ISSN: 0973-7510
E-ISSN: 2581-690X
This study investigated the antibacterial properties of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. extracts against various pathogenic bacteria. The fruiting bodies of fresh oyster mushrooms were collected from Mae Phai Boon Mushroom Farm in Ban Thung Nang Rao, Mueang District, Mahasarakham Province, and subjected to extraction using 95% ethanol and 95% ethyl acetate solvents. The effectiveness of the extracts in suppressing the proliferation of pathogenic microorganisms, encompassing Bacillus cereus, Enterobacter cloacae, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Serratia marcescens, and Staphylococcus aureus, was assessed through the utilization of the paper disk diffusion technique. The results revealed that the crude extract obtained from the 95% ethanol solvent exhibited significant inhibitory effects against B. cereus, E. cloacae, P. aeruginosa, S. marcescens, and S. typhi, yielding inhibition zone diameters from 9.22 to 12.33 mm. In contrast, the crude extract from the 95% ethyl acetate solvent showed inhibitory activity only against E. coli, revealing an inhibition zone diameter of 12.00 mm. Additionally, the determination of the minimum inhibitory concentration (MIC) of the 95% ethanol crude extract against P. aeruginosa and S. marcescens established a value of 150 mg/ml, and concomitantly, the minimal bactericidal concentration (MBC) was also established at 150 mg/ml. However, it was observed that the 95% ethanol crude extract at a concentration of 15 mg/ml was incapable of suppressing the proliferation of E. coli in the MIC evaluations. These findings imply that the extracts originating from P. ostreatus possess inherent capacity as organic antibacterial agents targeting specific pathogenic bacteria. Therefore, these findings justify the necessity for additional scrutiny into their conceivable utilities within the domains of medicine and food preservation.
Crude Extract, Minimum Inhibitory Concentration (MIC), Oyster Mushroom, Pathogenic Bacteria
Mushrooms, classified as macrofungi, have garnered increasing attention as functional food sources and as potential contributors to pharmaceutical and nutraceutical development. Edible mushrooms are valued for their nutritional and potential medicinal attributes.1 Apart from constituents such as terpenoids, steroids, polyphenols, polyketides, polyglucans, flavonoids, alkaloids, polysaccharides, and nutritional fibers, various mushroom species basidiocarps and mycelia harbor noteworthy bioactive compounds. These compounds contribute to an abundant reservoir of anti-inflammatory agents, antibiotics, and antioxidants.2,3 Pleurotus ostreatus, commonly known as the oyster mushroom, is not only consumed for its gustatory and nutritional merits but also for its therapeutic advantages. The exploration of pharmacologically significant substances has ignited substantial interest in oyster mushrooms.4,5 This mushroom variety, recognized for its widespread consumption and nutritional significance, ranks second among commercially cultivated mushrooms globally6 and stands as a repository of pivotal bioactive constituents.7
The escalated utilization of multiple antibiotic drugs for human disease management has induced a noteworthy surge in the development of resistance among pathogenic bacteria against diverse antimicrobial agents over the past decade. This has prompted extensive investigations to unearth novel bioactive compounds as countermeasures. The emergence of multidrug-resistant pathogens, a consequence of the excessive use of synthetic antibiotics, has engendered a global menace. Thus, there is a pressing need to explore herbal plant-derived antimicrobial agents. It is anticipated that enhanced antimicrobial modalities will yield effective strategies to counteract the emergence of drug-resistant bacteria that have recently surfaced.8 A comprehensive scrutiny of various fungal species, particularly mushrooms, has yielded significant insights into their antimicrobial capacities.9 Many mushroom species contain antimicrobial substances in both the fruiting body and the mycelium.10 For instance, Pleurotus ostreatus, commonly known as oyster mushroom, showcases discernible antibacterial and antifungal attributes.11 Specific Pleurotus strains have been demonstrated to synthesize both antifungal and antibacterial agents, thus offering potential avenues for addressing bacterial and fungal infections.12 To effectively extract the active ingredients from P. ostreatus fruiting bodies or mycelia, suitable solvents must be discovered because each of the aqueous and ethanolic extracts contains its own antimicrobial agents.13,14
Several edible mushroom species belonging to the Lentinula, Hericium, Grifola, Flammulina, Pleurotus, and Tremella genera have been documented for their therapeutic attributes, encompassing anti-cancer, anti-inflammatory, immune-regulatory, and antimicrobial properties.15 Notably, the immunomodulatory capabilities of cell wall glucans are well-established, while certain extracellular secondary metabolites, facilitated through mycelium-mediated extracellular secretion, exhibit activity against bacteria and viruses.16 This has led to the observation that dried mushroom extracts, derived through chloroform and ethyl acetate solvents, demonstrate antibacterial effects against Prevestella intermedia and Streptococcus mutans.17 The antimicrobial potential of Pleurotus ostreatus, recognized for its efficacy in curbing bacterial and fungal proliferation, has been explored. Extraction from the freeze-dried fruiting bodies, broth obtained from submerged culture, and mycelial biomass of P. ostreatus was undertaken using alcohol-based solvents and water. Subsequent evaluation of the extracted components revealed their efficacy against fungi and bacterial growth. Notably, the aqueous extracts obtained from fruiting bodies exhibited superior efficacy in inhibiting most fungi. Candida albicans, Cryptococcus humicola, and Trichosporon cutaneum emerged as the most susceptible fungi to inhibition, while Staphylococcus aureus and Escherichia coli showcased heightened sensitivity among the tested bacterial strains. The inhibitory effects on fungal and bacterial growth were also notable in extracts derived from culture broth or mycelial biomass. In comparison, microbial resistance was notably lower against alcohol-based solvent extracts. The minimum inhibitory concentrations of these extracts against fungi and bacteria were found to be 30 g/ml and 20 g/ml, respectively18. Additionally, Okafor et al.19 have reported on the inhibitory potential of p-anisaldehyde present in hexane-dichloromethane extracts of P. ostreatus against pathogenic bacteria such as Bacillus subtilis and Pseudomonas aeruginosa, alongside pathogenic fungi including Aspergillus niger and Fusarium oxysporum. Further investigations have indicated the antibacterial and antiparasitic attributes associated with the fruiting body of P. ostreatus. Extracts denoted as P. ostreatus EVFB1 and EVFB4 were tested for their ability to impede the growth of bacteria such as Listeria innocua, Bacillus cereus, and E. coli, underscoring the multifaceted antimicrobial potential inherent in this mushroom species.3,20
The assessment of antibacterial efficacy against human pathogens, specifically E. coli, B. subtilis, Streptococcus faecalis, Pseudomonas aeruginosa, and Salmonella typhi, was conducted through the disc diffusion technique. Notably, the methanolic extract derived from P. ostreatus cultivated on a substrate of sorghum grain residue exhibited the most pronounced antibacterial activity against E. coli and P. aeruginosa. Similarly, the methanolic extract obtained from Plurotus florida cultivated on a substrate of wheat grain demonstrated notable antibacterial activity, particularly against E. coli and S. faecalis. This observation assumes significance considering the inadvertent usage of commonly employed antibiotics for managing human infectious ailments, which has markedly augmented the potential of pathogenic bacteria to develop resistance against multiple antimicrobial agents.21 To cultivate the fruit bodies of P. ostreatus, a process involving the utilization of pasteurized dried Sudan grass (Sorghum bicolor L. Moench) submerged in hot water was employed, subsequently followed by the induction of fruit body development through controlled incubation conditions. After their processing which encompassed drying, comminution into powdered form, and subjecting to cold-water extraction, the specimens underwent an evaluation procedure. This evaluation involved an assessment of their antifungal efficacy against Candida albicans and their antibacterial effects against Enterococcus faecalis, E. coli, Klebsiella pneumonia, P. aeruginosa, and S. aureus. This evaluation was carried out utilizing the well diffusion method, whereby the susceptibility of all strains was examined. The crude extract derived from the fruit bodies of P. ostreatus displayed a notable inhibition percentage of 25% against C. albicans, P. aeruginosa, and S. aureus. The outcomes were analyzed in the context of the significance of naturally occurring bioactive compounds and the potential utility of these extracts as supplementary natural remedies against select pathogenic bacteria and fungi. Studying the effectiveness of oyster mushroom extracts made from ethanolic and ethyl acetate on the inhibition of pathogenic bacteria was the goal of this investigation. Future extracts from mushrooms may be used as dietary supplements that improve health and strength and can be classified as medicines.
Preparation of extracts
The fresh basidiocarp of oyster mushroom (P. ostreatus) was obtained from Mae Phai Boon Mushroom Farm, located in Ban Thung Nang Rao, Mueang District, Mahasarakham Province. Subsequently, both the pileus and stipes were cut into small pieces, followed by oven-drying at 50–55°C overnight. The dried samples were then stored at 4°C until further testing.22 For the extraction process, two different solvents, namely 95% ethanol and 95% ethyl acetate, were utilized. Specifically, 20 g of the dried basidiocarp was mixed with 180 ml of the respective solvent solution. The maceration process was carried out at room temperature using a sonicator bath for 30 minutes, followed by shaking at 150 rpm for 2 hours. The mixtures were then subjected to maceration and filtered through filter paper (Whatman No. 1). To ensure complete extraction, the same method was repeated to treat any remaining filter residue. The obtained filtrates were subjected to concentration utilizing a Rotary evaporator, with temperature control maintained below 40°C. After this, an additional drying step was executed using a freeze dryer. The resultant extract was subsequently stored at a temperature of -4°C until its subsequent application.
Bacterial species and inoculum preparation
The bacterial strains utilized in this research encompassed Gram-positive species, specifically Bacillus cereus and Staphylococcus aureus, alongside Gram-negative species including Enterobacter cloacae, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, and Serratia marcescens. These bacterial strains were procured from the Microbiology laboratory, Department of Biology, Faculty of Science, Mahasarakham University, Thailand. Prior to experimentation, the bacteria were cultured on nutrient agar (NA) plates and allowed to grow for a duration of 18 to 24 h at a temperature of 37°C. Subsequent to this incubation period, cell suspensions were prepared, achieving a density of 1 x 108 CFU/ml, based on the 0.5 McFarland standard.
Assessment of antibacterial activity using the paper disc diffusion method
The antibacterial potential of the P. ostreatus extract was assessed utilizing the paper disc diffusion technique. The extract was solubilized in 5% dimethyl sulfoxide (DMSO) to achieve a final concentration of 3,000 µg/ml. Nutrient agar (NGA) plates, previously inoculated with the relevant test bacteria, were employed for the experiment. An 8 mm sterile filter paper disc, impregnated with 20 µl of the extract, was positioned onto the surface of each plate, subsequently followed by an incubation period at 28 ± 2°C for -48 h. The extent of inhibition was assessed by measuring the diameter of the resulting inhibition zone in millimetres. The evaluation encompassed five replicates for each solvent extract and the respective tested bacterium. Streptomycin, administered at a concentration of 3,000 µg/ml, was employed as the positive control, while 5% DMSO was employed as the negative control.
Assessment of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
The assessment of both Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) adhered to the protocols outlined by the Clinical and Laboratory Standards Institute. Successive two-fold dilutions of the P. ostreatus extract, range from 2.93 µg/ml to 1,500 µg/ml, were meticulously prepared within 96-well microtiter plates. Following this, 100 µl of the test bacteria, standardized to a concentration of 108 CFU/ml, was introduced into each well. Specifically, well 1 contained 100 µl of Mueller Hinton broth (MHB), functioning as the negative control, while well 12 contained 100 µl of 300 µg/ml streptomycin, serving as the positive control. The microtiter plates were subsequently subjected to an incubation period of 24 h at a controlled temperature of 28 ± 2°C. To ascertain the Minimum Inhibitory Concentration (MIC), signifying the lowest concentration of the extract impeding the proliferation of the test bacteria, the optical density at 600 nm (OD600) was quantified using the Asys UVM 340 Microplate Reader (Biochrom).
For the determination of the Minimal Bactericidal Concentration (MBC), samples were extracted from the wells surpassing the MIC value, as well as from those with concentrations equivalent to or below the MIC value. These samples were subsequently cultured on nutrient agar (NA) Petri plates. The Petri plates were then incubated at a temperature of 28 ± 2°C for a duration of 24 h. The absence of observable colony growth on the NA plates was employed to compute the MBC value, representing the lowest concentration of the extract at which bacterial cell death was induced.
Assessment of antibacterial activity by the paper disc diffusion method
The antibacterial potential of 95% ethanol and 95% ethyl acetate extracts derived from P. ostreatus (commonly known as oyster mushrooms) was evaluated against a panel of eight pathogenic bacteria. This encompassed Gram-negative strains (E. cloacae, E. coli, P. mirabilis, P. aeruginosa, S. marcescens, and S. typhi) as well as Gram-positive strains (B. cereus and S. aureus). The assessment was conducted through the utilization of the paper disc diffusion technique. Inhibition zones were meticulously measured in millimetres, while streptomycin and 5% DMSO were respectively employed as positive and negative controls. The outcomes of this investigation unveiled that the 95% ethanol extract of oyster mushrooms exhibited noteworthy inhibitory effects against the proliferation of B. cereus (12.33 mm), E. cloacae (9.56 mm), P. aeruginosa (9.65 mm), S. marcescens (10.11 mm), and S. typhi (9.22 mm). Conversely, the 95% ethanol extract did not impede the growth of E. coli, P. mirabilis, and S. aureus. Conversely, the 95% ethyl acetate extract exhibited pronounced inhibitory activity specifically against E. coli (with an inhibition zone diameter of 12.00 mm), while its effect against the other bacterial strains was not significant. The positive control, streptomycin, demonstrated broad-spectrum antibacterial efficacy against all the tested bacterial strains.
The data presented in Table underwent rigorous statistical analysis, which revealed noteworthy variations among the inhibition zones observed across different bacterial strains following treatment with the respective extracts (P < 0.05). These findings provide substantial insight into the potential of extracts from oyster mushrooms as a potential reservoir of antibacterial agents targeted at specific pathogenic bacterial species. Consequently, this study strongly advocates for further exploration to harness these extracts latent capacity for the development of innovative antimicrobial agents.
Table:
Inhibition of growth of pathogenic bacteria by oyster mushroom extract using the paper disc diffusion method
| Pathogens | Inhibition zone diameter (mm) | |||
|---|---|---|---|---|
| 95% ethanol | 95% ethyl acetate | Streptomycin (3 mg) | 5% DMSO | |
| B. cereus | 12.33+1.15 Ba | 0.00+0.00 Cb | 19.07+1.10 Ab | 0.00+0.00 Cns |
| S. aureus | 0.00+0.00 Bc | 0.00+0.00 Bb | 23.89+0.84 Aa | 0.00+0.00 B |
| E. cloacae | 9.56+0.51 Ab | 0.00+0.00 Bb | 0.00+0.00 Bd | 0.00+0.00 B |
| E. coli | 0.00+0.00 Cc | 12.00+1.00 Ba | 19.53+1.36 Ab | 0.00+0.00 C |
| P. aeruginosa | 9.65+0.51 Bb | 0.00+0.00 Cb | 16.68+0.69 Ac | 0.00+0.00 C |
| P. milabilis | 0.00+0.00 Bc | 0.00+0.00 Bb | 19.10+1.15 Ab | 0.00+0.00 B |
| S. marcescens | 10.11+1.02 Bb | 0.00+0.00 Cb | 16.68+0.89 Ac | 0.00+0.00 C |
| S. typhi | 9.22+1.07 Bb | 0.00+0.00 Cb | 16.44+1.26 Ac | 0.00+0.00 C |
Note: Different capital letter within same row indicated significant difference (P < 0.05).
Different superscripts within same column indicated significant difference (P < 0.05).
Assessment of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
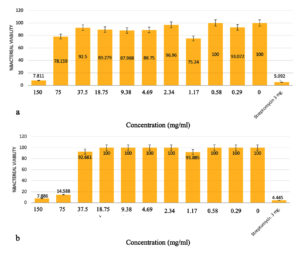
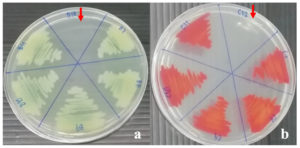
The investigation into the minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of the 95% ethanol-extracted oyster mushroom (P. ostreatus) extract against pathogenic bacteria (B. cereus, E. cloacae, P. aeruginosa, S. marcescens, and S. typhi) revealed interesting findings. Notably, the MIC values were discerned for P. aeruginosa and S. marcescens, amounting to 150 mg/ml. This determination signifies the minimal concentration necessary to curtail the proliferation of these bacterial species. At this concentration, the assessed percentage viability, relative to the negative control, stood at 7.81% for P. aeruginosa and 7.87% for S. marcescens. Furthermore, the results demonstrated that the oyster mushroom extract was unable to completely prevent the development of the tested bacteria at concentrations lower than the MIC. When the extract concentrations were reduced to 75 mg/ml for P. aeruginosa and 150 mg/ml for S. marcescens, the percentage viability increased to 78.16% and 14.56%, respectively (Figure 1). At even lower extract concentrations, the survival percentages for both bacteria approached 100%, indicating that the extract could not entirely inhibit their growth. Additionally, the effectiveness of the extract in suppressing pathogen growth decreased as its concentration decreased, suggesting a dose-dependent response. The MBC values for P. aeruginosa and S. marcescens at a concentration of 150 mg/ml were further determined, indicating that they were not completely destroyed at this concentration (Figure 2).
Figure 1. Minimum inhibitory concentration (MIC) of 95% ethanol extract of oyster mushroom to inhibit (a) P. aeruginosa (b) S. marcescens
Figure 2. Minimal bactericidal concentration (MBC) of 150 mg/ml (arrow) 95% ethanol extract of oyster mushroom to destroyed (a) P. aeruginosa (b) S. marcescens
These findings highlight the potential antimicrobial activity of the oyster mushroom extract against P. aeruginosa and S. marcescens but also suggest that higher concentrations of the extract may be necessary to achieve complete inhibition or destruction of these pathogenic bacteria. Subsequent investigations are imperative to elucidate the specific bioactive compounds accountable for the observed antimicrobial properties. Furthermore, there exists a need to enhance the extract’s effectiveness, thereby facilitating its potential utility in countering infections caused by pathogenic bacteria. The outcomes derived from this study significantly enrich our understanding of the prospective deployment of oyster mushroom extracts as a reservoir of antimicrobial agents. These findings not only shed light on this particular avenue but also lay the groundwork for future explorations within the realm of natural antimicrobial agents.
Foodborne pathogens constitute a category of bacteria responsible for inducing food deterioration, accompanied by the potential production of toxins, undesirable flavours, lytic enzymes, and spoilage. Among the bacteria of particular concern are Bacillus cereus, Enterobacter aerogenes, Escherichia coli, Micrococcus luteus, Proteus vulgaris, Staphylococcus aureus, and Salmonella typhimurium. These agents generate toxins that can trigger diarrhea in both humans and animals, primarily as a consequence of the consumption of contaminated food sources.23
The effect of oyster mushroom extract on the proliferation of pathogenic bacteria was assessed utilizing the paper disc diffusion method. Specifically, a 95% ethanolic extract derived from oyster mushrooms demonstrated the ability to impede the growth of B. cereus, E. cloacae, P. aeruginosa, S. marcescens, and S. typhi, resulting in inhibition zones measuring 12.33 mm, 9.56 mm, 9.65 mm, 10.11 mm, and 9.22 mm, respectively. However, this extract exhibited no inhibitory effect on the growth of E. coli, P. mirabilis, and S. aureus. Conversely, the oyster mushroom ethyl acetate extract displayed inhibitory activity against E. coli, with an observed inhibition zone of 12.00 mm. In contrast to the findings of this study, Han et al.24 reported contradictory results regarding the efficacy of 95% ethanolic oyster mushroom extract in inhibiting pathogenic bacterial growth. This discrepancy could potentially arise from variations in extract concentrations utilized in the experiments, which might have influenced the inhibition outcomes for certain pathogens. Consistent with the observations of Han et al.,24 the ethyl acetate extract from oyster mushrooms exhibited efficacy in inhibiting E. coli growth, marked by an inhibition zone of 8.33 mm. It is noteworthy that the 95% ethyl acetate extract of oyster mushroom demonstrated a broad spectrum of inhibitory activity against various bacterial strains, including B. cereus, E. cloacae, P. mirabilis, P. aeruginosa, S. aureus, S. marcescens, and S. typhi. Conversely, the 95% ethanol extract exhibited limited inhibitory potential, as it was ineffective against E. coli, P. mirabilis, and S. aureus.
Determination of the minimum inhibitory concentration of B. cereus, E. cloacae, P. aeruginosa, S. marcescens and S. typhi from oyster mushroom extract extracted with 95% ethanol showed that the MIC of P. aeruginosa was 150 mg/ml. The percentage viability compared to the negative control was 7.81%. The MIC for S. marcescens was established as 75 mg/ml, resulting in a percentage viability of 14.59% when compared to the negative control. Notably, at a concentration of 150 mg/ml, the MBC effectively eradicated P. aeruginosa and S. marcescens. Conversely, the MIC determination for the ethyl acetate extract sourced from oyster mushrooms could not be determined, as it displayed an inability to inhibit the growth of the subjected pathogens. It was found that as the concentration of the extract decreased, so did the effectiveness of the extract in suppressing the growth of pathogenic bacteria. This outcome paralleled the findings of Chaiharn et al.16 wherein they investigated the antimicrobial efficacy of desiccated extracts from edible mushrooms against a spectrum of foodborne pathogenic bacteria, encompassing B. cereus, Enterobacter aerogenes, E. coli, Micrococcus luteus, P. vulgaris, S. typhimurium, and S. aureus. The extracts sourced from Pleurotus ostreatus and Pleurotus pulmonarius exhibited inhibitory effects on both Gram-positive and Gram-negative bacterial strains. Specifically, water extracts from P. pulmonarius were particularly effective in impeding the growth of the Gram-positive species B. cereus and M. luteus, while E. coli and S. typhimurium exhibited resistance to the highest concentration of extracts. Notably, only the water extract derived from P. pulmonarius exhibited consistent antibacterial activity against the full panel of examined bacteria. The determination of the MIC showcased a varied range, with extracts targeting B. cereus demonstrating the lowest MIC range (1.25-5.00 mg/ml), whereas MIC values were relatively higher for P. ostreatus ethyl acetate extracts against S. typhimurium (12.5-22.5 mg/ml). Throughout this research endeavour, the potential utility of oyster mushroom extract as an agent to counteract microbial presence in food was identified. Nonetheless, the translation of these findings into practical applications necessitates further comprehensive investigation.
In summary, the outcomes of this investigation underscore the notable antibacterial prowess of P. ostreatus extracts against specific pathogenic bacterial strains. The observed differences in antibacterial effects between the ethanol and ethyl acetate extracts highlight the importance of solvent selection in extracting specific bioactive compounds from mushrooms.
The varying susceptibility observed between Gram-positive and Gram-negative bacteria underscores the intricate nature of bacterial cell wall structures and their consequential impact on antimicrobial efficacy. The determination of MIC and MBC values provides critical information for optimizing the use of oyster mushroom extracts as potential antimicrobial agents. Further investigation into the specific bioactive compounds responsible for the observed antimicrobial effects and their mechanisms of action is warranted to harness the full potential of oyster mushrooms in the development of novel antimicrobial therapies.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Gregori A, Svagel M, Pohleven J. Cultivation techniques and medicinal properties of Pleurotus spp. Food Technol Biotechnol. 2007;45(3):236-7.
- Breene WM. Nutritional and medical value of specialty mushrooms. J Food Protec. 1990;53(10):883-94.
Crossref - Akyuz M, Kirbag S. Antimicrobial activity of Pleurotus eryngii var. ferulae grown on various agro-wastes. EurAsia J BioSci. 2009;3:58-63.
Crossref - Waktola G, Temesgen T. Pharmacological activities of oyster mushroom (Pleurotus ostreatus). Nov Res Microbiol J. 2020;4(2):688-695.
Crossref - Bawadekji A, Mridha MAU, Ali AM, Jamith BW. Antimicrobial activities of oyster Mushroom Pleurotus ostreatus (Jacq. ex. Fr.) Kummer. J Appl Environ Biol Sci. 2017;7(10):227-231.
- Raman J, Jang KY, Oh YL, et al. Cultivation and nutritional value of prominent Pleurotus spp.: An Overview. Mycobiology. 2021;49(1):1-14.
Crossref - Ghosh T, Sengupta A, Das A. Nutrition, therapeutics and environment impact of oyster mushrooms: A low cost proteinaceous source. J Gynecol Women’s Health. 2019;14(1):555876.
Crossref - Karuppusamy S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res. 2009;3(13):1222-1239.
- Alves MJ, Ferreira IC, Dias J, Teixeira V, Martins A, Pintado M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012;78(16):1707-1718.
Crossref - Chiang SS, Wang LT, Chen SY, Mau JL. Antibacterial and anti-inflammatory activities of mycelia of a medicinal mushroom from Taiwan, Taiwanofungus salmoneus (higher Basidiomycetes). Int J Med Mushrooms. 2013;15(1):39-47.
Crossref - Hearst R, Nelson D, McCollum G, et al. An examination of antibacterial and antifungal properties of constituents of Shiitake (Lentinula edodes) and oyster (Pleurotus ostreatus) mushrooms. Complement Ther Clin Pract. 2009;15(1):5-7.
Crossref - Vamanu E. In vitro antimicrobial and antioxidant activities of ethanolic extract of lyophilized mycelium of P. ostreatus PQMZ91109. Molecules. 2012;17(4):3653-3671.
Crossref - Wolff ER, Wisbeck E, Silveira ML, Gern RM, Pinho MS, Furlan SA. Antimicrobial and antineoplasic activity of Pleurotus ostreatus. Appl Biochem Biotechnol. 2008;151(2-3):402-12.
Crossref - Wasser SP. Medicinal mushroom science: history, current status, future trends, and unsolved problems. Int J Med Mushrooms. 2010;12(1):1-16.
Crossref - Smith JE, Rowan NJ, Sullivan R. Medicinal mushrooms: a rapidly developing area of biotechnology for cancer therapy and other bioactivities. Biotech Lett. 2002;(22):1839-1845.
Crossref - Chaiharn M, Phutdhawong WS, Amornlerdpison D, Phutdhawong W. Antibacterial, antioxidant properties and bioactive compounds of Thai cultivated mushroom extracts against food-borne bacterial strains. Chiang Mai J Sci. 2018;45(4):1713-1727.
- Hirasawa M, Shoujii N, Neta T, Fukushima K, Takada K. Three kinds of antibacterial substances from Lentinus edodes (Berk.) Sing. (Shiitake, an edible mushroom). Int J Antimicrob Agents. 1999;11(2):151-157.
Crossref - Younis AM, Wu FS, El-Shikh HH. Antimicrobial activity of extracts of the oyster culinary medicinal mushroom Pleurotus ostreatus (higher basidiomycetes) and identification of a new antimicrobial compound. Int J Med Mushrooms. 2015;17(6):579-590.
Crossref - Okafor DC, Onuegbu NC, Odimegwu NE, et al. Antioxidant and antimicrobial activities of oyster mushroom. Am J Food Sci Technol. 2017;5(2):64-69.
Crossref - Egra S, Kusuma IW, Arung ET, Kuspradini H. The potential of white-oyster mushroom (Pleurotus ostreatus) as antimicrobial and natural antioxidant. Biofarmasi J Nat Prod Biochem. 2019;17(1):14-20.
Crossref - Gashaw G, Fassil A, Redi F. Evaluation of the antibacterial activity of Pleurotus spp. cultivated on different agricultural wastes in Chiro, Ethiopia. Int J Microbiolog. 2020;93189.
Crossref - Sutthisa W, Chaiyacham P. Antibacterial activity of ethanolic extracts of Lentinus squarrosulus Mont. against human pathogenic bacteria. J Pure Appl Microbiol. 2022;16(1):441-447.
Crossref - Kitzberger CSG, Smania JrA, Pedrosa RC, Ferreira SRS. Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J Food Eng. 2007;80(2):631-638.
Crossref - Han SR, Kim KW, Lim KO, Oh TJ. Biological activity analysis of different solvent extracts from Pleurotus ostreatus. Indian J Sci Technol. 2015;8(26):1-8.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.