct chemical reactions.
Test for saponins
The results showed that the M. oleifera seed extract contained the highest amount of saponin as indicated by the foam thickness in the test tubes, which reached 7 mm, compared to N. sativa seeds which gave a foam thickness of 2 mm. For U. dioica seed extracts, the foam layer was 1 mm thick (Table 3).
Table (3):
Saponin content of the test plants
Plant extract |
Foam thickness (mm) |
|---|---|
M. oleifera |
7 |
U. dioica |
1 |
N. sativa |
2 |
Test for flavonoids
The results exhibited that all plants contained varying amounts of flavonoids, mostly in U. dioica, then N. sativa, and finally M. oleifera, which gave the lowest indicator for containing this compound (Table 4).
Table (4):
Flavonoids content of the test plants
Plant extract |
Flavonoid content |
|---|---|
M. oleifera |
+ |
U. dioica |
+++ |
N. sativa |
++ |
(+): Slight color change, (++): Moderate color change, (+++): Intense color change, (-): No color change
Test for phenols and tannins
The results of the analysis revealed that the seeds of U. dioica contain a large amount of phenols and tannins, as they showed an intense dark green color, followed by M. oleifera, and N. sativa which also showed a slight dark green color (Table 5).
Table (5):
Phenols and tannins content of the test plants
Plant extract |
Phenols & tannins content |
|---|---|
M. oleifera |
(+) |
U. dioica |
(++) |
N. sativa |
(++) |
(+): Slight dark green, (++): Intense dark green, (-): Orange (negative result)
Test for glycosides
The results showed that the plants containing the most glucosides content were U. dioica seeds and N. sativa compared to M. oleifera, which revealed less content indicated in dark brown color (Table 6).
Table (6):
Glycosides content of the test plants
Plant extract |
Glycosides content |
|---|---|
M. oleifera |
(+) |
U. dioica |
(++) |
N. sativa |
(++) |
(+): Dark brown, (++): Reddish brown
Quantitative phytochemical analysis
The following quantitative tests were used to determine the exact amount of specific antibacterial substances through precise measurements.
Total phenolics, tannins and flavonoids
The quantitative test based on the gallic acid standard showed that the total phenolic content of U. dioica, N. sativam, and M. oleifera seed extracts were 301.13 ± 8.02, 198.95 ± 8.77 and 8.44 ± 1.13 mg GAE/g dry matter, respectively as shown in Table 7. Regarding tannins, the results of quantitative analysis based on gallic acid revealed that the percentage of total tannin contents for those seeds in the same order were 6.30 ± 0.77, 1.80 ± 0.48, and 0.64 ± 0.09 mg GAE/g dry matter (Table 8). Standard (mg Gallic Acid Equivalents/g dry matter) used for determining these compounds is shown in Figure 5. Concerning flavonoids, quantitative examination showed that the seed extracts of U. dioica, N. sativa, and M. oleifera contain 121.95 ± 3.55, 5.50 ± 0.94 and 2.72 ± 0.07 mg QE/g dry matter, respectively (Table 9). Values are expressed as means ± SD from four measurements. Quercetin equivalent (mg QE/g of dry matter) was used as standard for flavonoid (Figure 5).
Table (7):
Total phenol content of plant seed extract
Seed extract |
mg GAE/g dry matter |
|---|---|
M. oleifera |
8.44 ± 1.13 |
U. dioica |
301.13 ± 8.02 |
N. sativa |
198.95 ± 8.77 |
Table (8):
Total tannin content of plant seed extract
Seed extract |
mg GAE/g dry matter |
|---|---|
M. oleifera |
0.64 ± 0.09 |
U. dioica |
6.30 ± 0.77 |
N. sativa |
1.80 ± 0.48 |
Table (9):
Total flavonoid contents of plant seeds extract
Antibacterial compound |
mg QE/g dry matter |
|---|---|
M. oleifera |
2.72 ± 0.07 |
U. dioica |
121.95 ± 3.55 |
N. sativa |
5.50 ± 0.94 |
The results indicated that phenols content was dominant when compared to flavonoids and tannins in the extract of the test seeds, which is consistent with a previous report that showed a high content of phenolic compounds in those plants.46,47 The fact that such plants contain these substances is what explains their effective capabilities against bacteria.
Phenols are natural organic compounds that play a major role in protecting plants against bacterial pathogens. These compounds contain a carboxyl group linked to an aromatic ring and are characterized by their antioxidant and antimicrobial properties. Plant phenols have multiple mechanisms against bacteria, as they work to weaken or destroy the cell wall, causing loss of its internal balance and death. They also inhibit bacterial enzymes necessary for growth and reproduction, which limits their ability to spread. Moreover, phenols hinder the oxidation processes within bacterial cells, leading to their stress and death. They exhibit the potential to interfere with signaling systems within bacteria, causing a significant impairment in their ability to adapt to the surrounding environment.48
Plant tannins are natural polyphenolic compounds with antioxidant characteristics while exhibiting strong antibacterial effectiveness, making them a focus of interest in medical and industrial fields. These compounds inhibit the growth of bacteria by interfering with bacterial metabolic processes, preventing their growth and reproduction. These compounds damage the bacterial cell wall and interfere with the cell membrane permeability, leading to loss of vital substances and ultimately cell death. Furthermore, they impair bacterial enzymes essential for their life. They significantly affect cellular signaling and bacterial communication systems as well (Quorum Sensing), reducing their ability to form biofilms and increasing their resistance to antibiotics.49
Flavonoids are also classified as natural polyphenols and are found largely in plants. They exhibit protective functions against ultraviolet rays, pests, and diseases. These compounds are characterized by their antioxidant, antibacterial, and anti-inflammatory properties. Moreover, flavonoids found in plants exert their antibacterial properties by disrupting the bacterial cell walls and interfering with the metabolic processes.50 On the other hand, saponins are substances with detergent properties that can interact with the lipid portion of bacterial cell walls, increasing cell permeability, leading to its destruction by harmful substances.51,52 Regarding glycosides, which consist of a sugar group linked to a non-sugar aglycone, the antibacterial properties are related to their potential to disrupt the cell wall and plasma membrane, as well as to inhibit protein synthesis and metabolic processes in bacteria.53
U. dioica has exhibited pronounced effectiveness toward both bacterial groups. The seeds of this plant contain significant amounts of phenolic compounds, tannins, and flavonoids in addition to glycosides, which explains their great antibacterial efficiency, compared to other plants that showed a minimal effect. These phenolic compounds, especially caffeic acid, and flavonoids such as quercetin, contribute to their antimicrobial properties by interfering with the function of bacterial cell membranes and inhibiting enzyme activity.47 The current results were consistent with the study, which revealed that U. dioica extracts had a significant inhibitory capacity against E. coli, S. aureus, and Pseudomonas aeruginosa due to the presence of tannins that hinder the ability of bacteria to adhere and form biofilms.54
N. sativa has considerable amounts of phenolic compounds, flavonoids, and tannins, placing it second to U. dioica in antimicrobial qualities. This plant also contains high levels of glycosides and moderate levels of saponins, which enhance its ability against various bacteria. Studies have shown that thymoquinone and polyphenol components of N. sativa exert inhibitory effects on bacterial growth by interfering with DNA replication and protein synthesis.55 Furthermore, some studies have shown that N. sativa extracts are effective against multidrug-resistant bacteria, including methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa, due to their ability to disrupt bacterial membranes and impair biofilm synthesis.56 The flavonoid content of these plants induces bacterial cell damage by accumulating toxic reactive molecules inside the cell.
Concerning M. oleifera seeds, they contain minimal amounts of these biologically active compounds, though they consist of a considerable portion of saponins and a moderate quantity of glycosides, which explains their effective antibacterial effect.57 In addition, M. oleifera harbors different flavonoid species such as kaempferol and quercetin, which activate the oxidative stress in bacterial cells, causing their destruction.57,58 Furthermore, a study about M. oleifera extracts revealed that they exert significant effects against bacteria such as Salmonella typhi, B. subtilis, and Klebsiella pneumoniae, leading to disruption of bacterial cell walls and preventing protein synthesis. Moreover, the tannins in M. oleifera affect bacterial proteins, impairing their ability to colonize and infect host tissues.58
Interestingly, the results showed that B. subtilis was the most susceptible bacterium, implying it was the least resistant to medicinal plant extracts, followed by S. aureus. Gram-positive bacteria were much more sensitive and less resistant than Gram-negative bacteria. The bacterial type, its defense mechanisms, in conjunction with the type and source of the active compounds extracted, play vital roles in the susceptibility of these microorganisms. Based on our results, P. vulgaris was sensitive to C. sinensis extracts, while it was resistant to other plant extracts, especially U. dioica (seed), M. oleifera, and N. sativa. Such findings are interesting results emerging from different antibacterial mechanisms of action from C. sinensis on one side and U. dioica (seed), M. oleifera, and N. sativa on the other side. In the context of these results, Gram-positive bacteria seemed more sensitive to the antibacterial action of medicinal plants. This result can be ascribed to the special construction and content of the cell wall. As outer layers, Gram-positive bacteria possess thick (20-80 nm) cell walls. In contrast, Gram-negative bacteria exhibit a different cell wall structure with a thickness of 10 nm and an outer membrane with many holes and appendages. This membrane protects bacteria from external danger, mediated mainly by chemicals, as it acts as a barrier that obstructs the movement of these substances into the cell.19,59
The antibacterial activities of U. dioica, N. sativa, and M. oleifera can be attributed to the fact that they contain rich phytochemicals, especially phenols, tannins, and flavonoids. These compounds have an effective antibacterial effect by damaging bacterial cell membranes, reducing enzyme activity, and preventing biofilm formation. Due to the increasing prevalence of antibiotic resistance, these plants provide potential alternatives as natural antibacterial agents. Therefore, there must be future research that focuses on clinical trials and formula development to explore their therapeutic applications in modern medicine.
On the other hand, the study demonstrated the great mistakes that people make when practicing folk medicine and the wrong prescriptions that herbal shops prescribe to treat food poisoning diseases. It has been shown that only three out of nine plants commonly prescribed to treat these diseases were effective, namely, U. dioica, N. sativa, and M. oleifera. This improper action can cause many problems, as people are exposed to the harmful effects of overusing these plants while wasting their money and time. Moreover, this condition may cause an increase in health problems and complications of infections due to patients’ beliefs based on the illusion of waiting for effective results to cure their disease, as prescribed by folk medicine, before seeking adequate treatment for their disease. This situation also facilitates the development of such infectious diseases and their spread in an epidemic manner among people.
In addition, future research should focus on isolating and studying the active components in the most effective plant extracts. Although there may be limitations with patenting natural products, finding strong bioactive extracts still offers great potential for developing new medicines. This could include making semi-synthetic or specially designed treatments with better drug properties. By concentrating on plants that clearly show antimicrobial effects, future studies can be more reliable and help confirm traditional uses. This work is especially important for creating natural remedies that target the specific bacteria common in the region. Over time, this approach could lead to the discovery of new drugs that work against drug-resistant bacteria found locally.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
HS conceptualized the study and performed supervision. HS and IQ collected resources. GQ performed formal analysis. HN and AK performed investigation. HS wrote the manuscript. IQ, GQ, HN, HS and AK reviewed the manuscript. IQ and HS edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was funded by the Arab American University of Palestine (AAUP), 2023/2024.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Manandhar S, Luitel S, Dahal RK. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J Trop Med. 2019;2019(1):1895340.
Crossref - Abubakar AR, Haque M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J Pharm Bioallied Sci. 2020;12(1):1-10.
Crossref - Bitwell C, Indra SS, Lukec C, Kakoma MK. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Scientific African. 2023;19:e01585.
Crossref - Anand U, Herrera NJ, Altemimi A, Lakhssassi NA. Comprehensive Review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites. 2019;9(11):258.
Crossref - Giri S, Chandra P. Therapeutic Potential of Natural Flavonoids: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective on Parkinson’s Disease. Current Drug Therapy. 2025;20(3):315-331.
Crossref - Frenț O-D, Stefan L, Morgovan CM, Duteanu N, Dejeu IL, Marian E, Vicaș L, Manole F. A systematic review: Quercetin-Secondary metabolite of the flavonol class, with multiple health benefits and low bioavailability. Int J Mol Sci. 2024;25(22):12091.
Crossref - Angane M, Swift S, Huang K, Butts AC, Quek SY. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods. 2022;11(3):464.
Crossref - Singh N, Yadav SS. A review on health benefits of Phenolics derived from dietary spices. Curr Res Food Sci. 2022;5:1508-1523.
Crossref - Elisha IL, Botha FS, McGaw LJ, Eloff JN. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern Med. 2017;17(1):1-10.
Crossref - Girish HV, Satish S. Antibacterial activity of important medicinal plants on human pathogenic bacteria-a comparative analysis. World Appl Sci J. 2008;5(3):267-271.
- Alam S, Anwar J, Maity MK, Azam F, Jaremko M, Emwas A. The Dynamic Role of Curcumin in Mitigating Human Illnesses: Recent Advances in Therapeutic Applications. Pharmaceuticals. 2024;17(12):1674.
Crossref - Embuscado ME. Bioactive compounds from culinary spices and herbs and: A review. J Food Bioact. 2019;6:68-99.
Crossref - Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70(3):461-477.
Crossref - Hasymi Y. Exploration of the Experience of Using Herbal Medicine in the Treatment of Chronic Diseases: A Phenomenological Approach to Patients’ Perceptions of Effectiveness, Safety, and the Mechanisms of Action of Natural Compounds. PhytoCare: Journal of Pharmacology and Natural Remedies. 2025;1(1):27-36.
- Qadi M, Kharraz L, Shaheen A, et al. Assessment of the hygiene-related risks of child illness at selected elementary schools in Nablus city: a cross-sectional survey. Lancet. 2019;393:S41.
Crossref - Abu Elamreen FH, Abed AA, Sharif FA. Detection and identification of bacterial enteropathogens by polymerase chain reaction and conventional techniques in childhood acute gastroenteritis in Gaza, Palestine. Int J Infect Dis. 2007;11(6):501-507.
Crossref - Hussain F, Shahid M, Zulfiqar S, Hafeez J. Probing the Comparative Cioefficacy of Allium sativum L. Bulb through Different Solvents. J Mex Chem Soc. 2021;65(4):469-479.
Crossref - Constantin CA, Mikkola R, Andersson, MA, Teplova V, Suominen, I, Johansson T, Salkinoja-Salonen M. Bacillus subtilis and B. mojavensis strains connected to food poisoning produce the heat-stable toxin amylosin. J Appl Microbiol. 2009;106(6):1976-1985.
Crossref - Tortora G, Funke B, Case C. Microbiology: An Introduction. 13th ed. New York: Pearson;2018.
- El-Maghraby MA, Awad A, Salime AO. Isolation and antimicrobial susceptibility of Proteus vulgaris isolated from milk and dairy products. Benha Vet Med J. 2024;47(2):63-67.
Crossref - Sawalha H, Khasib S, Mansour B, Awwad Y, Abu Arra Z, Kmail A. Phytochemical characterization and antibacterial evaluation of crude saps from medicinal plants in Palestinian cuisine. Canrea Journal: Food Technology, Nutritions and Culinary. 2024;7(1):15-32.
Crossref - Sawalha H, Abo-Mowais M. Bacterial Contamination of Paper Banknotes in Circulation; a Case Study in the Jenin District, Palestine. J Sci. 2012;1(2):36-9.
- Drzewiecka D. Significance and Roles of Proteus spp. Bacteria in Natural Environments. Microb Ecol 72(4):741-758.
Crossref - Logan NA. Bacillus sp. of medical and veterinary importance. J Med Microbiol. 1988;25(3):157-165.
Crossref - Tsonis I, Karamani L, Xaplanteri P, et al. Spontaneous cerebral abscess due to Bacillus subtilis in an immunocompetent male patient: A case report and review of literature. World J Clin Cases. 2018;6(16):1169-1174.
Crossref - Jamous R. Ethnobotany of Palestinian Herbal Medicine in the Northern West Bank and Gaza Strip: A Review and a Comprehensive Field Study. Biodiversity & Environmental Research Center. 2006;4(1):1-122.
- Shahzadi I, Abdullah, Mehmood F, Ali Z, Ahmed I, Mirza B. Chloroplast genome sequences of Artemisia maritima and Artemisia absinthium: Comparative analyses, mutational hotspots in genus Artemisia and phylogeny in family Asteraceae. Genomics. 2020;112(2):1454-1463.
Crossref - Ahmad A, Husain A, Mujeeb MF, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trrop Biomed. 2013;3(5):337-352.
Crossref - Saleem A, Saleem M, Akhtar M. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. S Afr J Bot. 2020;128(2020):246-256.
Crossref - Imtara H, Elamine Y, Lyoussi B. Honey antibacterial effect boosting using Origanum vulgare L. essential oil. Evid Based Complement Alternat Med. 2018;7842583l.
Crossref - Strohl W, Rouse H, Fisher B. Lippincott’s Illustrated Review: Microbiology. 2nd ed. Lippincott Williams & Wilkins, 2007.
- Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharmacol. 2004;91(1):105-108.
Crossref - Benlyas M, Alem C, Filali-Zegzouti Y. Evaluation of antioxidant, antibacterial and antifungal activities of eleven monofloral honey samples collected from Morocco. J Chem Pharm Res. 2016;8(3):299-306.
- Hulankova R. The Influence of Liquid Medium Choice in Determination of Minimum Inhibitory Concentration of Essential Oils against Pathogenic Bacteria. Antibiotics. 2022;11(2):150.
Crossref - Mahamat O, Oulianovie GK, Christopher T, Albert K. Total Flavonoid Extract of Pseudocedrela kotschyi (Schweinf) Harms (Meliaceae) Inhibits the Pro-Inflammatory Function of Asthmatic Patients Neutrophils. Int J Immunol Immunother. 2020;7(1):040.
Crossref - Hussien A, Abdellattif AH, Abumunshar AA, et al. Food Safety Concerns and Practices Among Palestinian University Students: A Cross-Sectional Study. SAGE Open. 2022;12(3):21582440221119490.
Crossref - Engelkirk PG, Duben-Engelkirk JL eds. Laboratory Diagnosis of Infectious Diseases “Essentials of Diagnostic Microbiology”. Philadelphia: Lippincott Williams & Wilkins. 2008.
- Mohammed S, Abd Manan F. Analysis of total phenolics, tannins and flavonoids from Moringa oleifera seed extract. J Chem Pharm Res. 2015;7(1):132-135.
- Tamilselvi P, Krishnamoorthy R, Dhamotharan R, Arumugam P, Sagadevan E. Analysis of total phenols, total tannins and screening of phytocomponents in Indigofera aspalathoides (Shivanar Vembu) Vahl EX DC. J Chem Pharm Res. 2012;4(6):3259-3262.
- Proestos C, Boziaris IS, Nychas GJ, Komaitis M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006;95(4):664-671.
Crossref - Lind D, Marchal W, Wathen S (eds.). Statistical Techniques in Business & Economics. 2nd ed. New York: McGraw-Hill Irwin. 2005.
- Montgomery DC. Design Analysis of Experiments. 7th ed. Arizona: John Wiley & Sons. 2008.
- Korpe DA, Isen OD, Sahin FI, Cabi E, Haberal M. High-antibacterial activity of Urtica spp. seed extracts on food and plant pathogenic bacteria. Int J Food Sci Nutr. 2013;64(3):355-362.
Crossref - Eid AM, Elmarzugi NA, Abu Ayyash LM, Sawafta MN, Daana HI. A Review on the Cosmeceutical and External Applications of Nigella sativa. J Trop Med. 2017;2017(1):7092514.
Crossref - Rahman M, Sheikh ., Sharmin A, Islam S, Rahman A, Rahman M, Alam F. Antibacterial activity of leaf juice and extracts of Moringa oleifera Lam. against some human pathogenic bacteria. CMU J Nat Sci. 2009;8(2):219.
- Amin MF, Ariwibowo T, Putri SA, Kurnia D. Moringa oleifera: A Review of the Pharmacology, Chemical Constituents, and Application for Dental Health. Pharmaceuticals (Basel). 2024;17(1):142.
Crossref - Fialova SB, Rendekova K, Mucaji P, Nagy M, Slobodnikova L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, Considering European Legislation and Folk Medicine-A Review. Int J Mol ScI. 2021;22(19):10746.
Crossref - Kauffmann AC, Castro VS. Phenolic Compounds in Bacterial Inactivation: A Perspective from Brazil. Antibiotics. 2023;12(4):645.
Crossref - de Melo LFM, de Melo AVG, de Silva AP, Rocha HAO, Scortecci KC. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023;414(1):135645.
Crossref - Hasnat H, Shompa SA, Islam M, et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon. 2024;10(6):e27533.
Crossref - Arabski M, Wegierek-Ciuk A, Czerwonka G, Lankoff A, Kaca W. Effects of saponins against clinical E. coli strains and eukaryotic cell line. J Biomed Biotechnol. 2012;2012(1):1-6.
Crossref - Khan MI, Ahmed A, Shin HJ, Baek JS, Kim MY, Kim JD. Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram-positive and Gram-negative Bacteria, a Comprehensive Study In Vitro and In Vivo. Hindawi Evid Based Complement Alternat Med. 2018;2018:3486106.
Crossref - Koen V. Glycoside vs. Aglycon: The Role of Glycosidic Residue. In: Fraser-Reid BO, Tatsuta K, Thiem J (eds). Glycoscience in Biological Activity. Springer, Berlin, Heidelberg. 2008.
Crossref - Harrison F, Furner-Pardoe J, Connelly E. An assessment of the evidence for antibacterial activity of stinging nettle (Urtica dioica) extracts. Access Microbiol. 2022;4(3):000336.
Crossref - Abbas M, Gururani MA, Ali A, et al. Antimicrobial Properties and Therapeutic Potential of Bioactive Compounds in Nigella sativa: A Review. Molecules. 2024;29(20):4914.
Crossref - Elmowalid GAE, Ahmad AAM, El-Hamid M, et al. Nigella sativa Extract Potentially Inhibited Methicillin Resistant Staphylococcus aureus Induced Infection in Rabbits: Potential Immunomodulatory and Growth Promoting Properties. Animals. 2022;12(19):2635.
Crossref - Vergara-Jimenez M, Almatrafi M, Fernandez M. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants (Basel). 2017;6(4):91.
Crossref - El-Sherbiny GM, Alluqmani AJ, Elsehemy IA, Kalaba MH. Antibacterial, antioxidant, cytotoxicity, and phytochemical screening of Moringa oleifera leaves. Sci Rep. 2024;14(1):30485.
Crossref - Mai-Prochnow A, Clauson M, Hong J, Murphy AB. Gram-positive and Gram-negative bacteria differ in their sensitivity to cold plasma. Sci Rep. 2016;6(1):38610.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.