ISSN: 0973-7510
E-ISSN: 2581-690X
Treatment with herbal drugs is considered one of the oldest and safest methods for treating various diseases. Mentha arvensis is a medicinal plant used worldwide in the pharmaceutical industry for its antibacterial, anticancer, antiallergic, and anti-inflammatory properties. In the present study, the antibacterial activity of the methanolic extract of M. arvensis leaves was tested at different concentrations (50, 100, 150, and 200 µg/ml) against bacteria isolated from human burn wound infections, including Pseudomonas aeruginosa, Klebsiella pneumoniae and Staphylococcus aureus. The results demonstrated that at concentrations of 100, 150, and 200 µg/ml, the methanolic extract exhibited strong antibacterial activity against S. aureus and P. aeruginosa, while K. pneumoniae isolates were resistant to the extract at all tested concentrations. Further, the cytotoxic activity of the methanolic extract was assessed at various concentrations (100, 140, 160, 180, 220, and 250 µg/ml) against two cell lines: the RD tumor cell line and Vero normal cell line. The higher concentrations of the extract (220 and 250 µg/ml) showed potent cytotoxic effects on the RD cell line after 72 hours in a dose- and time-dependent manner. In contrast, the extract exhibited lower cytotoxic effects on Vero cells at the same concentrations after 72 h.
Klebsiella pneumonia, Mint, Pseudomonas aeruginosa, Staphylococcus aureus, Tumor Cell Line, Vero Cells
Burn wound infections are one of the most prevalent and severe health issues worldwide and lead to high morbidity and mortality rates.1 These infections can delay burn wound healing and promote the proliferation of microorganisms, which may lead to invasive infections. The high rate of burn wound infection results from the loss of skin integrity and decreased immunity and can develop into sepsis. The severity of the infections depends on various factors, such as the depth of the burn injury, the amount and virulence of microbial flora, and the length of hospital stays.2 The most common serious complications of burn injuries include impetigo, cellulitis, pulmonary infection, bacteremia, septicemia, and meningitis.3
Mentha arvensis, a common edible and aromatic perennial herb, belongs to the Lamiaceae family, which comprises over 30 species. The plant’s dark green quadrangular stem, measuring 55-91 cm in height, has opposite leaves at each node. M. arvensis emits a sharp peppermint odor and has a pleasant acrid taste.4 Its large-scale cultivation began in Japan and has spread to India, Australia, Asia, and South Africa.5 Plants contain various flavor compounds and are well-known worldwide as cold-relieving plants.6 It is also widely used in the cosmetic and pharmaceutical industries.7 Numerous studies have focused on the phytochemical compounds found in most plant parts, highlighting their significance in medical practice.8 The leaves of M. arvensis are considered a richer source of secondary metabolites than other plant parts such as the stems and roots.9 The primary constituents of the leaves include flavonoids, alkaloids, polyphenols, tannins, menthol, glycosides, isomenthone, steroids, sugars, carbohydrates, cineole, and saponins.10 Additionally, the plant contains minerals, fibers, trace elements, and vitamins such as vitamin C, niacin, riboflavin, and folic acid, all of which offer nutritional value.11 M. arvensis leaf extracts possess antimicrobial, antifungal, antiseptic, antioxidant, anti-inflammatory, sedative, and antitumor activities.12,13
Nazim et al. reported the antibacterial activity of M. arvensis leaves’ methanolic, ethyl acetate, and water extracts on various pathogenic bacteria, such as Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa. Using the well diffusion method, they found that bacterial isolates were highly sensitive to the methanolic extract of M. arvensis leaves at concentrations of 100–250 µg/ml with a maximum inhibition zone of 21.37 ±1.17 mm for S. aureus.14
Cytotoxicity assays are widely used in the pharmaceutical industry to screen cytotoxic compounds of interest for the development of drugs that target rapidly dividing cancer cells.15 In these assays, cells in the exponential growth phase are exposed to cytotoxic drugs or anticancer agents. Plants contain bioactive secondary metabolites characterized by complex structures and anticancer activities.16,17 Scientists have collected different parts of many plants, prepared extracts, and tested them to discover new chemotherapeutics for the treatment of cancer and microbial infections. The cytotoxic screening of plants serves as a preliminary method for identifying active plant compounds.18,19 In cytotoxicity assays, the treatment of cells with cytotoxic compounds can result in various cell fates. Cells may undergo necrosis, lose membrane integrity, and die rapidly due to cell lysis. Alternatively, cells can stop growing and dividing, leading to decreased cell viability or the activation of programmed cell death (apoptosis).20
Despite existing research on the antibacterial and cytotoxic properties of M. arvensis leaf extracts, there is still a knowledge gap concerning the effectiveness of these extracts against specific bacterial strains isolated from human burn wound infections and their cytotoxic effects on different cell lines. Therefore, this study aimed to investigate the activity of the methanolic extract of M. arvensis leaves in various concentrations on bacteria isolated from burn wounds, such as P. aeruginosa, K. pneumoniae, and S. aureus, and to assess the cytotoxic effects of the extract on RD tumor and Vero normal cell lines. This study contributes to a better understanding of the potential therapeutic applications of M. arvensis in the treatment of burn wound infections and cancer.
Bacterial isolation and diagnosis
Ninety specimens were obtained from cases of burn and wound infections in four hospitals in Baghdad from October 20, 2021, to January 20, 2022. Samples were collected using sterile swabs, and streaked on MacConkey, nutrient, and mannitol-salt agar, before being incubated overnight at 37°C. Lactose-fermenting colonies from MacConkey agar were subcultured on Eosin Methylene Blue (EMB) agar plates to isolate Klebsiella pneumonia, whereas non-lactose-fermenting colonies were transferred to cetrimide agar for Pseudomonas aeruginosa isolation. Selected colonies isolated from the nutrient agar were subcultured on mannitol salt agar for the isolation of Staphylococcus aureus. Bacterial isolates were identified through several steps, including examination of morphological characteristics on different culture media, conducting microscopic examinations after staining with Gram stain, and finally analyzing the biochemical reactions using the VITEK 2 system.
Preparation of the methanolic extract
Mentha arvensis seedlings were collected fresh from nurseries and taken for authentication in the National Herbarium of Iraq, after which they were dried and used. The methanolic extract was prepared from the leaves according to the method described by Sures and Rathishkumar by grinding the plant leaves in an electric grinder. Ten grams of the M. arvensis powder were immersed in 500 mL of 95% methanol for 72 h with intermittent shaking. The methanolic extract was filtered through a Whatman filter paper no. 1 and subjected to hot extraction using a Soxhlet extraction apparatus for 5 h. Afterward, the leaf extract was transferred to a rotary evaporator at 45°C for concentration. The M. arvensis leaf methanolic extract powder was preserved in a Falcon tube. A 1:2 stock solution was prepared from the extract; filtered using 0.45 µm and 0.22 µm Millipore filters, and then used in subsequent steps.
Phytochemical screening
Test for phenols
In this test, 3 mL of plant extract was added to 2 mL of 1% ferric chloride solution in a test tube. The formation of a bluish-green color indicates the presence of phenolic compounds. To confirm these results, 3 mL of the test sample was transferred to another test tube, followed by the addition of basic aqueous lead acetate, which should lead to the formation of a red-brown precipitate.9
Test for flavonoids
To 1 mL of M. arvensis leaf methanolic extract, 1 mL of 10% lead acetate solution was added. The formation of yellow color indicated a positive flavonoid result.5
Screening for saponins
A small quantity of 2N HCl was added to 2 mL of the methanolic extract and shaken vigorously. The aqueous layer was then decanted and two drops of Mayer’s reagent were added. The appearance of a deep-colored foaming layer indicated the presence of saponins.10
Screening for alkaloids
To test for alkaloids, 2 mL of the methanolic extract was heated after adding 10 % of sodium hydroxide solution to the test tube. The formation of a white precipitate indicates the presence of alkaloids.10
Screening for tannins
To detect tannins, 2 mL of the methanolic extract was heated after adding concentrated nitric acid and excess ammonia. The formation of a white precipitate indicates the presence of tannins.9
Screening for glycosides
To screen for glycosides, 0.5 g of M. arvensis leaves’ methanolic extract was diluted with 5 mL distilled water, followed by the addition of 2 mL of CH3COOH containing one drop of ferric chloride solution. Subsequently, 1 mL of concentrated H2SO4 was carefully added. The appearance of a brown ring at the interface indicated the presence of cardiac glycosides.10
Antimicrobial activity testing
The well diffusion method was employed to determine the antimicrobial activity of the methanolic extract, following the protocol described by Sharma et al. A sterilized cork borer was used to create five holes on Mueller-Hinton agar plates, with four outer holes designated for different concentrations of the methanolic extract. The central hole contained normal saline as a negative control. Bacterial isolates of 1.5×108 CFU/ml concentration were spread on the agar surface using a sterile cotton swab, and the plates were left to dry for 10 min. Different concentrations of the methanolic extract (50, 100, 150, and 200 µg/ml) were prepared from the stock solution to study their antibacterial activity. Then, 100 µL from each concentration were placed in the designated holes, while 0.1 mL of normal saline was added to the central hole. Three plates were used to detect the antibacterial activity of the extract against each bacterial isolate, which were incubated overnight at 37°C, and the antibacterial activity was evaluated by measuring the diameter of the inhibition zones around each hole in millimeters. The mean diameter of the inhibition zones at each concentration was recorded.4
Cell lines
The two cell lines used in the current study were provided by the Iraqi Center for Cancer and Medical Genetics Research (ICCMGR; Baghdad, Iraq). The Vero cell line was prepared from the normal kidney of an adult African green monkey in 1962, and passage 66 was used for the experiments. The cells were maintained in MEM containing 10% fetal calf serum (FCS). Rhabdomyosarcoma (RD) is a cancer cell line obtained from a biopsy specimen of an 8-year-old Caucasian girl with pelvic rhabdomyosarcoma. The RD cell line was used after 64-66 passages, and cells were maintained in MEM supplemented with 10% FCS.
Cytotoxicity assay
This method was performed according to Deepavali et al. on two cell lines: RD and Vero.21 Monolayer cells in the Falcon flask were examined using an inverted microscope. The excess medium was removed and 1-2 ml of warm trypsin–versin was added for one minute to harvest the cells, followed by the addition of culture media to stop trypsinization. Cell viability was assessed to achieve a final concentration of 1×106 cells/well, and then 200µl of cell suspension was transferred to wells within a microtiter plate. The plates were tightly sealed with adhesive parafilm and incubated at 37°C for 24 h. After incubation, the plates were examined under an inverted microscope to ensure the formation of a confluent monolayer without contamination. The excess medium was carefully removed, and different concentrations of the methanolic extract (100, 140, 160, 180, 220, and 250 µg/ml) were prepared from the stock solution to study its antitumor activity. Then, 200 µl of each concentration was added to the wells in a microtiter plate in triplicate. The two middle columns of the microtiter plate were used as controls (cells treated with serum-free medium alone). The plates were incubated in a CO2 incubator at 37°C for 24, 48, and 72 h. After exposure, the medium was carefully removed and the cells in the wells were washed three times with 0.1 ml of sterilized warm PBS. Finally, 200µl of crystal violet was added to each well, and the plates were incubated at 37°C for 30 min. Excess dye was removed by washing the wells thrice with PBS and allowing them to dry at room temperature. The absorbance was measured using an ELISA reader at a wavelength of 492 nm. The inhibition rate (IR) was estimated using the following equation:
IR(%) = (AB / A) x 100 …(1)
IR: inhibition rate; A: optical density of negative control; B: optical density of test extract.
Statistical analysis
The statistical program GenStat was used to analyze the resulting data according to a factorial experiment. The experimental design was a Completely Randomized Design (CRD) with 10 replicates for each treatment, and P-values of 0.05 or less were considered statistically significant.
Bacterial isolation and identification
The morphological characteristics of different selective media revealed that K. pneumonia isolates produced large pink mucoid colonies on MacConkey agar due to lactose fermentation. On EMB agar, they appeared as dark pink colonies without the production of a green metallic sheen. P. aeruginosa isolates on MacConkey agar, P. aeruginosa isolates appeared as circular, non-fermented colonies. Cetrimide agar, a selective medium for the isolation of P. aeruginosa, produced yellow-green colonies, indicating pigment production. These results are similar to those reported by Chauhan and Jindal.22 S. aureus colonies appeared as yellow opaque on mannitol-salt agar due to mannitol fermentation, a result compatible with that mentioned by Hanselman and his colleagues.15
The final diagnosis of selected isolated bacteria using the Vitek2 system showed that out of the 90 total specimens collected from infections of burns and wounds, 41 isolates (45.5%) were P. aeruginosa, 23 isolates (25.7%) were related to S. aureus, and 10 isolates (11.1%) were K. pneumoniae. The remaining 16 isolates (17.7%) belonged to different bacterial species. A report by the Control Center for Diseases and Prevention from 2019 to 2020 revealed that multidrug-resistant bacteria causing burn infections included P. aeruginosa, A. baumannii, K. pneumoniae, and S. aureus at rates of 41.4%, 25.9%, 21.3%, and 11.4%, respectively.3 Another study by Ken et al. indicated that the most common bacteria associated with burn wound infections were P. aeruginosa, Methicillin-Resistant S. aureus (MRSA), A. baumannii, K. pneumoniae, P. vulgaris, and E. coli.23
Antibacterial activity
The results in Table 1 demonstrate that the inhibition zone of the S. aureus isolate (S1) was significantly larger (14.2 mm) than the P. aeruginosa isolate (P1) that recorded (13.4 mm). Moreover, the S1, S2, S3, P1, P2, and P3 isolate’s inhibition zones were significantly larger than those of the K. pneumonia isolates (K1, K2, and K3). According to the effect of plant extract concentrations on different bacterial species. The largest mean inhibition zone was 16.5 mm corresponding to a concentration of 200 µg/ mL compared to the lower concentrations of 50, 100, and 150 µg/ml that resulted in inhibition zones of 9.3, 11.6, and 14.8 mm in diameter, respectively.
Table (1):
Antibacterial activity of M. arvensis leaves’ methanolic extract on the growth of S. aureus, P. aeruginosa and Klebsiella pneumonia isolates.
| Bacterial isolates | Zones of inhibition (cm/100 μl) | Mean | ||||
|---|---|---|---|---|---|---|
| M. arvensis leaves methanolic extract (µg/ml) | Control | |||||
| 50 | 100 | 150 | 200 | PBS | ||
| S1 | 11.3 | 14.7 | 21.0 | 24.0 | 0 | 14.2 |
| S2 | 12.0 | 14.3 | 20.7 | 23.7 | 0 | 14.1 |
| S3 | 11.7 | 14.7 | 20.3 | 23.7 | 0 | 14.1 |
| P1 | 12.0 | 15.3 | 19.3 | 20.3 | 0 | 13.4 |
| P2 | 12.7 | 16.3 | 19.0 | 21.3 | 0 | 13.9 |
| P3 | 13.0 | 16.7 | 19.7 | 21.7 | 0 | 14.2 |
| K1 | 3.3 | 4.0 | 4.7 | 4.7 | 0 | 3.3 |
| K2 | 3.7 | 4.7 | 4.7 | 4.7 | 0 | 3.6 |
| K3 | 3.7 | 4.0 | 4.0 | 4.0 | 0 | 3.1 |
| Mean | 9.3 | 11.6 | 14.8 | 16.5 | 0.0 | |
| LSD= 0.05 | Bacterial Spp.= 0.91; Concentrations of M. arvensis extract= 1.63 Bacterial Spp. * Concentrations of M. arvensis extract= 3.91 | |||||
S1, S2, S3= S. aureus isolates; P1, P2, P3= P. aeruginosa isolates; K1, K2, K3= K. pneumonia isolates.
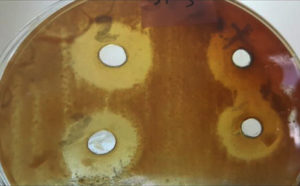
Additionally, the interaction between bacterial isolates and the plant methanolic extract concentrations revealed that the concentration of 200 µg/ml exhibited the highest average inhibition zone (24.0 mm) for the S1 isolate, as shown in Figure 1. The concentrations of 200 and 150 µg/ml demonstrated a significant increase in the mean inhibition zones in S1, S2, S3, P1, P2, and P3 bacterial isolates compared to the effects of 50 and 100 µg/ml concentrations for the same isolates. Conversely, there was a significant decrease in the mean inhibition zones recorded in K1, K2, and K3 bacterial isolates at all methanolic extract concentrations compared to other bacterial isolates. Numerous studies have focused on the antibacterial activity of the plant’s leaf extracts. The efficacy of M. arvensis leaf and internodal callus extract was screened against S. typhi, S. paratyphi, A. baumannii, and P. mirabilis, demonstrating that M. arvensis leaf extract had maximum antibacterial efficiency against Acinetobacter baumannii, with an inhibition zone of 19 mm.24
Figure 1. Antimicrobial activity for the methanolic extract of M. arvensis leaves against S. aureus isolate
Another study revealed that M. arvensis leaves extract at concentrations of 120–200 µg/mL exhibited strong antimicrobial activities against P. aeruginosa, S. pyogenes and S. aureus isolated from different clinical cases that were comparable to the standard kanamycin antibiotic.4 Junali and Saikial reported that the activity of Mentha leaf extract may be due to the presence of active compounds, such as flavonoids, saponins, tannins, alkaloids that stimulate the generation of reactive oxygen species (ROS), and protein leakage, leading to bacterial membrane damage.5
The methanolic extract of M. arvensis leaves was screened for antimicrobial activity using microdilution and well diffusion methods against various bacterial pathogens such as S. aureus, P. vulgaris, E. coli, S. typhi, P. aeruginosa, and K. pneumoniae. The extract was effective at various concentrations (1:2, 1:4, and 1:8) against most of the tested bacteria, except K. pneumoniae and E. coli. The activity of the leaf extract may be due to the presence of active phenolic compounds which have strong antimicrobial activity.25 A study that tested aqueous and methanolic extracts of Ocimum sanctum, Mentha arvensis, and O. basilicum leaves at different concentrations against various pathogenic bacteria using the well-diffusion method found that methanolic extracts exhibit higher inhibitory activity than aqueous extracts. This may be due to the presence of some active constituents in the alcoholic extract, which were detected at lower concentrations in the aqueous extracts. Additionally, some active compounds are only soluble in organic solvents and are therefore not present in water extracts.4
Studies on isopropyl alcohol, petroleum ether, and methanolic extracts of M. arvensis leaves have demonstrated that petroleum ether and methanolic extracts display good antibacterial activity against Proteus spp. and S. aureus isolated from different clinical cases.10 A study on M. arvensis leaf extract in different solvents, such as 20% and 50% methanol, chloroform, and ethyl acetate, tested against human cariogenic bacteria like S. mutans, S. sanguinis, S. mitis, and S. aureus isolated from patients with dental caries revealed that 50% methanolic extract has a broad spectrum antibacterial activity against isolated bacteria higher than other extracts.7
Methanolic extracts acted in a dose-dependent manner against bacterial pathogens. This result is consistent with another study that reported the concentration-dependent inhibitory effects of Mentha leaf extract against different bacterial species.26 However, the toxicity of different plant extract concentrations may vary among microorganisms, as leaves are a rich source of bioactive compounds that differ in their antimicrobial activities.8
The analysis of the plant’s active constituents by GC-MS revealed the presence of secondary metabolites in the leaves, such as alkaloids, tannins, flavonols, steroids, menthol, and eucalyptol, which are phytochemicals of pharmaceutical interest.13 Additionally, the phytochemicals present in hydrodistilled essential oils exhibit strong antimicrobial activities against pathogenic bacteria.27,28
Cytotoxic effect
The cytotoxic effect of the methanolic extract of M. arvensis leaves at different concentrations (100, 140, 160, 180, 220, and 250 µg/ml) on a confluent monolayer of the Vero cell line, passage number 66, at three exposure times (24, 48, 72 hours) is shown in Table 2. Estimation of the inhibitory rate (IR) on a confluent monolayer of the Vero cell line revealed that the M. arvensis leaf methanolic extract had a lower cytotoxic effect on the viability of the Vero cell line, which depended on the extract concentration. A minor reduction in cell viability was observed at all concentrations after 24 h. Based on the time factor, there was a slight decrease in cell viability with extended exposure times of 48 and 72 hours. Additionally, there was a very low increase in growth inhibition at the higher concentrations (220, 250 µg/ml) after 72 hours. P3 was statistically significant (p<0.05) as shown in Table 2.
Table (2):
Inhibition rates of Vero cell line following exposure to M. arvensis leaf methanolic extract at various concentrations and time points.
| Mean Percentage of growth inhibition (IR) within time | ||||||
|---|---|---|---|---|---|---|
| 24 hrs | 48 hrs | 72 hrs | ||||
| Concentration of leaves extract µg /ml | Mean (IR) | SE | Mean (IR) | SE | Mean (IR) | SE |
| 100 | 6.05 | 3.01 | 7.50 | 0.97 | 9.40 | 3.52 |
| 140 | 7.86 | 0.72 | 8.64 | 2.65 | 9.88 | 3.10 |
| 160 | 9.20 | 2.75 | 10.35 | 2.41 | 10.95 | 1.78 |
| 180 | 9.98 | 0.83 | 10.51 | 3.09 | 11.06 | 1.48 |
| 200 | 10.90 | 1.51 | 11.3 | 1.41 | 12.58 | 0.32 |
| 250 | 10.98 | 0.27 | 11.87 | 0.53 | 13.10 | 0.43 |
SE: standard Error.
A study of the cytotoxic effect of M. arvensis leaf methanolic extract on a confluent monolayer of the RD cell line (Figure 2) revealed that the methanolic extract of plant leaves had a concentration-dependent effect on the viability of the RD cell line. Viability significantly decreased with increasing methanolic extract concentrations after 24 h of treatment, as shown in Figure 3. Regarding the time factor, prolonging the treatment time to 48 and 72 hours exhibited a time-dependent pattern of inhibition rate, with IC50 ranging from 140 to 160 µg/mL. The higher concentrations of 220 µg/mL and 250 µg/ml of the extract showed a higher inhibition rate on the RD cell line after 72 hours compared to 24 and 48 hours, reaching 84.51% and 90.26%, respectively, as shown in Figure 4. P3 represents a comparison of the inhibition rates at three-time points: 24, 48, and 72 h. P3 showed significant differences (p<0.05), as shown in Table 3.
Table (3):
Inhibition of RD cell line growth following exposure to M. arvensis leaf methanolic extract at various concentrations and time points.
| Mean Percentage of growth Inhibition (IR) within time | ||||||
|---|---|---|---|---|---|---|
| 24 hrs | 48 hrs | 72 hrs | ||||
| Concentration of leaves extract µg /ml | Mean (IR) | SE | Mean (IR) | SE | Mean | SE |
| 100 | 34.13 | 3.20 | 41.46 | 1.73 | 48.11 | 2.19 |
| 140 | 48.66 | 0.47 | 52.32 | 1.81 | 56.41 | 2.11 |
| 160 | 54.71 | 1.50 | 63.27 | 1.99 | 70.80 | 2.32 |
| 180 | 58.17 | 1.20 | 67.20 | 1.89 | 73.33 | 1.88 |
| 220 | 67.21 | 1.42 | 80.67 | 1.60 | 84.51 | 1.53 |
| 250 | 76.51 | 1.33 | 83.61 | 1.63 | 90.26 | 1.90 |
SE: standard error
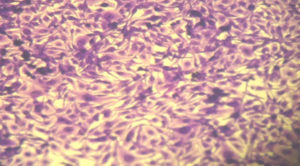
Figure 2. RD cell line (control) showing a confluent monolayer of cohesive malignant cells with no empty spaces
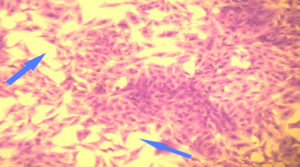
Figure 3. RD cell line shows a loss in the confluent monolayer with a reduction in the number of viable cells after exposure to M. arvensis leaves methanolic extract at 200 µg/mL for 24 h
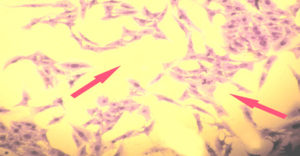
Figure 4. RD cell line reveal a great loss in the cellular features and a large reduction in number of viable cells after exposure to high concentration (250 µg/mL) of wild mint leaves methanolic extract for 72 hrs
The antitumor activity for M. arvensis, M. longifolia, and M. viridis methanolic and aqueous leaves extracts at concentrations of 50–200 µg/mL were tested for in vitro antitumor cytotoxicity against seven human cancer cell lines: A549, COLO-200, HCT-118, MCF-7, NCI-H324, PC-3, and THP-1. The results revealed that methanolic extracts of M. arvensis leaves at concentrations of 160–200 µg/mL had the strongest antitumor activity compared to other extracts, suppressing the growth of four cancer cell lines: COLO-200, MCF-7, NCI-H324, and THP-1, in the range of 75-98%.29 Ameen and Shafi indicated that in M. arvensis leaf extract, the main components with cytotoxic activities were phenolic compounds and flavonoids, which have specific antitumor and apoptosis-inducing activities in cell lines.30
M. arvensis leaf extracts (petroleum ether, benzene, chloroform, ethyl acetate, and methanol) were examined for cytotoxic antitumor activity in six human cancer cell lines (HeLa, MCF-7, Jurkat, T24, HT-29, and MIAPaCa-2) and two normal cell lines (IMR-90 and HEK-293). Among the tested extracts, ethyl acetate and methanolic extracts of M. arvensis leaves demonstrated significant dose- and time-dependent antitumor activities. The IC50 ranged from to 120–160 µg/ml for the methanolic extract and 100-140 µg/ml for the ethyl acetate leaf extract on tumor cell lines. This led to cell-cycle arrest at the G1 phase, induction of the mitochondrial apoptosis pathway, perturbation of oxidative balance, Bax gene upregulation, increased expression of p53 and p21, acquisition of a senescence phenotype, and induction of a response mediated by pro-inflammatory cytokine in treated cells.31
The cytotoxic effects of the purified total flavonoids from M. arvensis leaves were tested in two cancer cell lines, L20B and MCF-7. The maximum growth inhibition in the L20B cell line reached 75.5% at a concentration of 30 µg/mL after 72 hours, while the maximum growth inhibition in the MCF-7 cell line reached 82.4% at the same concentration after 72 hours. Polyphenolic compounds in plants, particularly flavonoids, prevented all stages of cancer (initiation, promotion, and progression). This suggests that flavonoid compounds possess cancer-blocking and suppressing capabilities.16 Flavonoids have been reported to exhibit anticancer activity through several mechanisms, including the inhibition of DNA topoisomerase I/II activities, reduction of reactive oxygen species (ROS), modulation of signaling pathways, and downregulation of nuclear factor-kappa B (NF-kB).18
A study evaluating the cytotoxic effect of M. lonifolia methanolic extract at various concentrations (100–300 µg/ml) on the viability of HepG2 and HeLa tumor cell lines showed that the plant methanolic extract inhibited the viability of both cancer cell lines in a dose-dependent manner.29 The highest inhibition rate (85% for HepG2 and 76% for HeLa) was observed at a concentration of 300 µg/ml. These inhibitory effects were concentration-dependent. The mechanism of antitumor cytotoxicity may be related to the phenolic and flavonoid contents of the extract. Essential oils from M. arvensis have demonstrated significant cytotoxic activity against various human cancers, including cervical, lung, and breast cancers. This is achieved by modulating the MAPK and PI3K/Akt pathways, inducing apoptosis, inhibiting tumor cell invasiveness and migration, causing cell cycle arrest, upregulating Bax and p53, and inducing a senescent phenotype.14
In conclusion, the present study demonstrated that the methanolic extract of M. arvensis is effective against pathogenic bacteria isolated from medical samples. In addition, it showed promising results in terms of a high rate of inhibition of the growth of cancer cell lines compared to normal cell lines. These findings suggest the potential of developing treatments for pathogenic bacteria and cancer cells using natural and environment-friendly sources. This could serve as an alternative to traditional chemicals, which are increasingly resistant to microorganisms owing to constant mutations and modifications.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Norbury W, Herndon DN, Tanksley J, Jeschke MG, Finnerty CC. Infection in burns. Surg Infect. 2016;17(2):250-255.
Crossref - Ali KM, Al-Jaff BMA . Source and antibiotic susceptibility of gram-negative bacteria causing superficial incisional surgical site infections. Int J Surg Open. 2021;30:100318.
Crossref - Ahmed AA, Juyee NA, Hasan SMA, Abedin MZ. Microbiological Screening and Antimicrobial Sensitivity Profiling of Wound Infections in a Tertiary Care Hospital of Bangladesh. European Journal of Medical and Health Sciences. 2020;2(5):101-106.
Crossref - Sharma U, Agnihotri R, Ahmad S, Mahajan S, Sharma R. Antibacterial activity of some medicinal plants of family Lamiaceae from Braj region. Glob J Med Plants Res. 2013;1:72-76.
- Chetia J, Saikia LR. Antimicrobial Activity Assay and Phytochemical Study of Different Aerial Parts of M. arvensis L. Collected from Dibrugarh, Assam. J Sci Res. 2020;64(1):103-112.
Crossref - Kapp K, Pussa T, Orav A, et al. Chemical composition and antibacterial effect of Mentha spp. grown in Estonia. Nat Prod Commun. 2020;15(12).
Crossref - Saleem MN , Idris M. Podina (Mentha arvensis): transformation from food additive to multifunctional medicine. ARC Journal of Pharmaceutical Sciences. 2016;2(2):6-15.
Crossref - Thakur S, Walia B, Chaudhary G. Mentha arvensis (Pudina): A Review Based upon its Medicinal Properties. Research Journal of Pharmacognosy and Phytochemistry. 2021;13(3):143-148.
Crossref - Malik F, Hussain S, Sadiq A, et al. Phyto-chemical analysis, anti-allergic and anti-inflammatory activity of M. arvensis in animals. Afr J Pharm Pharmacol. 2012;6(9):613-619.
Crossref - Patel D, Upadhye V, Upadhyay TK, Rami E, Panchal R. Phytochemical Screening and Antimicrobial Activity of M. arvensis L.Pudina: A Medicinal Plant. Can J Med. 2021;3:67-76.
- Thawkar BS, Jawarkar AG, Kalamkar PV, Pawar KP, Kale MK. Phytochemical and pharmacological review of Mentha arvensis. International Journal of Green Pharmacy (IJGP). 2016;10(2):71.
- Sevindik M. Pharmacological properties of Mentha species. J Tradit Med Clin Nat. 2018;7(2):259.
Crossref - Mancuso M. The antibacterial activity of Mentha. Herbs and spices. IntechOpen. 2020.
Crossref - Nazim M, Nawaz A, Anjum S, Ali M, Maryam H. Mentha arvensis, a medicinal and aromatic plant, has high nutritional value and several-uses: A review. Buletin Agroteknologi. 2020;1(2):37-49.
Crossref - Hanselman BA, Kruth SA, Rousseau J, Weese JS. Coagulase positive staphylococcal colonization of humans and their household pets. Can Vet J. 2009;50(9):954.
- Hasan ZYM, Al-Halbosiy MMF, Al-Nauimi E. Biological activity and cytotoxic effect of Iraqi Wild Mentha arvnesis total flavonoid. International Journal of Engineering and Technology. 2018;7(4):26-29.
Crossref - Yousuf T, Akter R, Ahmed J, et al. Evaluation of acute oral toxicity, cytotoxicity, antidepressant and antioxidant activities of Japanese mint (Mentha arvensis L.) oil. Phytomedicine Plus. 2021;1(4):100140.
Crossref - Tafrihi M, Imran M, Tufail T, et al. The wonderful activities of the genus Mentha: Not only antioxidant properties. Molecules. 2021;26(4):1118.
Crossref - Gonzalez HRM, Romero MV, Delgado MM, Yudit MA. Larberto JGH. Antibacterial activity of extract of Mentha arvensis L. (field mint) against strains causing pyodermitis. Rev Cubana Plant Med. 2020;25(4):e1164
- Al-Ali K, Abdelrazik M, Alghaithy A, Diab A, El-Beshbishy H, Baghdadi H. Antimutagenic and anticancer activity of Al Madinah Alhasawy mint (Mentha longifolia) leaves extract. Pak J Biol Sci. 2014;17(12):1231-1236.
Crossref - Thanekar DR, Tupe P, Dhodi J, Juvekar AR. Evaluation of in vitro cytotoxic activity of petroleum ether and methanol extract of M. arvensis (whole plant) on human cancer cell lines. Int J Pharm Pharm Sci. 2014;6(8):169-172.
- Chauhan A, Jindal T. Biochemical and molecular methods for bacterial identification. Microbiological Methods for Environment, Food and Pharmaceutical Analysis. 2020:425-468.
Crossref - Keen III EF, Robinson BJ, Hospenthal DR, et al. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns. 2010;36(6):819-825.
Crossref - Johnson M, Wesely EG, Kavitha MS, Uma V. Antibacterial activity of leaves and inter-nodal callus extracts of M. arvensis L. Asian Pac J Trop Med. 2011;4(3):196-200.
Crossref - Dwivedi D, Khandelwal G, Patidar RK, Singh V. Antimicrobial activity of M. arvensis against clinical isolates of human cariogenic pathogens-an in-vitro study. Int J Pharm Sci Res. 2012;3(5):1355-1360.
- Suresh SN, Rathishkumar S, Rajeshwari V, Sagadevan P, Gayathri S, Eswari DV. Studies on phytochemical composition and antibacterial potential of methanolic leaf extract of Mentha arvensis. Linn. IJPRD. 2012;4(8):001-004.
- Petretto GL, Fancello F, Zara S, et al. Antimicrobial activity against beneficial microorganisms and chemical composition of essential oil of Mentha suaveolens ssp. insularis grown in Sardinia. J Food Sci. 2014;79(3):M369-M377.
Crossref - Horvath P, Koscova J. In vitro antibacterial activity of Mentha essential oils against Staphylococcus aureus. Folia Veterinaria. 2017;61(3):71-77.
Crossref - Sharma V, Hussain S, Gupta M, Saxena AK. In vitro anticancer activity of extracts of Mentha spp. against human cancer cells. Indian J Biochem Biophys;2014;51(5):416-419.
- Ameen N, Shafi S. Pharmacognostical Review of M. Arvensis Linn. International Journal of Current Research. 2016;8(7):34120-34123.
- Jain D, Pathak N, Khan S, et al. Evaluation of cytotoxicity and anticarcinogenic potential of Mentha leaf extracts. Int J Toxicol. 2011;30(2):225-236.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.