ISSN: 0973-7510
E-ISSN: 2581-690X
Azadirachta indica, generally called as neem, margosa or Indian lilac is an Indian subcontinent native species, it ie well known for its various bioactivity In this study, neem leaves were collected and extracted. The extract was subjected for TLC and antibacterial activity against Pseudomonas aeruginosa. The extract was subjected for green synthesis of silver nanoparticles and it was able to reduce silver to silver nanoparticle of size around 65nm. The silver nanoparticle was also exhibiting antibacterial activity against P.aeruginosa.
Neem extracts, silver nanoparticles, antibacterial activity.
Over two millennia, neem trees products have been utilized in India as traditional medicines due to their novel properties1. Basically, neem is called arishtha in Sanskrit2,3 which means ‘reliever of sickness’ 3 and in India, Neem is also called as the ‘village pharmacy’ 4 for its properties to relieve from various infections, pains and fever. These most popularly known Neem tree is scientifically called as Azadirachta indica and is found in tropical regions indigenous to Indian subcontinents5 . The other species closely related to A. indica is Melia azedarac also known as the Persian lilac3. The biological activities of neem include anti-inflammatory, anti-pyretic, hypoglycaemic, anti-fungal, anti-gastric ulcer, diuretic, antibacterial, antitumour, antimalarial, anticancer, hypolipidemic etc1,3,6,7. Thus in Ayurveda, Neem (Nimba) has been long used for the control of malarial fever, blood disorders, leprosy, wounds, eye disorders and ulcer8 and hence is referred to as ‘Sarva-Rogha-Nirvarini’ which means ‘curing ailment’ in Ayurveda3,8,9. In a few experimental reports, the different approaches for the production of polymeric resins using neem is documented10. Neem is best known for its pesticide activity11,12. One of the primary mode of action of neem against insects is the disruption of its metamorphosis and the bitter flavour aids in keeping them away from feeding the host plants making it an exemplary anti-feedent and repellant13. Recently neem has acquired a great deal of global attention towards it12 for its versatile ability in medicine and agriculture. As a sign of global acceptance, US national academy of sciences has titled neem as ‘The tree for solving global problems’ 9 and the ability of neem extracts to prevent from tooth decaying, inflammation of gums are proved by various tests in Germany and it is currently exploited as an active ingredient in toothpastes in Germany and India. In 1985, neem based insecticide called Margosan-O was accepted by the Environmental protection agency for non-food uses and ever since various other neem based products were also approved by various administrations and agency for food and feed crops14. Neem can also be used to produce nanoparticles for its ability to reduce the metallic ions to yield nanoparticles15. Silver salts is known widely for its anti-bacterial effects having high toxicity towards micro organisms and is used for the same in many applications like dental work, catheters and burn wounds16, 17. The novel properties of silver are also exploited in the field of nanotechnology. Nanoparticles are clusters of atoms that are very small ranging from the size 1-100nm and are known for their high surface area to volume ratio which aids its applications in many fields18. Silver nanoparticles (AgNPs) are used as anti-microbial agents in health, food, textile industries and their products are also approved by US FDA, US EPA, SIAA of Japan, FITI testing and research institute, etc19. Though there are various ways to synthesise nanoparticles, the most non-toxic and eco-friendly method is the bio-based method which utilises various biologically products such as microbes and plants20,21. Hence this study focuses on the evaluation of the bioactivity of the neem extract and its ability to produce silver nanoparticles. The antibacterial effect of both the extract and the AgNPs is also discussed.
Chemicals used
All the solvents and chemicals used, chloroform, ethanol, methane, dichloromethane, hexane, iodine, silver nitrate, DPPH (1, 1-diphenyl 2-picrylhydrazyl) were of analytical grade. Sterile distilled water was used throughout the procedure.
Sample collection and extraction
Leaves of A. indica was collected from Chennai, Tamil Nadu, India and cleaned, then it was shade dried. The powdered leaves were added with ethanol and left for 3 days. The solvent was then filtered using Whatmann no.1 filter and then dried.
Thin layer chromatography
Thin Layer Chromatography was performed with TLC silica plate (Merk F245) for the sample and various solvents like chloroform, methane, ethanol, dichloromethane and hexane were used as mobile phase. The plates were exposed to iodine
Agar well diffusion assay
Agar well diffusion method was done to identify and evaluate the anti-bacterial activity of the extract obtained against Pseudomonas aeruginosa22.
Synthesis of AgNPs
The extract was mixed with 3mM Silver nitrate solution in water in the ratio of 1:5 and was kept in the dark for 24h in room temperature in order to produce and settle silver nanoparticles.
Characterization of AgNPs
The neem extract and the nanoparticles so produced were subjected to UV-Visible spectroscopy (Shimadzu 1800, Japan) and Fourier Transform Infra-red Spectroscopy (Shimadzu, Japan). Silver nanoparticles were subjected to EDX spectroscopy and Scanning electron microscopy (SEI and BSI).
Agar well diffusion assay of AgNPs
The nanoparticles were subjected to agar well diffusion assay to evaluate the antibacterial activity of it against Pseudomonas aeruginosa 22 with a concentration range from 2 to 16µg/ml. The positive control used was Ciproflaxacin.
Thin layer chromatography
The ethanol extracted samples were run using TLC with various mobile phases and the plates were exposed to iodine. Four bands were seen when chloroform was used as the mobile phase (Figure 1c).
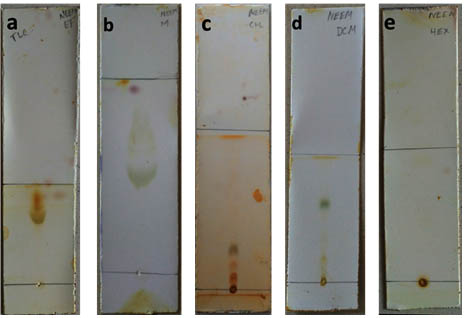
Fig. 1. TLC ran with different mobile phase and exposed to iodine a) Ethanol b) Methanol c) Chloroform d) Dichloromethane e) Hexane
Agar well diffusion assay
When the extract was subjected to antibacterial assay, it was found that the concentrations above 15µg/ml were acting against the bacterial cultures from the zone of inhibition (Table. 1).
Table (1):
Antibacterial activity of neem extract against Pseudomonas aeruginosa
Concentration (µg/ml) |
Zone of Inhibition (cm) |
|---|---|
+ve control |
4.4 |
-ve control |
– |
5 |
– |
10 |
– |
15 |
0.4 |
20 |
0.6 |
Characterisation of AgNPs
The absorbance spectra for the neem extract and the nanoparticles were obtained and it was found that the peaks near 360, 375, 380 and 440nm in the spectrum obtained for neem extract (Figure 2a) had a shift to 385, 440, 495 and 500nm in the spectrum obtained for the AgNPs (Figure 2b). In another study24 on the biologically synthesised nanoparticles, absorption maxima was seen at the range of 440-500nm which is in par with the current study.
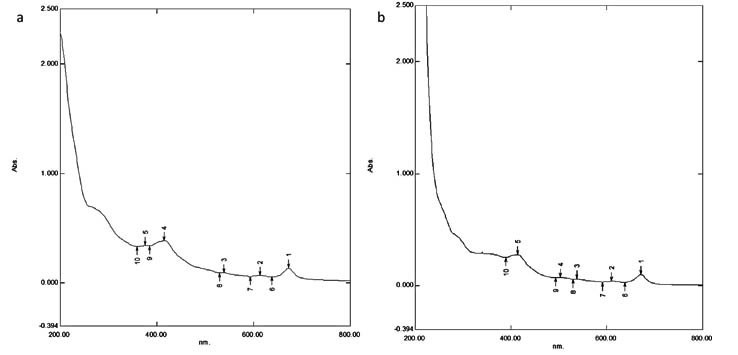
Fig. 2. UV-Visible spectroscopy analysis; a) Neem extract b) silver nanoparticle
Table (2):
Antibacterial activity of silver nanoparticles
Concentration (µg/ml) |
Zone of Inhibition (cm) |
|---|---|
+ve control |
2.7 |
-ve control |
– |
2 |
0.4 |
4 |
0.5 |
8 |
0.6 |
16 |
0.6 |
The IR spectrum of both the neem extract and the neem based silver nanoparticles were evaluated (Figure 3a, b). They both had a few bands in common with amide groups (at around 1640 cm–1)25, hydroxyl group ( around 3330 and 1045 cm–1)26. The extract was found to have band at 1085 which corresponds to the -C-N bonds of alcohols, esters, ethers and carboxylic acid while the AgNP had bands at 1339 and 1396 which corresponds to the C-N stretching of aromatic amine groups27. From the IR specctrum it can be confirmed that the amino acid residue and the protein groups present might have the strongest ability to bind with the metal nanoparticle succesfully aiding in both formation and stabilisation of the particles28.
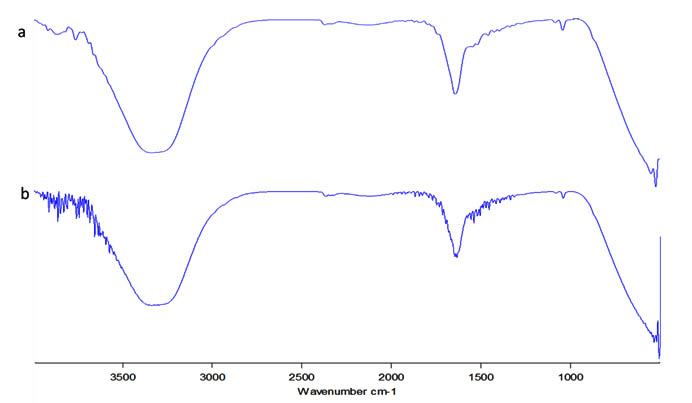
Fig. 3. FTIR analysis; a) Neem extract b) Silver nanoparticle
The spectrum obtained confirms the presence of silver in the analysed sample with the presence of the typical range for the absorption of metallic silver nanocrystals at 3KeV (Figure 4b). The weak signals showing the presence of C, O suggests the capping of AgNPs with the organic compound from neem extract29,30.
The Scanning electron micrographs of the silver nanoparticle shows the size of the ranging from 46 to 94nm and the presence of highly aggregated nanoparticles. (Figure 4a). In a study, less than 20nm spherical nanoparticles were produced using neem gum30 and 12nm sized nanoparticles were prepared using neem plant extracts31.
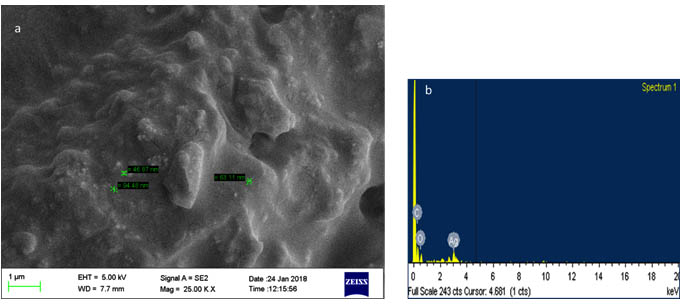
Fig. 4. SEM-EDX analysis of silver nanoparticle (a) SEM (b) EDX
Agar well diffusion assay of AgNPs
From Figure 5, it is evident that the antibacterial activity of the AgNPs increased with the increase in concentration when checked against Pseudomonas aeruginosa. This shows that the nanoparticles are bactericidal.
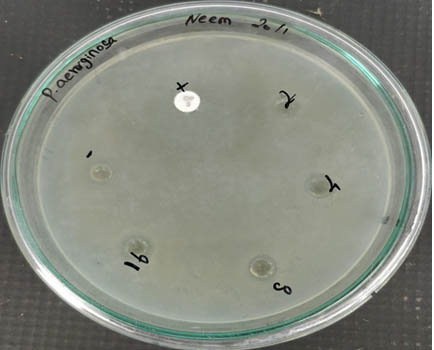
Fig. 5. Antibacterial activity of silver nanoparticle against Pseudomonas aeruginosa
From the above study it is clear that the neem extract and its silver nanoparticles have antibacterial activity against P.aeruginosa. The nanoparticles were found to be capped by the organic compounds present in the neem extract which could be a reason for the production of nanoparticles and also for its stability.
- Kumar, R., Mehta, S., Pathak, S. R. Bioactive constituents of neem. In Synthesis of Medicinal Agents from Plants. 2018; 75-103.
- Girish, K., & Shankara Bhat, S. Neem–a green treasure. Electronic journal of Biology, 2008; 4(3), 201-111.
- Agrawal, D. P.: Medicinal properties of neem: new findings. History of Indian science and 2001.
- Prashanth, G. K., & Krishnaiah, G. M. Chemical composition of the leaves of Azadirachta indica Linn (Neem). International Journal of Advancement in Engineering and Technology, Management and Applied Science. 2014; 1: 21-31.
- Joshi, M., Ali, S. W., Rajendran, S. Antibacterial finishing of polyester/cotton blend fabrics using neem (Azadirachta indica): a natural bioactive agent. Journal of Applied Polymer Science. 2007; 106(2), 793-80.
- SU, M., & Mulla, M. S.: Activity and biological effects of neem products against arthropods of medical and veterinary importance. Journal of the American Mosquito Control Association, 1999; 15(2), 133-152.
- Subapirya R, Nagini S. Medicinal properties of Neem leaves: a review. Curr Med Chem Anticancer Agents. 2005; 5: 149-156.
- Govindarajan, R., Vijayakumar, M., & Pushpangadan, P. Antioxidant approach to disease management and the role of ‘Rasayana’herbs of Ayurveda. Journal of ethnopharmacology,. 2005; 99(2): 165-178.
- Sultana, S., Khan, M. A., Ahmad, M., Bano, A., Zafar, M., Shinwari, Z. K. Authentication of herbal medicine neem (Azadirachta indica A. Juss.) by using taxonomic and pharmacognostic techniques. Pakistan Journal of Botany. 2011; 43: 141-150.
- Rahman, M. M., Ho, K., & Netravali, A. N. Bio based polymeric resin from agricultural waste, neem (Azadirachta indica) seed cake, for green composites. Journal of Applied Polymer Science. 2015; 132(1).
- Siddiqui, B. S., Afshan, F., Gulzar, T., Sultana, R., Naqvi, S. N., Tariq, R. M: Tetracyclic triterpenoids from the leaves of Azadirachta indica and their insecticidal activities. Chem Pharm Bull. 2003; 51: 415-417.
- Gajalakshmi, S., & Abbasi, S. A. Neem leaves as a source of fertilizer-cum-pesticide vermicompost. Bioresource Technology,. 2004; 92(3), 291-296.
- Waghmare, J. T., Ware, A. M., Momin, S. A. Neem oil as pesticide. Journal of dispersion science and technology. 2007; 28(2), 323-328.
- National Research Council. Neem: a tree for solving global problems. The Minerva Group, Inc.. 2002.
- Lalitha, A., Subbaiya, R., & Ponmurugan, P. Green synthesis of silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. Int J Curr Microbiol App Sci. 2013; 2(6), 228-235.
- Tripathi, A., Chandrasekaran, N., Raichur, A. M., & Mukherjee, A. Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leaves. Journal of Biomedical Nanotechnology. 2009; 5(1), 93-98.
- Sondi, I., & Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. Journal of colloid and interface science. 2004; 275(1), 177-182.
- Rai, M., Yadav, A., & Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnology advances. 2009; 27(1): 76-83.
- El-Nour, K. M. A., Eftaiha, A. A., Al-Warthan, A., Ammar, R. A. Synthesis and applications of silver nanoparticles. Arabian journal of chemistry. 2010; 3(3): 135-140.
- Bar, H., Bhui, D. K., Sahoo, G. P., Sarkar, P., Pyne, S., Misra, A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2009; 348(1-3): 212-216.
- Singh, A., Jain, D., Upadhyay, M. K., Khandelwal, N., & Verma, H. N. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig J Nanomater Bios,. 2010; 5(2): 483-489.
- Lalitha, A., Subbaiya, R., Ponmurugan, P. Green synthesis of silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. Int J Curr Microbiol App Sci. 2013; 2(6): 228-235.
- Tripathy, A., Raichur, A. M., Chandrasekaran, N., Prathna, T. C., Mukherjee, A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. Journal of Nanoparticle Research. 2010; 12(1): 237-246.
- Tripathi, A., Chandrasekaran, N., Raichur, A. M., & Mukherjee, A. Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leaves. Journal of Biomedical Nanotechnology. 2009; 5(1): 93-98.
- M.M. Ganesh Babu, P. Gunasekaran Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate Colloid Surf. B. 2009; 74: 191-195.
- P.D. Shashi, L. Manu, S. Mika Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa Colloid Surf. A, 2010; 364: pp. 34-41.
- Mallikarjuna, K., Narasimha, G., Dillip, G. R., Praveen, B., Shreedhar, B., Lakshmi, C. S., et al. Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization. Digest Journal of Nanomaterials and Biostructures. 2011; 6(1): 181-186.
- Sathyavathi, R., Krishna, M. B., Rao, S. V., Saritha, R., & Rao, D. N. Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Advanced science letters. 2010; 3(2): 138-143.
- Chandramohan, B., Murugan, K., Panneerselvam, C., Madhiyazhagan, P., Chandirasekar, R., Dinesh, D., et al. Characterization and mosquitocidal potential of neem cake-synthesized silver nanoparticles: genotoxicity and impact on predation efficiency of mosquito natural enemies. Parasitology research, 2016; 115(3); 1015-1025.
- Velusamy, P., Das, J., Pachaiappan, R., Vaseeharan, B., Pandian, K. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Industrial crops and products. 2015; 66: 103-109.
- Arumugam, N., Thulasinathan, B., Pasubathi, R., Thangavel, K., Muthuramalingam, J. B., & Arunachalam, A. Biogenesis of silver nanoparticles using selected plant leaf extract; characterization and comparative analysis of their antimicrobial activity. Nanomedicine Journal. 2017; 4(4): 208-217.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


