ISSN: 0973-7510
E-ISSN: 2581-690X
A total of 11 bacteria were isolated from sea water and screened for their antibacterial activity against five bacterial pathogens. The most potent isolate (S1) was identified as Bacillus cereus S1 using 16S rRNA sequence analysis. B. cereus S1 exhibited antibacterial potentiality against, Pseudomonas florescence, P. aeruginosa, Staphylococcus aureus, Vibrio damsela and Aeromonas hydrophila. The inhibition zones diameter were 11, 12, 12, 12 and 16 mm respectively. Maximization of the productivity with 1.3 fold increase was achieved using Plackett Burman experimental design. The optimized medium was formulated as (g/l): peptone, 7; beef extract, 1.5; sea water concentration (50%) with pH 5 and inoculum size (0.5ml for each 25 ml medium) and incubated for 12 h. Immobilization using adsorption on pumice improved the productivity by 1.6 fold compared to the basal medium while loss of antibacterial activity was up on using entrapment technique. The bioactive compounds were characterized by thermal stability even at 100oC while they were inactivated up on exposing to ultraviolet radiation for 20 min. Moreover, the anticoagulant activity of B. cereus S1 was tested using activated Partial Thromboplastin Time (APTT) and Prothrombin Time (PT) tests. It succeed to prolong the clotting time to 40 sec and 253 sec respectively which represents about 3.3 fold and 7.2 fold compared to the control. Hexane extract was compared with other standard antibiotics and it was superior in its antibacterial effect. The crude extract of B. cereus S1 was analyzed using gas chromatography–mass spectrometry (GC–MS). The main constituents were Cycloheptasiloxane, tetradecamethyl-, cyclooctasiloxane hexadecamethyl-, cyclononasiloxane octadecamethyl- , Heptasiloxane, hexadecamethyl- and 1,2-Benzenedicarboxylic acid, diisooctyl ester (phthalate).
Antibacterial, Anticoagulant, Antiinflammatory, Bacillus cerus S1.
The resistance of pathogenic bacteria became the upcoming challenge in therapeutic agents, which is increasing with time due to misuse of antibiotics.1,2 Gram-negative bacteria such as Aeromonas, Vibrio, Flavobacterium, Pseudomonas, and Francisella and Gram-positive bacteria from the genera Streptococcus and Lactococcus, are some of the pathogens responsible for different diseases and economic losses.3-12
Recently, side effects of new therapeutic agents which have entered the clinical area, have been reported13. The challenge for scientists is to explore new antimicrobial agents from natural sources including bacterial strains with broad antimicrobial activity against different
pathogens14,15.
Most of the biological activities have borne from oceans. Every year, marine organisms produce hundreds of new compounds 16 with photo protective, anti-microtubule, anti-proliferative, anti-tumor, cytotoxic, and antifouling properties. Isolation of new microbes from marine environment has been reported17,18
Marine bacteria associated to invertebrate surfaces often produce secondary metabolites with amazing biological activities including antivirulence, anticancer and antibacterial19. Search of secondary metabolites produced by Bacillus species has under laid objectives by the scientific community in the past two decades and obtained therapeutic enzymes, novel metabolites and bactericidal agents.20,21,22 All these natural compounds with broad biological activities enable the bacterium to survive in its natural environment.
The environmental and nutritional conditions have a great influence on production of the antimicrobial substances. In order to develop an efficient product of antimicrobial substances, knowledge regarding the environmental factors affecting this process needs to be well identified.23,24 Optimal production is achieved by using experimental designs as an excellent tool for optimization of culture conditions.
Thus the aim of the present study was isolation of marine bacteria with antibacterial properties, maximization and characterization of the bioactive compounds in addition to studying its anticoagulation and anti-inflammation activities.
Microorganism and culture conditions
Eleven bacterial isolates representing different colony morphologies were isolated from seawater samples collected from Alexandria sea shore, Egypt. Nutrient broth medium used for isolation consisted of (g/l); peptone, 5, yeast extract, 2, agar, 20, pH was adjusted to 7 and the incubation was for 24-48 hours.25
Pathogenic indicators
Different Gram positive and Gram-negative pathogens including Staphylococcus aureus ATCC 6538, Streptococcus faecalis, Pseudomonas aeruginosa ATCC 8739, Vibrio anguillarum and Vibrio fluvialis were used as target strains for detecting the antagonistic properties. These pathogenic indicators were kindly provided from the Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University
Antibacterial activity
The marine isolate (S1) was precultured in marine nutrient broth at 30oC on a rotary shaker incubator until the absorbance of the culture at A 550=1. The culture broth was centrifuged at 10000 g for 15 minutes to remove bacterial cells.26 The ability of the cell free supernatant to inhibit the growth of the indicator bacteria was performed using the well-cut diffusion technique. Briefly, five-millimeter-diameter wells were punched in agar plates (using a sterile gel puncher) inoculated with bacterial pathogenic strains, 50 µl of the tested compound was added in each well. After incubation at 30oC for 24h, the radius of the clear zone around each well was linearly measured in mm27.
Bacterial identification
NA was isolated, purified using standard procedures28 and the region of 16S rDNA was amplified using universal primers (27F (5‘-AGAGTTTGATCCTGGCTCAG-3‘), 1492R (5‘-GGTTACCTTGTTACGACTT-3‘).16S sequence analysis was used to perform the genotypic characterization. Multiple alignments with the most closely sequences and similarity levels were carried out using Blast program (http://www.ncbi.nlm.nih. gov/blast). Sequences of rRNA genes, for comparison, were obtained from the NCBI database. A phylogenetic tree was reconstructed by Bioedit software. All the previous mentioned work of DNA extraction, PCR reaction and sequencing of PCR-DNA product was carried out at Macrogen (Seoul, Korea).
Optimization of fermentation conditions using Plackett Burman experimental design
The Plackett -Burman experimental design29,30 was applied to reflect the relative importance of various factors on the production of the bioactive compounds by B. cereus S1. High (+) and low (-) levels for each variable were tested. The examined variables in this experiment and their levels are shown in Table 1. Duplicate of the eight different trials were performed. The different trials (Row no. 9 represents the basal control). The main effect of each variable was determined with the following equation:
Exi= (Mi+–Mi-)/N
Where Exi is the variable main effect, and Mi+, Mi- are the inhibition zone (mm) in the trials, each independent variable was used in high and low concentrations, respectively, and N is the number of trials divided by 2. Microsoft Excel was used to calculate t-values for equal unpaired samples to determine the variable significance.
Table (1):
The applied Plackett–Burman experimental design for seven cultural variables.
| Trial | Factors | |||||||
|---|---|---|---|---|---|---|---|---|
| Peptone (P)(g/l) |
Beef (B) (g/l) |
pH | Inoculum size (IS) (ml) |
Incubation period (IP) (ml) |
Seawater concentration (SW) (%) |
Culture Volume (CV) (ml) |
Diameter of inhibition zone (mm) | |
| 1 | – [3] | -[1.5] | -[5] | +[1.5] | +[48] | +[150] | -[25] | 13 |
| 2 | +[7] | – [1.5] | −[5] | -[0.5] | -[12] | +[150] | +[75] | 13 |
| 3 | −[3] | +[4.5] | -[5] | −[0.5] | +[48] | -[50] | +[75] | 15 |
| 4 | +[7] | +[4.5] | −[5] | +[1.5] | −[12] | -[50] | -[25] | 17 |
| 5 | −[3] | _[1.5] | +[9] | +[1.5] | -[12] | −[50] | +[75] | 13 |
| 6 | +[7] | −[1.5] | +[9] | −[0.5] | + [48] | -[50] | −[25] | 13 |
| 7 | −[3] | +[4.5] | +[9] | _[0.5] | −[12] | + [150] | _[25] | 14 |
| 8 | +[7] | +[4.5] | +[9] | +[1.5] | +[48] | + [150] | +[75] | 0 |
| 9 | 0[5] | 0[3] | 0[7] | 0[1] | 0[24] | 0[100] | 0[50] | 16 |
| Main effect | 3.75 | -1.5 | -4.5 | -3 | -4 | -4 | -4 | |
| t-value | -0.8 | -0.39 | -1.1 | 0.1 | -1.12 | -1.3 | -1.12 | |
Standard t-values are obtained from statistical methods (Cochran & Snedecor, 1989)31
Effect of immobilization technique on production of antibacterial agents
Immobilization was carried out by using adsorption of B. cereus S1 cells on different solid porous supports including luffa pulp, pumice, sponge and clay, 0.5 ml of bacterial suspension were added to 250 ml sterile flasks containing five grams of porous support materials in 25 ml of optimized culture medium. Luffa pulp and sponge pieces were around 0.5 cm in diameter. The flasks were then shacked slowly at 120 rpm under the optimized conditions. The antibacterial activity was estimated using well cut diffusion technique and compared with the free cells. Entrapment was done in 3% sodium alginate. A twenty ml sodium alginate solution was prepared by dissolving 0.3 g in 16 ml distilled water and autoclaved at 121oC for 10 min. 0.5 ml of B. cereus S1 suspension was added to the sterilized alginate solution. 10 ml of alginate-bacterial combination was introduced into a cross linking solution (sterile 2% CaCl2) using sterilized syringe to obtain spherical beads of calcium alginate beads entrapping the bacteria. The beads were left in the solution for two hours, washed several times and transferred to 25 ml optimized medium.32
Preliminary characterization of the bioactive compounds
Effect of temperature
Ten millilitres each of the cell free culture supernatant of B. cereus S1 were dispensed in various 50 ml screw capped conical flasks. The flasks were subjected to different temperature treatments (4, 30, 60, 100oC) for 20 minutes.33 The antibacterial activity was estimated using well cut diffusion technique.
Effect of ultraviolet radiation
The effect of UV light on antibacterial activity was determined as follows: 10 ml of filter-sterilized cell-free supernatant was exposed to UV irradiation at a distance of 25 cm for 15, 30, 45 and 60 min.34 After each time interval, antimicrobial activity was analyzed by well-cut diffusion technique.
Assay of bacteriolytic activity
The culture filtrate of B. cereus S1 was used to test its bacteriolytic activity using a method previously described by Than et al.35 A 24 hrs culture of A. hydrophila was centrifuged at 6000 g for 25 min to obtain the cell pellets. This was dispersed in 3 ml of sterile artificial sea water (ASW) and A mixture consisting of 7 ml of filtrate and 3 ml of A. hydrophila cell suspension in sterile seawater was adjusted to an initial absorbance of 0.5 (O.D 540 nm) using spectrophotometer then incubated at 30ºC for 12 h investigating cell lytic activity. A scanning electron microscope (SEM) was used to observe the cells and a positive control is kept without addition of the filtrate.
Extraction of bioactive compounds
Different organic solvents including ethanol, chloroform, methanol and hexane were used for extraction of the antimicrobial agents. 50 ml of each solvent was added to 50 ml fermented broth in a 250 ml separating funnel. The mixture was shaken vigorously for 20 min and kept to separate the solvent from aqueous phase. The organic phase was collected and evaporated by using a rotary evaporator and then dissolved in appropriate solvent.36 Antibacterial activity was determined each time using well cut diffusion technique.
Antibiogram
Sensitivity of A. hydrophila to different standard antibiotics including Streptomycin (10µg), Chloramphenicol (30 µg) and Ampicillin sulbactam (20 µg), Tetracyclin (30 µg) and Amoxixicillin (10 µg) was tested. The antagonistic effect of these antibiotics were compared to that of the active metabolite using disk diffusion method37
Scanning electron microscopy
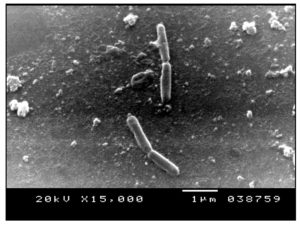
Preparation of cells for microscopic examination was carried out according to Sakat et al.38 Scanning-electron micrographs of B. cereus 1 and viewing the bacteriolytic effect of B. cereus S1 filtrate on A. hydrophila were produced using a JEOL JSM-5300 scanning electron microscope (JEOL Technics Ltd., Tokyo, 196-8558, Japan) at the Electron Microscope Unit, Faculty of Science, Alexandria University, Egypt.
Anticoagulant activity
Evaluation of activated partial thromboplastin time (APTT)
All clotting assays were carried out using normal citrated human plasma according to the manufacturers’ specifications. For this, 50 µL of citrated normal human plasma was mixed with 50 µL of a supernatant solution before adding 50 µL of APTT reagent. The mixture was then incubated at 37°C for 3 min. Then, 50 µL of 0.025 M calcium chloride reagent was added to the mixture to trigger the coagulation cascade. The clotting time was recorded in seconds. Heparin was used as the standard.39
Evaluation of prothrombin time (PT)
All clotting assays were carried out using normal citrated human plasma according to the manufacturers’ specifications. For this, 50 µL of human plasma was mixed with 50 µL of a supernatant and incubated at 37°C for 3 min. Then, 50 µL of 0.025 M PT reagent was added to the mixture to trigger the coagulation cascade. The clotting time was again recorded in seconds.39
Anti-inflammatory activity
Inhibition of albumin denaturation
Anti-inflammatory activity of the supernatant was estimated using methods of Sakat et al (2010).40 The reaction mixture was consisting of test supernatant and 1% aqueous solution of bovine albumin fraction. The samples were incubated at 37°C for 20 min and then heated to 51°C for 20 min after cooling of the samples, the turbidity was measured spectrophotometrically at 660 nm. The experiment was performed in triplicate. Percent inhibition of protein denaturation was calculated as follows:
% inhibition= [{Abs control- Abs sample}/Abs control] ×100
Spectral analysis
The active extract was analyzed using Gas –liquid chromatography mass spectra (GC–MS). The peaks were identified by comparing with WILEY MASS SPECTRAL DATA BASE LIBRARY.40
Infrared analysis
Pellets for infrared analysis were prepared by grinding a mixture of 1mg sample with 200 mg dry potassium bromide, followed by pressing the mixture into a 16 mm diameter mold. The Fourier transform-infrared (FT-IR) spectra were recorded on a Bruker Vector 22 instrument with a resolution of 4 cm-1 in the 4000-400 cm-1 region.
Screening for antibacterial activity
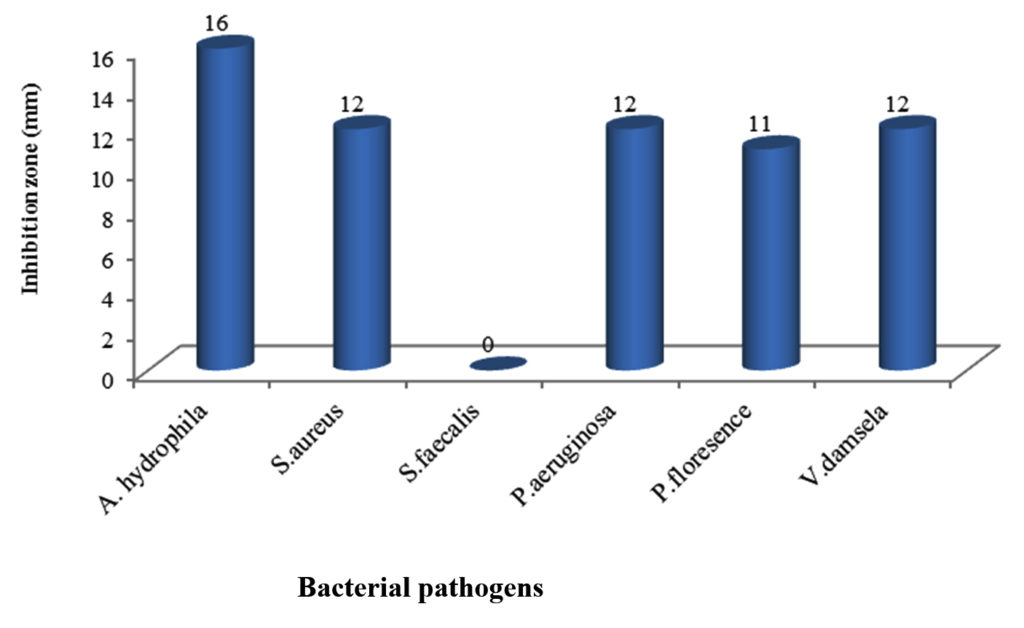
The marine bacterium S1 isolated from sea water was tested for its potentiality in production of antibacterial agents against some pathogens including V. damsela, P. aeruginosa, P. florescence, S. faecalis, S. aureus and A. hydrophila. Results in Figure 1 showed that the highest antibacterial activity was against A. hydrophila with inhihition zone diameter (16mm) followed by P. aeruginosa, V. damsela and S. aureus (12 mm), while the lowest antibacterial activity was against P. florescence (11 mm). On the other hand there was lack of activity against S. faecalis and thus A. hydrophila was chosen to complete the study as it is the most susceptible bacterial pathogen. Results concluded that Gram negative bacteria was the most susceptible for the antagonistic compounds which is coincide with Telesmanich et al. (2000).41 The difference in the susceptibility may be due to physiological characteristic and metabolism of each strain, cell wall structure and degree of contact.41
Antimicrobial activity by Bacillus spp. was proven in previous studies.43,44,45 The results obtained in the present study indicated the potential production of bioactive compounds by marine Bacillus sp. Anand et al (2006)46 reported the production of a highly active metabolites by marine Bacillus sp. Mohan et al (2016)47 stated that Bacillus sp. isolated as sponge endosymbiotic bacteria showed antimicrobial activity with broad range against virulent marine fish pathogens such as Vibrio alginolyticus,Vibrio vulnificus, Vibrio parahaemolyticus, Aeromonas salmonicida, Flavobacterium sp., Edwardsiella sp., Proteus mirabilis and Citrobacter brackii with zones of inhibition (16-23 mm). Many scientists isolated Bacillus sp. from diverse group of invertebrates and confirmed the production of novel active metabolites against aquatic fish pathogens.20,21,48
Molecular identification of the marine isolate (S1)
DNA of the promising Bacillus sp. was extracted and the extracted 16SrRNA gene was amplified using the universal primers. The produced amplicons was analyzed using agarose gel electrophoresis as shown in Figure 2a. The amplified DNA was partially sequenced. This sequence was compared with those which gave the highest homology using Blast search computer based program. The resulting data indicated that the isolate under study was identified as B. cereus S1.The obtained similarity was 99% with accession number KX683220. The phylogenetic relationships of this experimental isolate and the closely related relatives were analyzed as shown in Figure 2b. Figure 3 shows the scanning electron micrographe of B. cereus S1
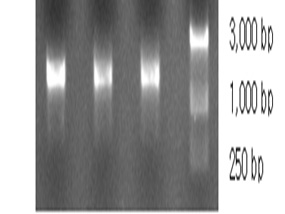
Fig. 2a. 1 6S agarose gel electrophoreses of the extracted and amplified DNA. Lanes 1, 2 are the purified PCR products. Lane 3 is molecular weight marker
 Fig. 2b. Phylogenetic relationships among the representative experimental strain and the most closely related Bacillus species. The dendogram was generated using tree view program
Fig. 2b. Phylogenetic relationships among the representative experimental strain and the most closely related Bacillus species. The dendogram was generated using tree view programOptimization of fermentation conditions using Plackett- Burman experimental design
Two phases of the application of Plackett-Burman statistical design was carried out. The first step was to screen for important factors and their levels that affect production process in shake flasks (Table 1). The second was the verification experiments to validate the results under specific optimized medium. All experiments were carried out in duplicates. Table 1 shows results of the experimental design. The main effect of each variable on the production of the bioactive compounds as well as t-values were estimated for each independent variable as shown in Table 1 and graphically presented in Figure 4. It was observed that the production of the antibacterial agent was negatively affected by beef extract, inoculum size, incubation period, sea water (%), culture volume and positively affected by peptone concentration, which means that increasing levels of peptone cause increase in the antagonistic activity which is in accordance with other previous studies,49 while decreasing levels of the other variables cause increase in the antagonistic activity. The effect of some of these factors on the production was similar to that reported by Ali (2012)50 who stated that beef extract, culture volume, inoculum size and incubation period had negative effect on antimicrobial agents production by marine Pseudomonas piscicida B12. The pH optimum for antimicrobial agent production was 6 which is in accordance with Wen Zhou et al (2010)24 who stated that optimum pH for production of bioactive compounds by B. cereus was in the acidic range as was also reported by Sana et al (2008).51 Regarding effect of NaCl, Balakrishnan et al (2014)45 observed that effect of NaCl is dependent on the mechanism of expression of the bacterium to the particular salt concentration. Results of t-test indicated that variations in sea water (%), culture volume and incubation period in the tested ranges had the most considerable effects on production of the antimicrobial agents by B. cereus S1. The interacting effect of sea water (%), culture volume and incubation period in three -dimensional representation is illustrated in Figure 5.
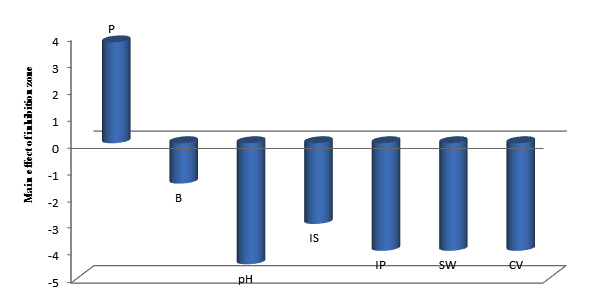 Fig. 4. Elucidation of fermentation conditions affecting the production of the antagonistic agents by B. cereus S1.
Fig. 4. Elucidation of fermentation conditions affecting the production of the antagonistic agents by B. cereus S1.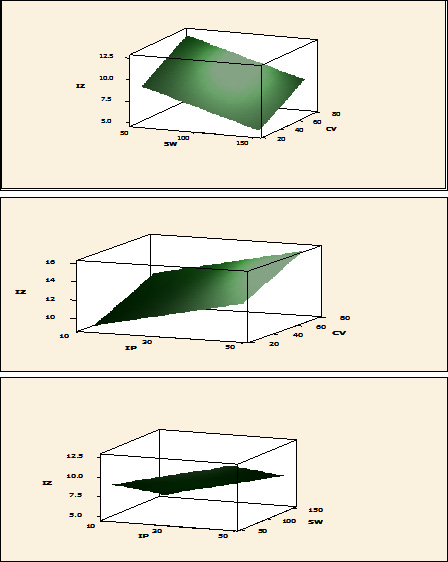
Fig. 5. Interaction effect between culture volume (CV) and sea water (%) (SW) (a); culture volume (CV) and incubation period (IP) (b) and seawater (%) (SW) and incubation period (IP) (c) Levels, with respect to inhibition zone (IZ)(mm) based on Plackett-Burman results
Abd-Elnaby et al (2016)52 reported that increasing levels of pH, inoculum size caused an increase in the antagonistic activity by about 1.3 fold for Streptomyces parvus. Conversely to the present study, this finding was reported by Wefky et al. (2009)48
According to the obtained results, the predicted medium for cultivation of B. cereus S1 to enhance maximum production of the bioactive compounds was formulated as follows: (g/l) : peptone,7; beef extract, 1.5; concentrated seawater (50%), adjusted pH 5 and inoculum size (0.5ml for each 25 ml medium) all of which are incubated for 12 h.
Verification experiment
A verification experiment was carried out in order to evaluate the accuracy of the applied Plackett-Burman statistical design, predict the near optimum levels of independent variables. The obtained data were examined and compared to the basal and anti-optimized medium. Data revealed that the inhibition zone diameter produced by B. cereus S1 raised to 21 mm and realized 1.3 fold increase when growing in optimized medium (Table 2 and Figure 6).
Table (2):
A verification experiment: Antibacterial activity of B. cereus S1 grown on basal versus optimized medium.
Response |
Basal Optimized |
medium medium |
|---|---|---|
Inhibition zone diameter (mm) |
16 |
21 |

Fig. 6. Verification experiment of the applied Plackett-Burman statistical design by comparing the antagonistic activity of B. cereus S1 growing on the resulting optimized medium, (a) the basal medium and (b) the anti-optimized medium.
Effect of immobilization on production of the antibacterial agents
Immobilization on different support materials was investigated to enhance the production of the antibacterial agents. As shown in Table 3, the antibacterial activity of cells adsorbed on pumice was increased up to 1.6 fold than the cells grown in the basal medium, while adsorption on luffa pulp and clay caused complete disappearance of the antagonistic activity. On the other hand, antagonistic activity was also disappeared up on using entrapped cells. These results may be due to poor mechanical stability of the support. Diffusion limitation of the bioactive compounds is an important factor affecting this process too52. Abd-Elnaby et al. (2016)52 also reported the potentiality of the immobilized cells in raising the antimicrobial activity of Streptomyces parvus compared to free cells.
Table (3):
Effect of immobilization on the antibacterial activity of B. cereus S1.
| Diameter of inhibition zones using different support materials and Ca -alginate beads | ||||||
|---|---|---|---|---|---|---|
| Free cells | Luffa pulp | Sponge | Pumice | Clay | Ca-alginate beads | |
| Inhibition zone diameter (mm) | 21 | 0 | 11 | 25 | 0 | 0 |
Preliminary characterization of the bioactive compounds in cell free supernatant
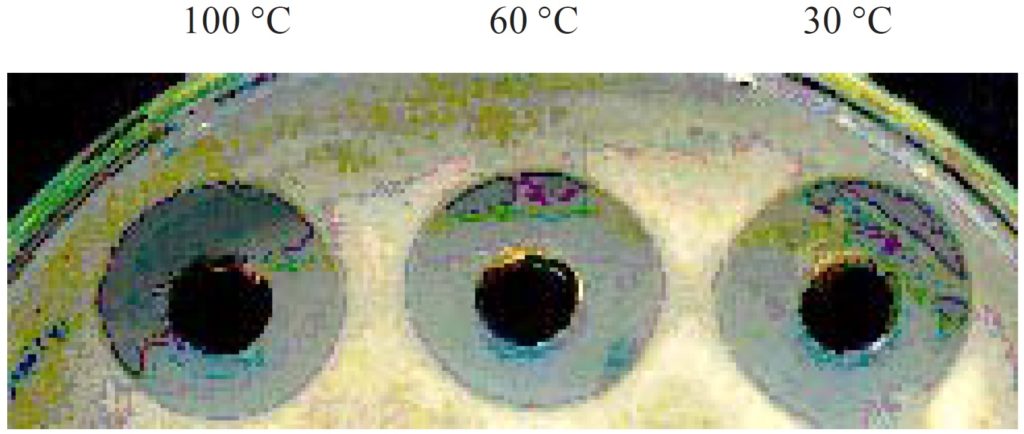
Stability of antibacterial compounds produced by B. cereus S1 toward temperature and UV was examined. Screening the effect of different temperature on stability of the bioactive components (Table 4 and Figure 7) showed that the antibacterial compounds produced by B. cereus S1 were relatively stable even after boiling at 100oC while they lost their antibacterial activity at 4oC. They retained about 84% and 44% of the antibacterial activity after exposing to 60oC and 100oC respectively for 20 min. Extreme temperatures, boiling for 10 min, had no effect on the antimicrobial agents produced by B. subtilis as was reported by Sabaté and Audisio (2013)54. The same finding was observed by Risøen et al. (2004)55 who stated that the antimicrobial compound retained activity over a wide range of temperatures even up to 100oC. in other study by Chalasani et al (2015),56 the antimicrobial compound was also stable at different temperatures 80% activity was retained a 80°C for 1 h, 75% at 100°C for 30 min and 60% at 121°C for 20 min. On the other hand, exposing the cell free supernatant to UV radiation for different time intervals caused complete absence of antagonistic activity. It can be stated that bioactivity of the compounds is dependent on the mutation effect on the active gene which responsible for the production of antimicrobial agents.57
Table (4):
Effect of temperature and UV on antibacterial activity of B. cereus S1.
| Temperature (oC) | UV exposing time (min) | |||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 30 | 60 | 100 | 15 | 30 | 45 | 60 | |
| Inhibition zone diameter (mm) | 0 | 25 | 21 | 11 | 0 | 0 | 0 | 0 |
Assay of bacteriolytic activity
The bacteriolytic activity of a 0.45 µm culture filtrate from 12 hrs of B. cereus S1culture against A. hydrophila was detected using SEM. Figure 8 represents the morphological changes of the nontreated and treated A. hydrophila. Figure 8a shows the SEM micrographs of bacterial cells without the supernatant treatment. The figure revealed the normal rod shape cell structure without any shrinkage or cavity formation as the surface was smooth and regular. Figure 8b shows the morphology of the cell after 24 h of treatment with the supernatant. The bacterial cells started to show multiple defects with many of cells exhibited crumpled or shrunken cell surface. Figure 7c revealed that some cells showed more formation of crumpled cells and cells lysis were formed up on treatment with supernatant for 12 h. It was reported that the cell wall of Gram negative bacteria (A. hydrophila) is surrounded by an outer membrane consisting of lipopolysaccharides, phospholipids and lipoproteins and thus they are less sensitive to bacteriolytic enzymes than Gram positive bacteria.58 Only a few active compounds have been reported to lyse cells of Gram negative bacteria;59 therefore, the present study is interesting that the isolate B. cereus S1 was active against A. hydrophila which can be applied as probiotic in aquaculture.
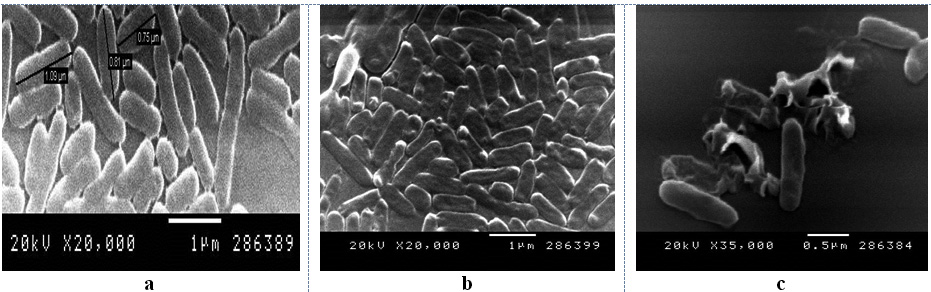 Fig. 8. SEM photomicrographs showing effect of culture filtrate of B.cereus S1 on A. hydrophila a) control (no addition of the filtratet); (b) and (c) treatment with the filtrate.
Fig. 8. SEM photomicrographs showing effect of culture filtrate of B.cereus S1 on A. hydrophila a) control (no addition of the filtratet); (b) and (c) treatment with the filtrate.Extraction of the bioactive compounds
Different solvents were screened for their efficiency in extracting the bioactive compounds. Hexane was the most efficient solvent exhibiting the highest value of inhibition zone diameter (25 mm) followed by chloroform (11 mm) while the diameter of inhibition zones were 10 mm up on using methanol and ethanol (Table 5). This may have been a consequence of the organic solvents denaturing the compound during the procedure or that the compound is labile or that the compound is a type of molecule not extracted into these solvents.60
Table (5):
Efficiency of different solvents for extraction of the active compounds.
Solvent |
Inhibition zone diameter (mm) |
|---|---|
Ethnol |
10 |
Methanol |
10 |
Chloroform |
11 |
Hexane |
25 |
Antibiotic susceptibility test
Sensitivity of A. hydrophila to different standard antibiotics including Streptomycin (10 µg), Chloramphenicol (30 µg) and Ampicillin sulbactam (20 µg), Tetracyclin (30 µg) and Amoxixicillin (10 µg) was tested. The antagonistic effect of these antibiotics were compared to that of the active fraction. It was superior in its effect than Streptomycin (10 µg), Chloramphenicol (30 µg) and Ampicillin sulbactam (20 µg) with 2.7, 2.1 and 1.5 fold respectively and also than Tetracyclin (30 µg) and Amoxixicillin (10 µg) which showed no antibacterial activity against A. hydrophila (Figure 9) this was confirmed by study of Barakat and Beltagy (2015)61 who reported the sensitivity of A. hydrophila to the bioactive compounds produced by Streptomyces ruber EKH2 rather than that of some tested commercial antibiotics.
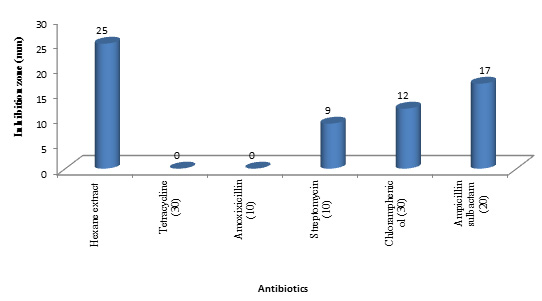 Fig. 9. Antibacterial activity of hexane extract compared with other different standard antibiotics
Fig. 9. Antibacterial activity of hexane extract compared with other different standard antibioticsAnticoagulant activity
Pharmaceutical importance of the bioactive compounds depends up on the positive chemical interactions with microorganisms.61 Bacterial supernatant was tested for blood coagulation effects in normal human plasma using heparin as standard. Results in Table 6 revealed that the supernatant exhibited greater activity with prolonged the clotting time 40 sec which represents about 3.3 fold compared to the control which suggests that the metabolites produced by B. cereus S1 is an effective antithrombotic agent. On the other hand the tested supernatant was capable of increasing the normal coagulation time up to 253 sec with 7.2 fold in relation to normal APTT time. Hassanein et al (2011)63 reported that the clotting time of human blood serum in the presence of active metabolites produced by B. subtilis K42 reached a relative PTT of 241.7% with a 3.4-fold increase, Similarly Wei et al (2011)64 stated that the fermented chickpeas from B. amyloliquefaciens showed anticoagulant activity, and the purified anticoagulant component showed higher anticoagulant activity than heparin sodium.
Table (6):
Anticoagulant and anti-inflammatory activities of the produced compounds
| Anticoagulant activity | Anti-inflammatory activity |
||
|---|---|---|---|
| Prothrombin time (PT) (sec.) |
Partial Thromboplastin Time (APTT) (sec.) | ||
| Supernatant | 40 | 253 | 86% |
| Heparin | 87 | ||
Anti-inflammatory activity
Inhibition of albumin denaturation
Denaturation of proteins is a well documented cause of inflammation. As part of the investigation on the mechanism of the invitro anti inflammation ability of the metabolites produced by B. cereus S1, protein denaturation was studied. It showed relatively good anti-inflammatory activity (86%) compared to the control without addition of the supernatant (Table 6). Our finding is supported by study of Kurian et al (2015)65 who proved the anti-inflammatory activity of Bacillus spp. BTCZ31
Spectral analysis
GC-MS analysis of hexane extract was carried out to identify the components in the extract. As shown in Figure 10 a, the main constituents in the hexane extract at retention time 22.61, 26.53, 29.92, 35.72 and 41.85 sec. and correspond to a molecular weight 519.0776, 593.231,667.38546, 533.14722 and 390.5561 and formula C14H42O7Si7, C16H48O8Si8, C18H54O9Si9, C16H48O6Si7 and C24H38O are identified as Cycloheptasiloxane, tetradecamethyl-, cyclo-octasiloxane hexadecamethyl-, cyclononasiloxane octadecamethyl- , Heptasiloxane, hexadecamethyl- and 1,2-Benzenedicarboxylic acid, diisooctyl ester (phthalate). The major chemical component (Phthalate) is reported to have a known biomedical value in the pharmacological fields. Antibacterial activity of phthalate against Staphylococcus aureus (ATCC 6538), Streptococcus faecalis (ATCC8043), Pseudomonas aeruginosa (A TCC 8739), Escherichia coli (ATCC 8739), Micrococcus luteus (ATCC 10240) and Candida albicans was evidenced in previous studies.66,67 Barakat and Beltagy (2015)61 also stated that phthalate showed antibacterial activity with broad spectrum against the fish pathogens A. hydrophila, Edwardsiella tarda, P. aeruginosa and V. ordalii. The antibacterial properties of siloxanes have been earlier reported by Lessoy et al.68 and also exhibit bioactivity as antibacterial and antioxidant agents as was documented by Avci and Dik69. Anti-inflammatory activity of siloxane was also reported.70 The mass spectra of the major compound (Phthalate) is shown in Figure 10b. IR spectra of phthalate showed peaks at 2994 (CH2), 1763 (C‚O), 1637 (C‚C), 1242 (C–O) and 1056 (C– H) cm-1 (Figure 10c).
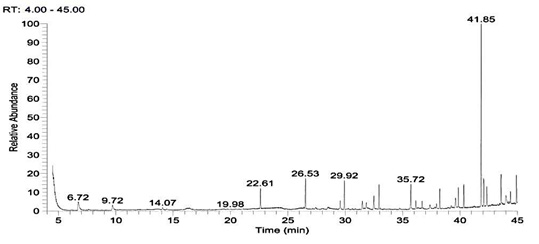
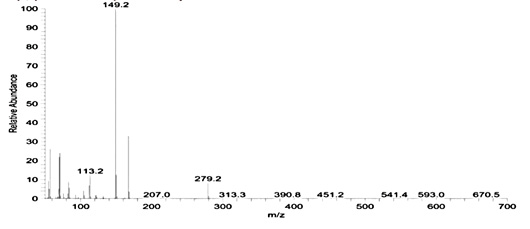
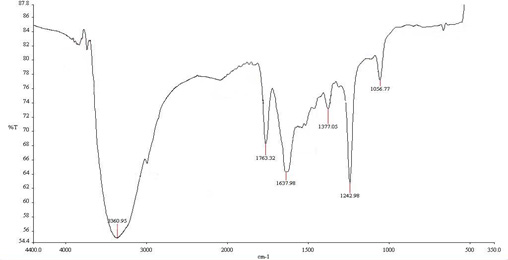
Fig. 10. Gas chromatogram spectral analysis showing the major peak of the active compound (a), mass spectral analysis of phthalic acid in the crude extract (b) and IR spectrum of the phthalic acid derivatives (c)
The present research indicates that the marine bacterium B. cereus S1 has the potentiality to target the growth of both Gram negative and Gram positive bacteria which supports the successful use of the strain as a biological control agent. Moreover, it was proved as anticoagulant and anti-inflammatory agent. One of the challenges in future will be the large scale production of these compounds to meet the demand for different applications which can be applied as probiotic in aquaculture system. Heat stability of the inhibitory substances produced by B. cereus S1 can effectively be used as bio-preservative in food. Further studies are needed for complete identification of the active compounds.
- Talpur, A. D., Memon, A. J., Khan, M.I., Ikhwanuddin, M., Danish, M.M., Daniel, A.B. Abol-Munafi, A.B. Inhibition of pathogens by lactic acid bacteria and application as water additive multi isolates in early stages larviculture of P. pelagicus (LINNAEUS, 1758). J. Anim. Plant Sci., 2012; 22(1): 54-64.
- Akbar, A., Anal, K.A. Prevalence and antibiogram study of Salmonella and Staphylococcus aureus in poultry meat. Asian Pac. J. Trop. Biomed., 2013; 3(2): 163-168.
- Vendrell, D., Balcazar, J.L., Ruiz-Zarzuela, I., Blas, I.D., Girones, O., Muzquiz, J.L. Lactococcus garvieae in Fish: A Review. Comparative immunology, microbiology and infectious diseases., 2006; 29: 177 -198.
- Olivares-Fuster, O., Klesius, P.H., Evans, J., Arias, C.R. Molecular typing of Streptococcus agalactiae isolates from fish. J.Fish Dis. 2008; 31: 277-283.
- Staroscik,A.M., Hunnicutt, D.W., Archibald, K.E., Nelson, D.R. Development of methods for the genetic manipulation of Flavobacterium columna. BMC Microbiol., 2008; 8:115.
- Evans, J.J., Klesius, P.H., Shoemaker, C.A. First isolation and characterization of Lactococcus garvieae from Brazilian Nile Tilapia, Oreochromis niloticus (L.), and Pintado, Pseudoplathystoma corruscans (Spix & Agassiz). J. Fish Dis. 2009; 32: 943-951.
- Birkbeck, T.H., Feist, S.W., Verner-Jeffreys, D.W. Francisella infections in fish and shellfish. J.Fish Diseases., 2011; 34: 173-187.
- Burr, S.E., Goldschmidt-Clermont, E., Kuhnert, P., Frey, J. Heterogeneity of Aeromonas populations in wild and farmed Perch, Perca fluviatilis L. J.Fish Dis. 2012; 35: 607-613.
- Figueiredo, H.C.P., Nobrega-Netto, L., Leal, C.A.G., Pereira, U.P., Mian, G.F. Streptococcus iniae Outbreaks in Brazilian Nile Tilapia (Oreochromis niloticus L.) farms. Braz.J. Microbiol., 2012; 43: 576-580.
- Silva, B.C., Mouriño, J.L.P., Vieira, F.N., Jatobá, A., Seiffert, W.Q. Martins, M.L. Haemorrhagic septicaemia in the hybrid surubim (Pseudoplatystoma corruscans , Pseudoplatystoma fasciatum) caused by Aeromonas hydrophila. Aqu. Res., 2012; 43: 908-916.
- Sebastião, F.A., Furlan, L.R., Hashimoto, D.T., Pilarski, F. Identification of bacterial fish pathogens in Brazil by direct colony PCR and 16S rRNA gene sequencing. Adv. Microbial., 2015; 5: 409-424.
- Rajan, B.M., Kannabiran, K. Extraction and identification of antibacterial secondary metabolites from marine Streptomyces sp. VITBRK2. Intern. J. Mol. Cell. Med., 2014; 3: 130–137.
- Ananou, S., Garriga, M. A., Jofré, T., Aymerich, A., Gálvez, M., Maqueda, M., Martínez-Bueno, Valdivia E. Combined effect of enterocin AS-48 and high hydrostatic pressure to control food-borne pathogens inoculated in low acid fermented sausages. Meat Sci., 2010; 84: 594-600.
- Nicoletti, O.R., Trincone, A. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar. Drugs., 2016; 14(2): 37.
- Hu, Y., Chen, J., Hu, G., Yu, J., Zhu, X., Lin Y., Chen, S.,Yuan, J. Statistical research on the bioactivity of new marine natural products discovered during the 28 Years from 1985 to 2012. Mar. Drugs., 2015; 13: 202-221.
- Bhatnagar, I., Kim S. Immense essence of excellence: Marine Microbial Bioactive Compounds. Mar Drugs., 2010; 9(8): 1391–1402. 12.
- Datta, D., Nath S., Talapatr, S. Swarnakar, B. Bioactive compounds from marine invertebrates for potential medicines – An overview . Int. Lett. Nat. Sci., 34; 42-61.
- Penesyan, A., Tebben, J., Lee, M., Thomas, T., Kjelleberg, S., Harder, T., Egan, S. Identification of the antibacterial compound produced by the marine epiphytic bacterium Pseudovibrio sp. D323 and related sponge-associated bacteria. Mar Drugs., 2011; 9(9): 1440-68.
- Jin, L., Ma Peng, W.C., Xu, B.Y., Zhang, F., Guo, Y. Li, Z. Bacillamide C. Bacillamide production by the optimized cultivation of the Bacillus atrophaeus strain C89 associated with the South China Sea sponge Dysidea avara. Process Biochem., 2011; 46: 1153–1159.
- Phelan, R.w., C. Charles, J.P. Morrissey. Tetracycline resistance-encoding plasmid from Bacillus sp. strain 24 isolated from the marine sponge Haliclona simulans, Appl. Environ. Microbiol., 2011; 77 (1): 327–329.
- Fang Liu, Sun, W., Su, F., Zhou, K., Li, Z. Draft Ggenome sequence of the Sponge-associated strain Bacillus atrophaeus C89, a potential producer of marine drugs. J. Bacteriol., 2012; 18: 6539- 6544.
- Leal-Sánchez, M.V., Jiménez-Diaz, R., Maldonado, A., Barragán, A., Fernándezand, J.L., Ruiz-Barba. Optimization of bacteriocin production by batch fermentation of Lactobacillus plantarum LPCO10. Appl. Environ. Microbiol., 2002; 68(9): 4465-4471.
- Wen Zhou, W., He, Y., Ni ,T., Zhong, J. Optimization of fermentation conditions for production of anti-TMV extracellular ribonuclease by Bacillus cereus using response surface methodology. Bioprocess Biosystems Eng., 2010; 33(6): 657–663.
- Moriyama, H., Fukusaki, E., Cabreracrespo, J., Shinmyo, A.,Okada, H. Structure and expression of genes coding for xylan-degrading enzymes of Bacillus pumilus. Eur J Biochem., 1987; 166:539-545.
- Rattanachuay, P., Kantachote, D., Tantirungkij, M., Nitoda, T., Kanzaki, H. Inhibition of shrimp pathogenic vibrios by extracellular compounds from a proteolytic bacterium Pseudomonas sp. W3. Elect J. Biotechnol., 2010; 13(1): 1-11.
- El-Masry, M.H., Khalil, A.I., Hassouna, M.S.,Ibrahim, H.A. In situ and in vitro suppressive effect of agricultural composts and their water extracts on some phytopathogenic fungi. World. J. Microbiol. Biotechnol., 2002; 18: 551-558.
- Hall,T.A.,1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nu c l. Ac ids Symp. Ser. 95-98.
- Plackett, R.L., Burman, J.P., 1946. The design of optimum multifactorial experiments.Biometrica. 33: 305-325.
- Yu, X., Hallett, S.G., Sheppard J., Watson, A.K. Application of Plackett-Burman experimental design to evaluate nutritional requirements for the production of Colletorichum coccodes spores. Appl. Microbiol. Biotechnol., 1997; 47: 301-305.
- Cochran, W.G., Snedecor, G.W.Statistical methods .P.466.lowastate University Press , Ames, Lowa 50041. 1989.
- Eikmeier, H.J., Reh m,1987.Stability of calcium alginate during citric acid product ion by immobilized Aspergillus niger. Appl.M icrobiol. Biotechnol., 26: 105-111.
- Bharti, P., Anand, V., Chander, J., Singh, I., Singh, T., Tewari, R. Heat stable antimicrobial activity of Burkholderia gladioli OR1 against clinical drug resistant isolates. Ind. J. Med. Res., 2012; 135: 666-671.
- Shokri, D., Zaghian, S., Khodabakhsh, F, Fazeli, H., Mobasherizadeh, S., Ataei, B. Antimicrobial activity of a UV-stable bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium strain DSH20 against vancomycin-resistant Enterococcus (VRE) strains. J. Microbiol. Immunol Infect., 2014; 47: 371-376.
- Than, P., Del Castillo, C., Yoshikawa, T., Sakata, T. Extracellular protease production of bacteriolytic bacteria isolated from marine environments.. Fisheries Sci.., 2004; 70(4): 659-666.
- Bozzola, J.J., Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists. 2nded., 1999. Jones and Bartlett Publishers, Massachusetts, USA.
- Rodrigues, J.A., Vanderlei, E.S., Bessa, E., Magalhães, F., Paula, R.C., Lima, V., Benevides, N.M. Anticoagulant activity of a sulfated polysaccharide isolated from the green seaweed Caulerpa cupressoides. Braz.Arch. Biol Technol. Int., 2011; 54(4): 691-700.
- Sakat , S., Juvekar A.R., Gambhire, M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn.. J. Pharm. Pharm. Sci., 2010; 2(1): 146-155.
- Atta, H.M., Ahmad, M.S. Antimycin-A antibiotic biosynthesis produced by Streptomyces sp. AZ-AR-262: taxonomy, fermentation, purification and biological activities. Aust. J. Basic Appl. Sci., 2009; 3: 126-135.
- Abou-Elela, G.M.,.El-Sersy, N.A., Abd-Elnaby, H., Wefky S. Distribution and bio-diversity of faecal indicators and potentially harmful pathogens in North Delta (Egypt). Aust. J. Basic . Appl. Sci., 2009; 3(4): 3374-3385.
- Telesmanich, N.P., Vinokur, N.L., Men’shikova, E.A., Nepomniashchaia, N.B. Bactericidal properties of hemo-cytolysin from Vibrio choleraenon O1 P-11702 strain in a panel of indicator cultures for detection of vibriocins. Mikrobiol. Epidermiol. Immunobiol., 2000; 6: 74-76.
- Emami-Karvani, Z., Chehrazi, P. Antibacterial activity of ZnO nanoparticle on Gram positive and Gram-negative bacteria. Afr. J. Microbiol. Res., 2011; 5(12): 1368-1373.
- Zai, A.S., Ahmad, S., Rasool, S.A. Bacteriocin production by indigenous marine catfish associated Vibrio spp. Pakistan J. Pharma Sci., 2009; 22(2): 162-167.
- Gram, L., Melchiorsen, J., Bruhn, J.B. Antibacterial activity of marine culturable bacteria collected from a global sampling of ocean surface waters and surface swabs of marine organisms. Mar. Biotechnol., 2010; 12: 439–451.
- Balakrishnan, B., Kothilmozhian, J., Ranishree, J., Thadikamala, S., Prabakaran Panchatcharam, P. Purification, characterization and production optimization of a vibriocin produced by mangrove associated Vibrio parahaemolyticus. Asian Pac J Trop Biomed., 2014; 4(4): 253-261.
- Anand, T.P., Bhat, A.W., Shouche, Y.S., Roy, U., Siddharth, J., Sarma, S.P. Antimicrobial activity of marine bacteria associated with sponges from the waters off the coast of South East India. Microbiol. Res., 2006; 161: 252–262.
- Mohana, G., Kumar, A., Thangappanpillai T., Ramasamyd B. Antimicrobial activities of secondary metabolites and phylogenetic study of sponge endosymbiotic bacteria, Bacillus sp. at Agatti Island, Lakshadweep Archipelago. Biotechnol Reports. 2016; 11: 44–52.
- Liu,F., Sun, W., Su, F., Zhou, K., Li, Z. Draft genome sequence of the sponge-associated strain Bacillus atrophaeus C89, a potential producer of marine drugs, J. Bacteriol., 2012; 18: 6539–6544.
- Wefky, S. H, Abou- Elela, G., El- Bestawy, E. Optimization of fermentation conditions for bioactive compounds production by marine bacterium Enterococcus faecium. J.Appl.Sci. Res., 2009; 5(10): 1445-1454.
- Ali, M.A. Antimicrobial agents production from marine bacteria in Suez Bay. M.Sc. Thesis, 2012. Fac. Sci., Al-Azhar Univ
- Sana, B., Ghosh, D., Saha, M., Mukherjee, J . Purification and characterization of an extracellular, uracil specific ribonuclease from a Bizionia species isolated from the marine environment of the Sundarbans. Microbiol. Res., 2008; 163: 31–38.
- Abd-Elnaby, H., Abo-Elela, G., Abdel-Raouf, U. Antibacterial and anticancer activity of marine Streptomyces parvus: Optimization and application. Biotechnol. Equip., 2016; 30(1): 180-191.
- Shobier, A.H., Hassan, S.W.M., Abdel Ghani, S.A.H., El Ashry, S. Comparative study of the antibacterial activity of Ulva lactuca and Pterocladia capillacea extracts before and after encapsulation in Ca-alginate beads. Egypt J.Aqu. Res., 2010; 36(3): 395-402.
- Sabate, M., Carina Audisio, C. Inhibitory activity of surfactin, produced by different Bacillus subtilis subsp. subtilis strains, against Listeria monocytogenes sensitive and bacteriocin-resistant strains. Microbiol. Res., 2013; 168: 125–129.
- Risøen, P.A., Rønning, P., Hegna, I.K., Kolst, A.B. Characterization of a broad range antimicrobial substance from Bacillus cereus. J.Appl. Microbiol., 2004; 96: 648–655.
- Chalasani, A.G., Dhanarajan, G., Nema, S., Sen, R., Roy, U. An Antimicrobial metabolite from Bacillus sp.: significant activity against pathogenic bacteria including multidrug-resistant clinic strains. Front Microbiol., 2015; 6: 1335.
- Ashok, G., Karthikeyan, P., Panneerselvam, A., Senthilkumar, G. European. Effect of antimicrobial activity of UV mutated actinomycetes sp. isolated from mangroves. J. Exp.Biol., 2014; 4(5): 46-52.
- Wang, S., Kong, J., Zhang, X. Identification and characterization of the two-component cell lysis cassette encoded by temperate bacteriophage ÕPYB5 of Lactobacillus fermentum. J.Appl. Microbiol., 2012; 105 (6): 1939-1944.
- Shastry, S., Prasad, M.S. Extracellular protease from Pseudomonas sp. (CL 1457) active against Xanthomonas campestris. Process Biochem., 2002; 37(6): 611-621.
- Towse, S.J. Antimicrobial activity of marine Vibrio sp., isolate NI-22. Honours Thesis. Winnipeg University, 2005.
- Barakat, K.M., Beltagy, E.A. Bioactive phthalate from marine Streptomyces ruber EKH2 against virulent fish pathogens. Eg. J. Aqu. Res., 2015; 41: 49-56.
- Mahajan, R., more, D. Evaluation of anticoagulant activity of aqueous and ethenolic extracts and their phytochemicals of some medical plants. Int. J. Pharm. Pharmaceut., 2012; 4(1): 498-500.
- Hassanein, W. A., Kotb, E., Awny, N.M., El-Zawahry, Y.A. Fibrinolysis and anticoagulant potential of a metallo protease produced by Bacillus subtilis K42. J. Biosci., 2011; 36(5): 773–779.
- Wei X., , Luo, M., Xu L., Zhang, Y., Lin, Z., Kong, P., Liu, H. Production of fibrinolytic enzyme from Bacillus amyloliquefaciens by fermentation of chickpeas, with the evaluation of the anticoagulant and antioxidant properties of chickpeas. J. Agric. Food Chem., 2011; 59 (8): 3957–3963.
- Kurian, B.N., Nair, H.P., Bhat, S.G. Evaluation of anti-inflammatory property of melanin from marine Bacillus spp. BTC. Asian.J Pharm Clin Res., 2015; 8(3): 251-255.
- Hassan, S.W. Studies on the bioactive compounds from marine bacteria. PH.D. Thesis Fac.Sci.Alex.Univ., 2009.
- Janaki, M., Sanith, V., Kannagi, A. Bioactive potential of Fusinus nicobaricus from Gulf of Mannar. Int.J. Pharmaaceut. Res. Biosci., 2015; 4(5): 262-270.
- Lessoy, TZ., Micael, E.B., Jean, T.G., Betty, M.F., Sebastien, L.N. Two novel nonconventional seed oil extracts with antioxidant and antimicrobial activities. Tropical J. Pharma. Res., 2012; 11: 469-475.
- Avci, O., Dik, B (2014). Determination of invivo antiviral activity of Nerium oleander distillate against parainfluenza-3 virus. Anim Vet Sci,. 2(5): 150-153.
- Tang, L., Chu, T., Huag, Y. 1999. Anti-inflammatory properties of triblock siloxane copolymer-blended materials. Biomaterials., 1999; 20(15): 1365-1370.
© The Author(s) 2016. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.