ISSN: 0973-7510
E-ISSN: 2581-690X
Elizabethkingia, a genus of bacteria that includes opportunistic human pathogens, poses significant challenges in healthcare due to its association with high case-fatality rates worldwide. This study aims to assess the antibacterial activity of Aronia melanocarpa (black chokeberry) extracts against Elizabethkingia species, such as Elizabethkingia meningoseptica (isolated from Institut Jantung Negara (IJN)), E. meningoseptica (from National Collection of Type Cultures (NCTC)), and Elizabethkingia anopheles. Aqueous, ethanolic, and methanolic extracts of A. melanocarpa were examined for antibacterial activity using agar well diffusion, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) tests. The sensitivity of each bacterial strain to different extract concentrations was evaluated. The aqueous extracts showed negligible antibacterial activity against all tested strains. However, the methanolic and ethanolic extracts demonstrated significant antibacterial efficacy. Notably, both extracts inhibited the growth of E. meningoseptica and E. anophelis, with methanolic extracts showing the highest potency in MIC and MBC assays. The findings suggest that methanolic and ethanolic extracts of A. melanocarpa hold potential as alternative antimicrobial agents or candidates for developing pharmaceutical treatments targeting antibiotic resistant Elizabethkingia infections. Further studies are necessary to investigate their mechanisms of action and clinical applications.
Elizabethkingia, Aronia melanocarpa, Antibacterial Activity, Antibiotic Resistance, Alternative Therapeutics
Elizabethkingia is a Gram-negative bacterial genus first formally described in 2005. Species within this genus are characterized by their obligate aerobic nature, nonmotility, glucose-nonfermenting metabolism, and pale-yellow pigmentation.1 Notably, Elizabethkingia species have been found to inhabit diverse ecological niches, including soil, freshwater reservoirs, riversides, and healthcare environments such as intensive care units. Among the various species identified, Elizabethkingia meningoseptica and Elizabethkingia anophelis are important opportunistic bacteria that can cause high rates of morbidity and mortality.2
E. meningoseptica, initially identified in 1959 and reclassified under the genus in 2005, has been linked to infections such as urinary tract infections, skin and soft tissue infections, pneumonia, bacteremia, and catheter-related infections, particularly in vulnerable populations such as neonates, infants, and immunocompromised individuals. On the other hand, E. anophelis, first reported in 2011, has emerged as a pathogen associated with bloodstream and respiratory infections, contributing to notable outbreaks since its discovery.1 Clinical manifestations of Elizabethkingia infections typically include fever, respiratory distress, cellulitis, and septicemia, posing diagnostic challenges exacerbated by the intrinsic antimicrobial resistance exhibited by these organisms.3
Despite advancements in medical science, infections caused by pathogenic Elizabethkingia species remain notoriously difficult to treat, often resulting in poor clinical outcomes and elevated case-fatality rates. The limited therapeutic options available underscore the urgent need for an enhanced understanding of the epidemiology, clinical manifestations, and antimicrobial resistance mechanisms associated with these pathogens.4
Antimicrobials, commonly referred to as antibiotics, serve an important role in modern healthcare, serving as crucial instruments in the treatment of bacterial infections.5 However, the escalating threat of antibiotic resistance presents a formidable challenge to global public health, posing one of the most pressing risks to human well-being.6,7 In response to this crisis, an intricate approach encompassing various strategies has been advocated for the control of bacteria, especially Gram-negative species. These strategies include the development of antimicrobial adjuvants, structural alteration to available antibiotics, as well as the exploration of novel organic compounds with distinctive action mechanisms, aimed at targeting vulnerabilities in antibiotic-resistant pathogens.8,9
Aronia melanocarpa, well-known as black chokeberry, belongs to the Rosaceae family. It is native to eastern North America, including the United States and portions of Canada, as well as many European nations.10 Renowned for its nutritional richness, it serves as an abundant source of critical nutrients like organic acids, carbohydrates, proteins, lipids, minerals, and vitamins.11 Moreover, A. melanocarpa stands out as a reservoir of bioactive compounds, notably polyphenols like proanthocyanidins, anthocyanins, flavonoids, and phenolic acids, which confer a myriad of health advantages such as antioxidant, anti-inflammatory, antiviral, anticancer, anti-atherosclerotic, hypotensive, and antiplatelet properties.12,13 Its versatility extends beyond nutritional value, finding widespread application in the food industry for the production of various products such as nectars, juices, powders, jams, syrups, fruit desserts, jellies, and dietary supplements. Despite its well-documented antioxidant properties, the medicinal potential of this plant remains relatively understudied.14
Hence, the main aim of this study is to assess the antimicrobial efficacy of A. melanocarpa extracts obtained through distinct solvent extractions-ethanol, methanol, and aqueous-against strains of E. meningoseptica and E. anophelis. Both clinical isolates sourced from Malaysian hospitals and standard strains were included in the study. Furthermore, the study aimed to characterize the antimicrobial sensitivity profiles of each extract using minimum bactericidal concentration (MBC) and minimum inhibitory concentration (MIC) assays, elucidating their effectiveness against these pathogenic microbes.
Study design
The current study has been conducted within the laboratories of Management and Science University (MSU). Bacterial samples encompassed three distinct strains: two pure strains of E. meningoseptica procured from the National Collection of Type Cultures, UK (NCTC 10568), and Institut Jantung Negara (IJN)-Malaysia, while the third sample consisted of E. anophelis isolated from a local hospital and confirmed through identification by IJN.
Plant parts collection
The dried fruits of A. melanocarpa, sourced from a local supplier in Selangor, Malaysia, were obtained from Natherm Group Sdn. Bhd., 13A, Jalan Besi 1/1, Taman Perindustrian Sungai Purun, 43500 Semenyih, Selangor, Malaysia (GPS: 2.93954, 101.82553). These fruits underwent a purification procedure and were then processed into chokeberry fine powder, which was utilized for various extractions as outlined in previous methodologies.15
Aqueous extraction preparation
For the aqueous extraction, 40 g of A. melanocarpa powder was boiled in 400 mL of distilled water. After evaporation, one-fourth of the initial extraction volume was collected. The boiled mixture was filtered through cheesecloth and then centrifuged at 5000 rpm for 15 minutes. The supernatant was further filtered using Whatman No. 1 filter paper (UK, HP7 9NA), repeating this process twice under stringent aseptic conditions. The resulting filtrate was transferred to fresh sterilized dark bottles and stored at 4 °C until further use, following established protocols.16,17
Organic extraction preparation
Approximately 50 g of the dried powder was soaked in 200 ml (50% ethanol and 50% methanol, respectively) with continuous agitation for 48 hours, then filtered through cheesecloth. The filtrates were centrifuged for 10 minutes at 9000 rpm before being filtered using Whatman No. 1 filter paper to eliminate any unsuitable residues. To remove any leftover organic solvents, the filtrates were evaporated and dried under decreased pressure at 40 °C with a rotating vacuum evaporator. The extracts were dissolved in 10% dimethyl sulfoxide (DMSO). After the solvents had fully evaporated, the crude extraction yields were weighed to determine the concentrations (5 g, 10 g, and 20 g) and kept in a refrigerator at 4 °C. Subsequently, the different concentrations of alcoholic extracts were tested through a series of pilot studies, confirming the use of the 20 g concentration for this study. The yield percentages were calculated with the formula (R/S) x 100, where R is the weight of the recovered plant residues and S is the weight of the input plant material.17
Agar preparation
The preparation of different agar media used in this study followed the Clinical and Laboratory Standards Institute (CLSI) Guidelines and the manufacturer’s standard procedures.18 For the turbidity standard for inoculum preparation, CLSI recommends standardizing the inoculum density for antimicrobial susceptibility testing. Mueller-Hinton Broth was used to dilute the Gram-negative broth culture to a turbidity equivalent to 0.5 McFarland standard, which equals (1-2) × 108 CFU/mL.
Antibacterial susceptibility test (Agar Well Diffusion Method)
Plant extracts’ antibacterial activity is tested using the agar well diffusion technique.19 The agar plates were incubated at 37 °C for 24 hours. The inhibition zones were measured with a vernier caliper and recorded against the various concentrations of A. melanocarpa extracts.20
Minimum Inhibitory Concentrations (MIC) test
MIC of A. melanocarpa extracts was determined using the 96-well microtiter plate dilution method. A serial dilution of ethanol, methanol, and aqueous extracts, starting from 20,000 µg/ml down to 39.06 µg/ml, was prepared in Mueller-Hinton broth. Bacterial inocula were standardized to 1-2 × 108 CFU/ml following CLSI guidelines, adjusted to a 0.5 McFarland standard, and further diluted to 5 × 105 CFU/ml in each well. The microtiter plates were incubated at 37 °C for 24 hours. MIC values were determined based on visual turbidity, with clear wells indicating bacterial inhibition.16
Minimum Bactericidal Concentration (MBC) test
To determine the MBC, samples from clear wells (indicating no visible growth) were streaked onto sterile nutrient agar plates. The plates were incubated at 37 °C for 24 hours and then examined for bacterial growth. The lowest concentration of A. melanocarpa extract that showed no bacterial growth was recorded as the MBC.21
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 22® (IBM Corp., USA). Mean values and standard deviations were calculated for the inhibition zones of each extract. One-way analysis of variance (ANOVA) was employed to determine statistically significant differences among groups, followed by Tukey’s Honestly Significant Difference (HSD) post hoc test for pairwise comparisons. Results were considered statistically significant at p < 0.05. Data visualizations, including tables, charts, and graphs, were generated using Microsoft Excel.
Antimicrobial activity of A. melanocarpa extractions
A. melanocarpa extracts were prepared in triplicate using three different extraction solvents: 50% ethanol, 50% methanol, and hot water (aqueous extraction). The physical characteristics of each extract were documented and summarized in Table 1.
Table (1):
Physical Appearance of Different A. melanocarpa Extracts
Extracts |
Color |
Consistency |
|---|---|---|
Aqueous |
Pink |
Liquid |
Ethanol 50% |
Purple |
Viscous |
Methanol 50% |
Purple |
Viscous |
A series of pilot studies were conducted before the main experiment to identify the most effective antibacterial concentration among the tested ranges. The results indicated that 20 g/mL exhibited the highest antibacterial activity. Therefore, this concentration was selected for further experiments (Table 2).
Table (2):
Bactericidal Activity of A. melanocarpa Extracts Against E. meningoseptica and E. anophelis
| Organism Extract | Crude | Concentration Of Extracts (g/mL) | ||
|---|---|---|---|---|
| 20 | 10 | 5 | ||
| E. meningoseptica (IJN) | Aqueous | – | – | – |
| Ethanol | + | – | – | |
| Methanol | + | – | – | |
| E. meningoseptica (NCTC) | Aqueous | – | – | – |
| Ethanol | + | – | – | |
| Methanol | + | – | – | |
| E. anophelis (IJN) | Aqueous | – | – | – |
| Ethanol | + | – | – | |
| Methanol | + | – | – | |
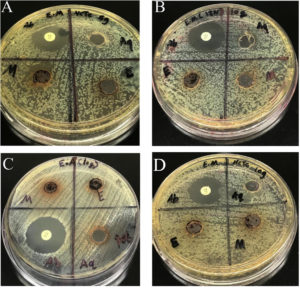
Figure 1. Bactericidal Activity of A. melanocarpa Extracts Against E. meningoseptica and E. anophelis After 24 Hours of Incubation. (A) E. meningoseptica (NCTC) at 5 g/mL, (B) E. meningoseptica (IJN) at 10 g/mL, (C) E. anophelis at 10 g/mL, and (D) E. meningoseptica (NCTC) at 10 g/mL. The antibacterial effects of A. melanocarpa extracts were assessed based on bacterial inhibition after 24 hours of incubation
Figure 1 illustrates the bactericidal activity of A. melanocarpa extracts against E. meningoseptica (IJN), E. meningoseptica (NCTC), and E. anophelis at concentrations of 5 g/mL and 10 g/mL after 24 hours of incubation. This figure provides a comparative visual representation of the antimicrobial efficacy of different extracts.
Antimicrobial sensitivity test
The results indicated that the 50% methanol extract exhibited the broadest antimicrobial activity, with the highest zone of inhibition observed against E. anophelis (16.7 mm), followed by E. meningoseptica (NCTC) (14.9 mm) and E. meningoseptica (IJN) (14.1 mm). In comparison, the 50% ethanol extract demonstrated inhibitory effects against E. meningoseptica (NCTC) (12.9 mm) and E. anophelis (12.6 mm). However, the aqueous extract showed no antibacterial activity against any of the tested strains, including E. meningoseptica (IJN), E. meningoseptica (NCTC), and E. anophelis (Figures 2-4).
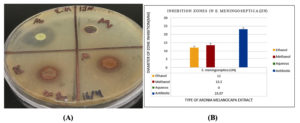
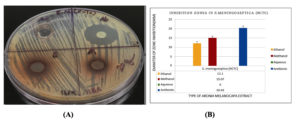 Figure 2. (A) Antimicrobial sensitivity test of E. meningoseptica (NCTC), showing no inhibition zone for the aqueous extract, while methanolic and ethanolic extracts exhibited clear inhibition zones ranging from 12-17 mm. Vancomycin (VA) as a positive control showed an inhibition zone of 23.3 mm. (B) Inhibition zone measurements of aqueous, methanolic, and ethanolic extracts, along with the positive control, against E. meningoseptica (NCTC)
Figure 2. (A) Antimicrobial sensitivity test of E. meningoseptica (NCTC), showing no inhibition zone for the aqueous extract, while methanolic and ethanolic extracts exhibited clear inhibition zones ranging from 12-17 mm. Vancomycin (VA) as a positive control showed an inhibition zone of 23.3 mm. (B) Inhibition zone measurements of aqueous, methanolic, and ethanolic extracts, along with the positive control, against E. meningoseptica (NCTC)Figure 3. (A) Antimicrobial sensitivity test of E. meningoseptica (IJN), showing no inhibition zone for the aqueous extract, while methanolic and ethanolic extracts exhibited clear inhibition zones ranging from 12-14 mm. Vancomycin (VA) as a positive control showed an inhibition zone of 20.2 mm. (B) Inhibition zone measurements of aqueous, methanolic, and ethanolic extracts, along with the positive control, against E. meningoseptica (IJN)
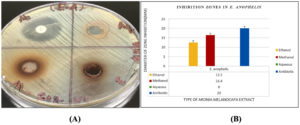
Figure 4. (A) Antimicrobial sensitivity test of E. anophelis, showing no inhibition zone for the aqueous extract, while methanolic and ethanolic extracts exhibited clear inhibition zones ranging from 12-14 mm. Vancomycin (VA) as a positive control showed an inhibition zone of 20.8 mm. (B) Inhibition zone measurements of aqueous, methanolic, and ethanolic extracts, along with the positive control, against E. anophelis
The statistical analysis of the data (Tables 3-5) presented descriptive statistics of the inhibition zones of various A. melanocarpa extracts against each tested strain. The results were considered statistically significant at a p ≤ 0.05 significance level.
Table (3):
Descriptive analysis of the inhibition zones of various A. melanocarpa extracts against E. meningoseptica (NCTC)
| Descriptive Analysis – Zone of Inhibition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extract | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Means | |||
| Lower Bound | Upper Bound | Minimum | Maximum | |||||
| Methanol | 3 | 15.07 | 0.21 | 0.12 | 14.55 | 15.58 | 14.90 | 15.30 |
| Ethanol | 3 | 12.10 | 0.20 | 0.12 | 11.605 | 12.60 | 11.90 | 12.30 |
| Aqueous | 3 | 0.00 | 0.00 | 0.00 | 0.005 | 0.00 | 0.00 | 0.00 |
| Antibiotic | 3 | 20.43 | 0.15 | 0.09 | 20.055 | 20.81 | 20.30 | 20.60 |
| Total | 12 | 11.90 | 7.83 | 2.26 | 6.93 | 16.87 | 0.00 | 20.60 |
| Anova: Zone of Inhibition | ||||||||
| Zone of Inhibition | Sum of Squares | df | Mean Square | F | Sig. | |||
| Between Groups | 673.49 | 3 | 224.50 | 8418.58 | 0.00 | |||
| Within Groups | 0.21 | 8 | 0.027 | |||||
| Total | 673.70 | 11 | ||||||
* The mean difference is significant at p ≤ 0.05
Table (4):
Descriptive analysis of the inhibition zones of various A. melanocarpa extracts against E. meningoseptica (IJN)
| Descriptive Analysis – Zone of Inhibition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extract | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Means | |||
| Lower Bound | Upper Bound | Minimum | Maximum | |||||
| Methanol | 3 | 13.50 | 0.79 | 0.45 | 11.53 | 15.47 | 12.60 | 14.10 |
| Ethanol | 3 | 12.00 | 0.10 | 0.06 | 11.75 | 12.25 | 11.90 | 12.10 |
| Aqueous | 3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Antibiotic | 3 | 23.07 | 0.21 | 0.12 | 22.55 | 23.58 | 22.90 | 23.30 |
| Total | 12 | 12.14 | 8.57 | 2.47 | 6.70 | 17.59 | 0.00 | 23.30 |
| Anova: Zone of Inhibition | ||||||||
| Zone of Inhibition | Sum of Squares | df | Mean Squares | F | Sig. | |||
| Between Groups | 805.92 | 3 | 268.64 | 1572.53 | 0.00 | |||
| Within Groups | 1.37 | 8 | 0.171 | |||||
| Total | 807.29 | 11 | ||||||
* The mean difference is significant at p ≤ 0.05
Table (5):
Descriptive analysis of the inhibition zones of various A. melanocarpa extracts against E. anophelis (IJN)
| Descriptive Analysis – Zone of Inhibition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extract | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Means | |||
| Lower Bound | Upper Bound | Minimum | Maximum | |||||
| Methanol | 3 | 16.47 | 0.2082 | 0.12 | 15.95 | 16.98 | 16.30 | 16.70 |
| Ethanol | 3 | 12.50 | 0.1000 | 0.058 | 12.25 | 12.75 | 12.40 | 12.60 |
| Aqueous | 3 | 0.00 | 0.0000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Antibiotic | 3 | 20.47 | 0.3055 | 0.18 | 19.71 | 21.23 | 20.20 | 20.80 |
| Total | 12 | 12.36 | 8.0137 | 2.31 | 7.27 | 17.45 | 0.00 | 20.80 |
| Anova: Zone of Inhibition | ||||||||
| Zone of Inhibition | Sum of Squares | df | Mean Squares | F | Sig. | |||
| Between Groups | 706.12 | 3 | 235.37 | 6419.24 | 0.00 | |||
| Within Groups | 0.29 | 8 | 0.04 | |||||
| Total | 706.41 | 11 | ||||||
* The mean difference is significant at p ≤ 0.05
The Minimum Inhibitory Concentration (MIC)
Different concentrations of active crude extracts were evaluated in a 96-well microtiter plate using the Micro Broth Dilution Assay, as recommended by CLSI.18 The results showed that preliminary screening identified ethanol and methanol extracts as having the highest antimicrobial activity, and these extracts were further analyzed to determine the MIC for each bacterial sample. The concentration-dependent effects of the active plant extracts are summarized in Table 6. The MIC values of the methanol extract were detected at 5000 µg/mL for E. meningoseptica (IJN), 2500 µg/mL for E. meningoseptica (NCTC), and 1250 µg/mL for E. anophelis. Meanwhile, the MIC values of the ethanol extract were detected at 10,000 µg/mL for E. meningoseptica (IJN), 2500 µg/mL for E. meningoseptica (NCTC), and 625 µg/mL for E. anophelis. The inhibitory effects of the extracts varied depending on the bacterial species, strain variations, and type of organic solvent. Both extracts demonstrated bacteriostatic properties, with methanol exhibiting a stronger inhibitory effect against E. anophelis, while ethanol also showed significant inhibition against E. anophelis.
Table (6):
The MIC of methanol, ethanol, and aqueous extracts against each susceptible bacterial strain
| Organism | Extract | The concentration of Extracts in µg/mL | MIC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20000 | 10000 | 5000 | 2500 | 1250 | 625 | 312 | 156.25 | 78.125 | 39.06 | +ve | -ve | Result | ||
| E. meningoseptica (IJN) | Aqueous | – | + | + | + | + | + | + | + | + | + | + | – | 20000 |
| Ethanol | – | – | – | + | + | + | + | + | + | + | + | – | 5000 | |
| Methanol | – | – | – | + | + | + | + | + | + | + | + | – | 5000 | |
| E. meningoseptica (NCTC 10568) | Aqueous | – | + | + | + | + | + | + | + | + | + | + | – | 20000 |
| Ethanol | – | – | – | – | + | + | + | + | + | + | + | – | 2500 | |
| Methanol | – | – | – | – | + | + | + | + | + | + | + | – | 2500 | |
| E. anophelis | Aqueous | – | + | + | + | + | + | + | + | + | + | + | – | 20000 |
| Ethanol | – | – | – | – | + | + | + | + | + | + | + | – | 2500 | |
| Methanol | – | – | – | – | + | + | + | + | + | + | + | – | 2500 | |
*. All values are expressed in µg/mL; (–) represents “No Growth Observed”; (+) represents “Growth Observed
Minimum Bactericidal Concentrations (MBC)
The MBC was determined by subculturing samples from the wells showing no visible bacterial growth in the MIC assay onto fresh Mueller-Hinton agar plates. The plates were then incubated to assess the presence or absence of bacterial colonies. The lowest concentration at which no bacterial growth occurred was recorded as the MBC. The methanol extract exhibited potential bactericidal activity against E. meningoseptica (IJN) with an MBC of 10,000 µg/mL; for E. meningoseptica (NCTC), the MBC was also 10,000 µg/mL, and for E. anophelis, it was 2,500 µg/mL. The ethanol extract showed bactericidal effects with identical MBC values for E. meningoseptica (IJN and NCTC) at 10,000 µg/mL, and 2,500 µg/mL for E. anophelis.
The findings of this study clearly indicate that hot distilled water is not an effective solvent for antimicrobial testing, as it failed to inhibit bacterial growth. This aligns with existing literature, which suggests that organic solvents, particularly ethanol and methanol, are more efficient in extracting antimicrobial compounds from plants. These solvents effectively dissolve aromatic and saturated organic compounds, which are often responsible for antimicrobial activity.22,23 Among the tested extracts, 50% methanol demonstrated the highest phenolic compound content, reinforcing its reputation as the preferred solvent for plant extractions.24,25 Our results suggest that A. melanocarpa methanolic and ethanolic extracts hold potential for future applications in treating infections caused by Elizabethkingia species. This supports previous work by Park and Hong, who proposed that these extracts could be useful in food preservation due to their antimicrobial properties.17 Additionally, studies have documented the antibacterial effects of A. melanocarpa against a range of pathogens, including Bacillus cereus, Pseudomonas aeruginosa, and Staphylococcus aureus. Mostafa et al. reported that ethanol extracts were particularly potent against foodborne pathogens when tested using the agar well diffusion method. These results are consistent with our findings and support the continued investigation of A. melanocarpa as a plant-based antimicrobial agent.21
Interestingly, our MIC results indicate that E. anophelis is more susceptible to A. melanocarpa extracts compared to E. meningoseptica (NCTC) and E. meningoseptica (IJN). The ethanolic extract at 5% concentration (125 mg/mL) produced an inhibition zone of 11 mm, which, while lower than the 15 mm zone observed with vancomycin, still shows promising activity. Notably, the ethanol extract produced inhibition zones ranging from 10 mm (5% v/v) to 12 mm (10% v/v), demonstrating a dose-dependent effect.
Comparing our MIC findings with previous research, Daoutidou et al. reported that E. coli exhibited a 13 mm inhibition zone at a 10% aqueous extract concentration (250 mg/mL).20 Similarly, Denev et al. found that proanthocyanidins from A. melanocarpa had a potent MIC (0.156 mg/mL) against Candida albicans, significantly outperforming standard antimicrobial agents.14 Their study attributed A. melanocarpa antibacterial activity primarily to proanthocyanidins, which exhibited a remarkable effect against Proteus vulgaris, surpassing ampicillin’s efficacy by 25-fold. These findings align with our results, further emphasizing the role of phenolic compounds in antimicrobial action.
Given the rising threat of antibiotic resistance, exploring plant-based alternatives is more crucial than ever. A. melanocarpa extracts not only demonstrate antimicrobial potential but also offer a natural alternative for combating pathogenic bacteria. Previous research by Salamon et al. highlighted that the MBC of ethanol extract against S. aureus was 10.0 mg/mL, whereas acetone extracts showed slightly lower efficacy (MBC: 15.0 mg/mL).26 Similar trends have been reported for other plant extracts, including Phaleria macrocarpa,27 Nigella sativa, Eucalyptus, and Swietenia macrophylla, all of which exhibit varying degrees of antimicrobial activity.26,28
One of the key factors contributing to A. melanocarpa antimicrobial efficacy is its rich phenolic content. Previous studies highlight that phenolic compounds not only exhibit direct antimicrobial activity but also interfere with bacterial adhesion to host cells by binding to bacterial cell walls.23,29 This mechanism may explain the inhibitory effect observed in our study.
While our findings add to the growing body of evidence supporting the antimicrobial properties of A. melanocarpa, further research is needed to isolate and characterize the specific bioactive compounds responsible for its activity. Additionally, in vivo studies and clinical trials would be essential to evaluate its therapeutic potential and safety profile. Nevertheless, this study provides a strong foundation for considering A. melanocarpa as a promising candidate for natural antimicrobial agents, potentially contributing to the development of alternative treatments for bacterial infections.
We also explored an alternative route by using A. melanocarpa plant powder with both aqueous and organic solvents to discover new antimicrobial agents. The methanolic and ethanolic extracts effectively inhibited clinically relevant Elizabethkingia species (E. meningoseptica NCTC/IJN and E. anophelis), reinforcing their potential as natural therapies. Although research on A. melanocarpa antimicrobial effects remains limited, our findings align with recent evidence. For example, a study on leaf extracts showed A. melanocarpa exerted bacteriostatic effects and significantly delayed bacterial growth in meat-related pathogens.30 Additionally, detailed investigations of black chokeberry polyphenols found that proanthocyanidins were the most potent antimicrobial agents in the fruit.14 Similar plant-based phenolic extracts have demonstrated notable efficacy: Nigella sativa and Syzygium aromaticum extracts presented minimum bactericidal concentrations between ~9 and 19 mg/mL against S. aureus and Propionibacterium acnes, showcasing the broader relevance of phenolic-rich antimicrobial agents. This supports our hypothesis since A. melanocarpa is among the richest natural sources of phenolics, which are known to disrupt bacterial cell membranes and inhibit adhesion.31 Such mechanisms help explain the significant antibacterial effects observed in our extracts and make a strong case for A. melanocarpa as a promising source of bioactive compounds for drug discovery, particularly in combating antibiotic-resistant infections.
This study highlights the practical potential of A. melanocarpa as a natural antimicrobial agent, particularly in an era of rising antibiotic resistance. While traditionally consumed in processed forms due to its astringent taste, A. melanocarpais rich in bioactive phytochemicals, especially phenolics, flavonoids, and tannins, that exhibit notable antibacterial effects.
Our findings demonstrate that methanolic and ethanolic extracts of A. melanocarpa significantly inhibit the growth of pathogenic Elizabethkingia species. These results suggest important real-world applications, such as the incorporation of A. melanocarpa extracts as natural preservatives in food products to enhance safety and shelf life, as well as their potential development into plant-based therapeutic agents to support or replace conventional antibiotics in the management of multidrug-resistant infections.
To fully translate these findings into practice, further studies should focus on identifying and purifying the most active antimicrobial compounds within the extracts, assessing their toxicity and efficacy in vivo, and exploring formulation strategies for pharmaceutical or nutraceutical use. If successful, A. melanocarpa could emerge as a valuable, sustainable source of antimicrobial agents for both food industry and medical applications.
ACKNOWLEDGMENTS
The authors acknowledge the Faculty of Health and Life Sciences, Management and Science University (MSU), and Department of Medical Laboratory Science (MLS), Komar University of Science and Technology (KUST) for providing necessary facilities and guidance to conduct the research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Lin J-N, Lai C-H, Yang C-H, Huang Y-H. Elizabethkingia Infections in Humans: From Genomics to Clinics. Microorganisms. 2019;7(9):295.
Crossref - Bernardet J-F, Hugo C, Bruun B. The Genera Chryseobacterium and Elizabethkingia. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, eds. The Prokaryotes: Proteobacteria: Delta, Epsilon Subclass. Springer New York; 2006;7:638-676.
Crossref - Feng M, Huang M, Fan Y, Liu G, Zhou S, Zhou J. Clinical Characteristics and Risk Factors for Infection and Death in Critically Ill Patients with Pulmonary Infection with Elizabethkingia Spp. Infect Drug Resist. 2024;17:2673-2683.
Crossref - Jiang B, Zhang W, Deng N, et al. A systematic review of reported symptomatic Elizabethkingia infection cases in children and adults. Acta Tropica. 2025;263:107544.
Crossref - Cook MA, Wright GD. The past, present, and future of antibiotics. Sci Transl Med. 2022;14(657):eabo7793.
Crossref - Deori C, Sonowal T, Das M. Antimicrobial resistance: a looming threat to public health and global well-being. Indian Journal of Community and Family Medicine. 2024;10(1):18-25.
Crossref - Mohammed MF, Kannan HB, Abdalqader M, Alwan MR, Alhoot MA, Ghazi HF. Antibacterial activities of watermelon (Citrullus lanatus) rind and seed extracts against selected gram-positive and gram-negative bacteria. Int J Med Toxicol Legal Med. 2020;23(3and4):95-100.
Crossref - Savanur SS, Gururaj H. Study of antibiotic sensitivity and resistance pattern of bacterial isolates in intensive care unit setup of a tertiary care hospital. Indian J Crit Care Med. 2019;23(12):547-555.
Crossref - Mohammed MF, Raman N, Alhoot MA, Alwan MR. Antibacterial activities of Allium sativum (Garlic) extracts against Staphylococcus aureus and Escherichia coli. Eur J Mol Clin Med. 2020;7(11):526-534.
- Jurikova T, Mlcek J, Skrovankova S, et al. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules. 2017;22(6):944.
Crossref - Sagandyk AT, Liberal A, da Silveira TFF, et al. Nutritional, phytochemical, and bioactive prospects of black chokeberry (Aronia melanocarpa) and saskatoon berry (Amelanchier ovalis) grown in the Republic of Kazakhstan. Appl Food Res. 2024;4(2):100564.
Crossref - Zhu Y, Zhang J-y, Wei Y-l, et al. The polyphenol-rich extract from chokeberry (Aronia melanocarpa L.) modulates gut microbiota and improves lipid metabolism in diet-induced obese rats. Nutr Metab. 2020;17:1-15.
Crossref - Nurnazira H, Nurul Azira I, Ernie Zuraida A. Systematic review on effectiveness of flavonoids against hypercholesterolemia: Insights from in silico, in vitro, and in vivo studies. Food Chem Adv. 2025;7:100981.
Crossref - Denev P, Ciz M, Kratchanova M, Blazheva D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019;284:108-117.
Crossref - Salih ND, Azmi N, Gopalan HK. The Protective Effects Of Phaleria Macrocarpa Leaves Methanol Extract On Pancreatic Islets Histology In Streptozotocin-Induced Diabetic Rats. Sci Int. 2015;27(5):4219-4224.
- Kaushik P, Goyal P. Evaluation of various crude extracts of Zingiber officinale rhizome for potential antibacterial activity: A study in vitro. Adv Microbiol. 2011;1(1):7.
Crossref - Park H-M, Hong J-H. Physiological activities of Aronia melanocarpa extracts on extraction solvents. Food Science and Preservation. 2014;21(5):718-726.
Crossref - Weinstein MP, Patel JB, Burnhman CA, ZImmer BL. Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Standard, Approval CDM-A. M07 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 2018:91.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71-79.
Crossref - Daoutidou M, Plessas S, Alexopoulos A, Mantzourani I. Assessment of antimicrobial activity of pomegranate, cranberry, and black chokeberry extracts against foodborne pathogens. Foods. 2021;10(3):486.
Crossref - Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci. 2018;25(2):361-366.
Crossref - Nortjie E, Basitere M, Moyo D, Nyamukamba P. Extraction methods, quantitative and qualitative phytochemical screening of medicinal plants for antimicrobial textiles: a review. Plants. 2022;11(15):2011.
Crossref - Liepioa I, Nikolajeva V, Jakobsone I. Antimicrobial activity of extracts from fruits of Aronia melanocarpa and Sorbus aucuparia. Environ Exp Biol. 2013;11(4):195-199.
- Radhia A, Hanen N, Abdelkarim B, Mohamed N. Phytochemical screening, antioxidant and antimicrobial activities of Erodium glaucophyllum (L.) L’Herit. J Biomed Sci. 2018;7(4):13-19.
Crossref - Azura A, Nurul Azira I, Faridah Y, Hamzah Mohd S. Expression, purification, and characterization of a recombinant stem bromelain from Ananas comosus. Process Biochemistry. 2011;46(12):2232-2239.
Crossref - Salamon I, Sezer EN, Kryvtsova M, Labun P. Antiproliferative and antimicrobial activity of anthocyanins from berry fruits after their isolation and freeze-drying. Appl Sci. 2021;11(5):2096.
Crossref - Saleh ID, Zain HHM, Ibrahim H, Salih ND, Gopalan HK. The impacts of treatment with newly developed probiotic versus Phaleria macrocarpa leaves extract on the histological features in immunocompromised New Zealand white rabbits. IOP Conf Ser Earth Environ Sci. 2021;761:012096.
Crossref - Basurra RS, Wang SM, Alhoot MA. Nigella sativa (black seed) as a natural remedy against viruses. J Pure Appl Microbiol. 2021;15(1):29-41.
Crossref - Kareem M, Khalaf JM, Hasan MS, Saleh EN, Salih ND. Effects of Eucalyptus alcoholic extracts on pathogenic E. coli, in vitro study. Int J Pharm Res. 2020;12(1):1033-1034.
Crossref - Efenberger-Szmechtyk M, Nowak A, Czyzowska A, Kucharska AZ, Fecka I. Composition and Antibacterial Activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. Leaf Extracts. Molecules. 2020;25(9):2011.
Crossref - Oulahal N, Degraeve P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front Microbiol. 2021;12:753518.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.