ISSN: 0973-7510
E-ISSN: 2581-690X
The current investigation aims to test the susceptibility of human pathogenic clinical isolates and MTCC strains to leaf and seed extracts of Terminalia catappa and Nigella sativa. Disc diffusion assay, micro dilution assay and minimum Bactericidal Concentration investigated the susceptibility of bacteria to the test extracts. The active extract was subjected to phytochemical screening, separation of the phytochemicals by Thin Layer Chromatography, bioactivity guided assay and Time- kill assay. Acetone and methanol extracts of T.catappa revealed, significant inhibition of clinical origin Staphylococcus aureus followed by Proteus vulgaris and the MTCC strains Staphylococcus aureus, Salmonella typhi, Pseudomonas aeroginosa and Bacillus subtilis. Nigella sativa inhibited the growth of clinical origin Staph.aureus and MTCC strain of Staph.aureus, Salmonella typhi and B.subtilis. Minimum inhibitory concentration for all the test bacteria was reported in the range of 5000μg/ml to 9 μg/ml in T. catappa extract. Most sensitive being the clinical isolate Staph. aureus and Proteus vulgaris. The bactericidal concentration for the test bacteria was found to be between 5000μg/ml and 625μg/ml. Phyto-chemical analysis of leaf extracts of T. catappa found to have dominated by polyphenols (Terpenoids, steroids, flavonoids, flavones, saponins and tannins) and N.sativa extracts recorded the presence of alkaloids, proteins and oils and fats. TLC profiling of the acetone extract revealed many antibacterial active bands. Bands having Retention factor 0.47 and 0.52 were active against the test bacteria. Time kill assay of the acetone extract of T. catappa were carried out for the first time. The extract exhibited dose dependent bactericidal and bacteriostatic activity against the clinical isolates.
Combretaceae, Terpenoids, Polyphenols, Thymoquinone, Bioautography, Time- kill assay
Recently there has been increase in the spread of untreatable microbial infections and bacteria cause 90% of the infections1. World Health Organization reports that infectious diseases cause 50% deaths. The non-selective use of synthetically originated antibiotics, microorganisms have led to the development of multidrug resistance, which poses a serious health concern2. The resistance to drug may be caused mostly by the unregulated use of antibiotics and poor hygienic conditions and affects severely in every aspect of life 3. The escalating resistance in microorganisms is due to the phenomenon of genetic mutations or acquired antibiotic resistant genes influenced by ill-suited use of antibiotics1. Additionally antibiotics are associated with unfavorable consequences on the host including hypersensitivity, allergic reactions and immune- suppression4. The multiple drug resistance has enforced researchers in search of new drugs with antimicrobial property from different sources like medicinal plants, which serve as acceptable source of novel antimicrobial agents5. Rational localization of biologically active components from folk medicines and systemic evaluation will result in finding of novel efficacious drugs, which are potentially active against pathogens6. Antimicrobial drugs with efficacious mode of action should be developed in order to surpass the downside of current antimicrobial drugs7. A large number of medicinal plants are reported to exhibit antimicrobial activity2. Reports of WHO states that medicinal plants would be the best root to acquire diverse of drugs1. In this context, plants promise a source of natural antimicrobial agents8. Antimicrobial activities of plants are ascribed to the presence of phytochemicals like tannins, phenols, alkaloids, terpenoids and flavonoids9. The application of plant based drugs in the treatment against pathogens is obtaining great acceptance because of scientific interest and non toxicity properties 10.
Approximately 119 drugs isolated from plants are used in treating infectious diseases worldwide11. Not less than 50% of the drugs which are used in treating clinically infectious diseases accounts to have originated from natural and natural product derivatives12. Since phytochemicals have the ability to inhibit the growth of infectious microbes, many plants are still being used for their antimicrobial properties and many other biological activities. Inspite of existence of vigorous antimicrobial drugs, the emergence of resistance microbes has created an immense interest in the exploration and the outcome of efficacious drugs13.
Terminalia catappa is a large tropical tree of the family Combretaceae1. Different parts of the plant are used in folklore medicine and studies have revealed various medicinal properties15. Various research disclose the medicinal uses like microbial inhibition of leaf aqueous and methanolic extract of the plant16. Bacterial inhibitory activity of leaf aqueous extract17, anti-inflammatory property of leaf ethanolic extract18, wound healing activity of bark, antioxidant and radical scavenging activity of leaf aqueous extract, anticancer and anti-aging activity of ethanol and aqueous extract of leaves have also been reported19. Anti-methicillin potential of phytochemicals of T.catappa have also been evident20. Insilico studies of compounds from T. catappa are responsible for hepatoprotective and hepatotoxic properties21. Extracts of the leaves of the plant also possess anticancer, anti-HIV reverse transcriptase, hepatoprotective, Anti-inflamatory, Anti-hepatitis and Aphrodisiac effects22. Phytochemicals include tannins (Punicalagin, Punicalin, Chebulagic acid, geranin, granitin B), flavonoids (Vitexin, Rutin, Isovitexin), and Terpenoids (Ursolic acid, Asiatic acid) which are responsible for therapeutic activity23 .
Nigella sativa, belongs to the family Rananculaceae24. It is native to Mediterranian regions such as South west Asia, Southern Europe and North Africa25. Seeds contain yellowish volatile oil, fixed oil, alkaloids, saponins, minerals and vitamins26. The reported studies related to Nigella sativa have illustrated wide range of biological activities such as antioxidant27, anti-inflamatory28, antidiabetic29, anti prostate cancer effects30, antibacterial31, immunomodulatory effect32, and hepatoprotective33.
Secondary metabolite analysis found that seeds contain two types of alkaloids, isoquinone alkaloids (Nigellimine – N oxide) and pyrazole alkaloids (Nigellicine and Nigellidine)34. Essential phytoconstituents also include thymoquinone, P-cymene, 4- Terpineol, carvacrol, t- anethol, longifoline, thymol, thymohydroquinone and dithymoquinone .addtionaly they have a water soluble pentacyclic triterpene, alpha- hedarin and saponins as anticancer agents 35.
Preparation of the plant extract
Mature leaf material was collected from fields in and around Mysuru, Karnataka. The leaves were shade dried and powdered using laboratory blender. N. sativa seeds were collected from the local market, powdered and used for successive solvent extraction by soxhlet extractor using polar and non-polar solvents. After extraction the solvents were evaporated to dryness under reduced pressure and preserved the extracts at 4°C for future analysis.
Microorganisms and growth conditions
Human pathogenic bacteria were obtained from MTCC Chandigarh, Viz, B.subtilis (MTCC 121), B.cereus (MTCC 1272), Pseudo.aeruginosa, (MTCC424) Salm. typhi (MTCC 733), Escherichia coli (MTCC 7410) and Staph.aureus (MTCC 7443). Human pathogenic clinical isolates were provided by Microbiology department, Government Medical College, Mysuru, Viz, Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, Staphylococcus aureus and Salmonella typhi. The pathogens were maintained on Muller Hinton agar in glass tubes. Bacteria were resuscitated by sub culturing from stock onto Muller Hinton agar plates followed by incubation overnight for their optimum growth.
Antibacterial activity of the plant extracts
Antibacterial susceptibility test by Disc diffusion assay
Antibacterial potency of the test plant extracts were done by disc diffusion method employing the CLSI M02-A document36. Inoculums were prepared and adjusted to McFarland standard with cell density of 1to 1-2 X108 CFU/ ml. Standardized inoculum (0.1ml) was swabbed to the previously solidified MH agar plate. Sterile discs were loaded with test plant extract (100mg/ml) and impregnated onto the plates followed by incubation for 18-24 hours at 35 ±2°C. Standard antibiotics and solvents were employed as positive and negative control. All experiments were carried out in triplicates. Zone of inhibition (ZOI) around the disc was measured in mm.
Microbroth dilution method for determining Minimum inhibitory concentration (MIC) and Minimum Bactericidal Concentration (MBC)
MIC was established by employing 96 well microtiter plate according to the CLSI M07-A9 document37 . The test plant extract, which showed the inhibitory activity, were selected for determining MIC and MBC. The test plant extract were serially diluted two fold to obtain concentration of 5000-9μg/ml. The experimental set up included reference drug controls and solvent as positive and negative controls respectively. Aliquot of standardized inoculum (1.5 X 108 colony forming units/ml) was added to all the wells. The micro dilution plate was covered to avoid drying followed by incubation. Inhibition of bacterial growth was confirmed by addition of 20μl of aqueous solution of 2, 3,5-Triphenyl tertrazolium chloride (TTC) and re-incubated for 4-5h. The colorless well was designated as the MIC, which inhibited the growth of bacteria. Change of colour from colourless to pink indicated the presence of viable cells. MBC was ascertained by sub culturing 10μl of test dilution from the lowest concentration well by streaking on the previously sterilized and solidified MH agar with overnight incubation at optimum temperature. The well, which gave no bacterial colony on the agar medium, was designated as MBC.
Phytochemical composition of the active test extracts
The test extracts were subjected to qualitative phytochemical analysis according to the established procedures to determine the presence of different class of secondary phytochemicals38,39.
Thin layer chromatography (TLC) of the active extracts
The active extracts were subjected for separation of phytochemicals. Based on the review of literature and slight modification, EMW (Ethyl acetate: methanol: water) with 40:5.4:4 ratio showed better separation of the compounds and it was best-suited eluent system for the separation of phytocompounds from members of Combretaceae. The retention factor (Rf) of the separated phytochemicals was calculated40.
TLC bioautography
Agar overlay technique41 was adopted with minor modifications to locate the antibacterial bands. The TLC plates (silica gel G f254, Merk) were spotted with test plant extract(acetone extract) and developed in the pre saturated developing chamber using EMW as eluent system. After the solvent front reached the optimum distance the chromatogram was removed and visualized under long and short UV to locate the separated compounds. The chromatogram was dried overnight and subjected to overlay bioautography. One milliliter of standardized test inoculum was mixed with 10ml molten Muller Hinton agar. TLC chromatograms were placed in the sterilized plates and thin layer of media inoculated with the test bacteria was flooded on the chromatogram. Plates were incubated overnight at 37°C. ZOI around the separated phytochemicals can be seen as a clear area without the growth of the test bacteria. As a confirmatory test the chromatograms were flooded with microbiological agar containing TTC and further incubates for 30 minutes for optimum colour development. The ZOI is seen as white zone against the pink background.
Time kill assay
Protocol of kill time assay was adopted42 with slight modifications. The active extract of T. catappa was set at a concentration of 4MIC and 8MIC. A control tube was maintained without the test extracts. Each tube was then inoculated with standardized suspension of clinical isolates of S.aureus and P.vulgaris At prefixed time points aliquots were withdrawn from each concentration diluted serially (10-1 to 10– 4) and aliquots were plated on agar plates. Colony counts were performed after overnight incubation at 37°C in ambient air. Colony counts were averaged and expressed as log10 cfu/ml.
Satatitical analysis
Values are presented as Mean ±SEM. ANOVA was employed to determine the difference in specific means followed by tukey’s post hoc test at P<0.05. Graphpad prism (version 8) and Microsoft excel 2007 was used in generating graphs and analyzing the data.
Disc diffusion assay
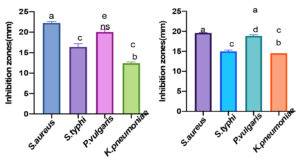
The test organisms exhibited different sensitivity for the extracts tested which is shown in Fig. 1 to 4. All the test pathogens exhibited significant sensitivity towards acetone and methanol extracts of T.catappa. Acetone extract had good inhibitory activity against the test organisms in contrast with the methanol extract. Significant inhibition was observed against the clinical isolate Staph.aureus (22.5mm) followed by P.vulgaris (20.5mm), Salm.typhi and Kleb.pneumoniae with inhibition zone 16.4mm and 12.4mm respectively. The MTCC strains also exhibited sensitivity towards the acetone and methanol extracts with zone of inhibition ranging between 11.7mm and 23.3mm. Hexane extract of N.sativa inhibited the growth of clinical isolate of Staph.aureus with inhibition zone 19.08mm, Staph.aureus (MTCC 7443) and Salm.typhi (MTCC 733) with zone inhibition 11.25mm and 15.9mm respectively.
Fig. 1 & 2. Antibacterial activity of Acetone extract (Fig:1) and Methanol extract (Fig :2) of T.catappa on human pathogenic clinical isolates. Data are represented in mean ±SEM. Different letters in the bars depict the significant difference at P<0.05. Error bars signify standard error. Inhibition zones are presented in millimeter (mm).
Fig. 3 & 4. Antibacterial activity of Methanol extract (Fig:3) and Acetone extracts (Fig :4) of T.catappa on human pathogenic bacteria (MTCC). Data are represented in mean ±SEM. Different letters in the bars indicate statistical difference (P<0.05). Error bars signify standard error. Inhibition zones are presented in millimeter (mm).
Micro dilution assay
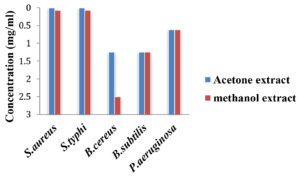
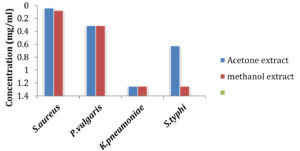
Considering the results obtained from disc diffusion assay, deducing the lowest inhibitory concentration (MIC) of active extracts was considered necessary and the results are shown in Fig. 5 and 6.
Of all the organisms tested, the clinical isolate Staph.aureus and P. vulgaris was the most sensitive to the acetone extract with lowest concentration of 39μg/ml and 312μg/ml respectively, Kleb.pneumoniae and Salm.typhi were less susceptible with concentration 1250μg/ml and 625μg/ml respectively. The acetone extract also exhibited inhibitory effect at the lowest concentration against all the MTCC strains tested except E.coli with concentrations between 1250μg/ml and 9μg/ml.
Table (1):
Minimum Inhibitory and Minimum Bactericidal Concentration and of acetone and methanol extract of T.catappa on human pathogenic bacteria (MTCC).
| MTCC strains | Acetone extract | Methanol extract | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| S.aureus | 9 | * | 78 | * |
| S.typhi | 9 | 625 | 78 | 5000 |
| B.cereus | 1250 | 2500 | 2500 | 5000 |
| B.subtilis | 1250 | * | 1250 | * |
| P.aeruginosa | 625 | 5000 | 625 | 5000 |
FN: Values presented in μg/ml; *: Indicates no MBC established
Methanol extract showed inhibitory activity towards the test pathogens but slightly less effective compared to the acetone extract having concentration between 5000μg/ml and 625μg/ml for clinical isolated bacteria. The minimum concentration of methanol extracts required against B.subtilis (MTCC 121) Salm.typhi (MTCC 733), Staph.aureus (MTCC 7443), Pseudo.aeruginosa (MTCC 424), and B. cereus (MTCC 1272) was in the range between 1250μg/ml and 78μg/ml.
Table (2):
Minimum Inhibitory and Minimum Bactericidal Concentration of acetone and methanol extract of T.catappa on human pathogenic clinical isolates.
| Clinical isolates | Acetone extract | Methanol extract | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| S.aureus | 39 | 1250 | 78 | 5000 |
| P.vulgaris | 312 | 5000 | 312 | 5000 |
| K.pneumoniae | 1250 | 5000 | 1250 | 5000 |
| S.typhi | 625 | 5000 | 1250 | 5000 |
FN: Values presented in μg/ml
Minimum Bactericidal Concentration
Acetone extract reported bactericidal effect at 1250μg/ml for clinical isolate Staph. aureus where as the bactericidal effect for Kleb.pneumoniae, Salm.typhi and P. vulgaris was 5000μg/ml. MBC against Salm.typhi (MTCC 733), B.cereus (MTCC 1272) and Pseudo.aeroginosa (MTCC 424) was at the concentration of 625 μg/ml, 2500 μg/ml and 5000 μg/ml respectively. On the contrast bactericidal action of methanol extract was 5000μg/ml for all the bacterial clinical isolates and the MTCC strains except Staph.aureus (MTCC 7443) and B.subtilis (MTCC 121), which did not show any bactericidal activity at the tested concentration.
Table (3):
Qualitative phytochemical analysis of the test plants.
| Secondary metabolites | Tests | T.catappa | N.sativa | |
|---|---|---|---|---|
| Acetone Methanol | N-hexane | |||
| Terpenoids | Salkowski test | + | + | – |
| Liebermann test | + | + | – | |
| Alkaloids | Mayers test | – | – | + |
| Wagners test | – | – | + | |
| Flavonoids | Lead acetate test | + | + | – |
| Shinoda test | + | + | – | |
| Alkaline test | + | + | – | |
| Saponins | Froth test | + | + | – |
| Tannins | Gelatin test | + | + | – |
| Oils and fats | Oils and fats | – | – | + |
| Phenols | Fecl3 test | + | + | – |
| Cardiac glycosides | Killer-kellani test | + | + | – |
FN: +: Present; –: Absent
Results of phytochemical analysis presented in Table 3. Broad group of phytochemicals were present in the acetone and methanol extract like terpenoids, flavonoids, saponins, cardiac glycosides, phenols and tannins. Hexane extract of Nigella sativa was dominated by alkaloids, oils and fats.
TLC profiling and bioautography
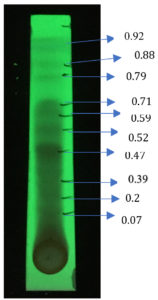
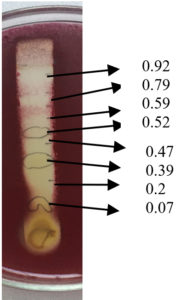
Thin layer chromatography of acetone extract using EMW solvent system was capable of eluting bands with Rf values 0.07, 0.20, 0.39, 0.47, 0.52, 0.59, 0.71, 0.79, 0.88 and 0.92.
Fig. 7. Chromatogram of acetone extract of T.catappa leaf eluted wih Ethyl acetate, Methanol and Water solvent system (40:5.4:4) showing clear seperation of phytocompounds.
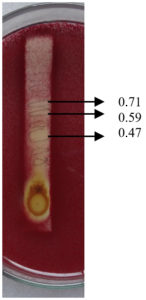
Fig. 8. Agar overlay bioautography of acetone extract of T.catappa leaf showing inhibition of P. vulgaris(Clinical isolate) at Rf values 0.71. 0.59 and 0.47.
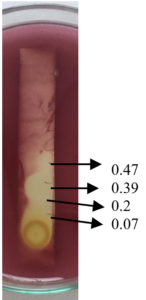
Fig. 9. Agar overlay bioautography of acetone extract of T.catappa leaf showing inhibition of S.aureus (MTCC 7443) at Rf values 0.47, 0.2 and 0.07.
Agar overlay bioautography of acetone extract of T. catappa showed clear zone of inhibition at Rf values 0.47, 0.2 and 0.07 against clinical isolate S. aureus (Fig. 11) and Proteus vulgaris (Fig. 8) was inhibited by the bands with Rf values 0.71, 0.59 and 0.47. S.typhi (MTCC 733) (Fig. 10) was also inhibited by the bands with Rf value 0.92, 0.79, 0.59, 0.52, 0.47, 0.39, 0.2 and 0.07. Bands with Rf value of 0.47, 0.2 and 0.07 strongly inhibited the Staph.aureus (MTCC 7443) (Fig. 9). Clear zone of inhibition around the separated bands was indicative of antibacterial property of the compounds present.
Colorless areas indicate inhibition of the test organisms by the separated phytocompounds.
Fig. 10. Agar overlay bioautography of acetone extract of T.catappa leaf showing inhibition of S.typhi (MTCC 733) at Rf values 0.92, 0.79, 0.59, 0.52, 0.47, 0.39, 0.2 and 0.07.
Fig. 11. Agar overlay bioautography of acetone extract of T.catappa leaf showing inhibition of S.aureus (clinical isolate) at Rf values 0.47, 0.39, 0.2 and 0.07.
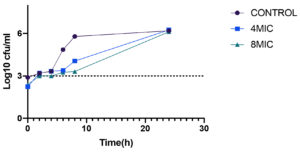
Kill time assay
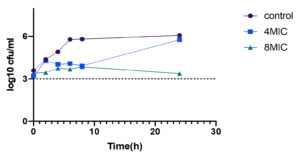
The time kill profile for clinical isolates Staph. aureus and P.vulgaris are presented in Table 4 showing the reduction in viable cell counts at particular time intervals. For both bacterial strains a separate time kill profile was produced over a period of 24 hours of incubation following inoculation. Difference in the viable counts for both bacterial strains were more after 4h post inoculation. At concentration corresponding to 8MIC bactericidal activity as observed at time point of 2-4h for Staph.aureus (Fig. 13) after which regrowth of the bacteria was observed indicating concentration dependent killing of the test bacteria. However, 4MIC concentration did not kill the bacteria but slowed down the growth compared to initial control. For P. vulgaris (Fig. 12) maximum reduction of viable cell counts was seen after 24h of incubation at 8 MIC concentration with near bactericidal effect of 3.37 log10 Cfu/ml and 4MIC concentration reduced the viable cell counts after 8h of incubation with 3.9log10 Cfu/ml exhibiting the bacteriostatic action of the extract. For both the bacteria tested the viable colonies were reduced post inoculation after which the colonies regrew until the end of time course. However, there was delay in the exponential growing phases of the test bacteria. 8MIC concentration was more effective than 4MIC for both the test bacteria indicating the extract is dose and time dependent killing effect irrespective of the bacteria.
Table (4):
Time kill profile of acetone extract of Terminalia catappa showing reduction of viable cell counts at different time intervals.
| Time intervals | 0hr | 2hr | 4hr | 6hr | 8hr | 24hr | 0hr | 2hr | 4hr | 6hr | 8hr | 24hr | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test bacteria | S.aureus (Log10cfu/ml) | P.vulgaris (Log10cfu/ml) | |||||||||||||
| Concentration | |||||||||||||||
| Control | 2.89 | 3.19 | 3.32 | 4.85 | 5.87 | 6.19 | 3.5 | 4.38 | 4.91 | 5.78 | 5.8 | 6.07 | |||
| 4MIC | 2.82 | 3.20 | 2.1 | 3.38 | 3.74 | 6.24 | 3.21 | 4.2 | 4.02 | 4.07 | 3.9 | 5.76 | |||
| 8MIC | 2.38 | 3.0 | 3.0 | 3.25 | 3.27 | 6.11 | 3.40 | 3.4 | 3.74 | 3.68 | 3.84 | 3.37 | |||
The present investigation reports the acetone and methanol extract of T.catappa leaf and hexane extract of N.sativa seeds demonstrated the antibacterial activity against the human pathogenic bacteria and clinical isolates. The preliminary method for evaluating the sensitivity of the test bacteria towards the plant extract is inhibition zone testing by disc and well diffusion assay. However, to test the efficacy of the extracts, broth dilution assays are also used preferably microbroth dilution assay41.
Acetone and methanol extract of T.catappa demonstrated varying level of activity with inhibition zones ranging between 11.66 and 23.33 mm for all the test bacteria.
The results are in accordance with the previous reports where in polar solvent (methanol and aqueous) extracts of T. catappa leaves in addition to twenty other plants were evaluated against bacteria (Gram positive and gram negative) and found that majority of the test strains were susceptible to methanol extract having inhibition zone 5-18mm16. The differences in the antibacterial activity can be due to the different chemical composition and the distinct mechanism of action of their bioactive constituents43. The results can also be attributed with previous reports, which recorded the aqueous extracts of T.catappa exhibited inhibitory activity against Staph.aureus, Kleb.pneumoniae, E.coli and Candida albicans 44. Several other reports have revealed the antimicrobial activity of leaves and fruits of T.catappa on different gram positive and negative bacteria45. The susceptibility results obtained by disc diffusion assay of leaf methanol extract of T. catappa against Staph.aureus, pseudo. aeroginosa, B.subtilis and a clinical isolate Staph.aureus demonstrated that gram-positive organisms were more susceptible46. Polyphenols are reported to have antibacterial activity47. Various polyphenols isolated from T.catappa like catappanin, geranin, gallantonic, leutolin, apigenin, orientin, isovitexin, catachien, kampferol, genistein, querccetin, ellagic acid, chlorogenic acid, ferulic acid , arjunetin,, ursolic acid, gallic acid, arjunoilc acid, betulinic acid and several compounds from T.catappa have been reported. The above isolated compounds from the plant could be acreditted to the invitro susceptibility of bacteria found in another study48. Several other workers49-51 have also reported the antibacterial properties of various other parts of T. catappa against human pathogenic bacteria. Our results exhibit a wide spectrum inhibitory activity towards both gram-positive and negative bacteria. We report strong antibacterial inhibition by polar solvent extracts (acetone and methanol) of T.catappa against clinical isolate Staph.aureus followed by Salm.typhi (MTCC 733) and clinical isolate P.vulgaris.
The hexane extract of N.sativa exhibited inhibitory activity against clinical isolate Staph.aureus, Salm. typhi (MTCC 733), Staph. aureus (MTCC 7443) and B. subtilis (MTCC 121). The hexane extract of N.sativa showed susceptibility against gram positive and negative bacteria indicating wide range inhibitory activity. The results are in agreement with other workers where methanol and N-hexane extract of N. sativa showed considerable good activity against the test pathogens showing varying zone of inhibition with varying dilutions. Gram-positive S.epidermidis was most sensitive to the extracts52. Aqueous and alcoholic extract of seeds of N.sativa against bacteria and fungi was reported and all the extracts posed different efficacy against the test organisms53.
Evaluation of essential oil for antibacterial activity, which showed the suppression of test pathogens exhibiting varying zone of inhibition54. The results can be related to other reports where the essential oil and oleoresins exhibited the significant inhibition of test organisms55. N-hexane extract and seed oil of N.sativa on selected human pathogenic bacteria was evaluated and found that the test microbes were inhibited by both extract and oil56. The results obtained in the study are in concordance with the previous studies. The methanol extracts obtained from the seeds were evaluated for antibacterial activity by and found that Staph. aureus and Pseudo.aeroginosa were sensitive to the extracts57. N-hexane, methanol and aqueous seed extract of N.sativa was evaluated for their invitro susceptibility activity where methanol extract showed considerable inhibition of Staph.aureus, Escherichia coli and Salm. enterica58. The efficacy of N.sativa oil is attributed to its quinone constitutes in the fixed and essential oil, which is, endowed with thymoquinone a significant bioactive constituent making up 30-48% of total constituents. Other functional constituents include p-cymeme, carvacrol, thymohydroquinone, dihydrothymoquinone, thymol, α-thujene,t-anthole, β-pinene, α pinene and γ-terpinene59.
The MIC values of T.catappa showed, that acetone extract was more potent in inhibiting the test bacteria and methanol extract was slightly less potent than the acetone extract. However, hexane and chloroform extract had no activity against the test strains owing to the fact that polar component of the extract is posing the antibacterial action. The acetone extract showed strong inhibition of clinical isolate Staph. aureus (MIC; 39μg/mL) and P. vulgaris (MIC; 625μg/mL). MIC values of acetone extract for MTCC bacteria ranged between 9μg/ml and 1250μg/ml. Methanol extract posed the lowest inhibitory concentration (MIC) from 78μg/ml to 1250μg/ml for both clinical isolates and MTCC strains. However, the methanol extract exhibited moderate inhibitory activity compared to acetone extract. MBC values were more than the MIC. When higher concentration used there might result in bactericidal effect. Some researchers have reported the antimicrobial agents having MIC values between 1.60mg/ml to 8mg/ml as frail microbial inhibitors60. None of the extracts showed MIC values greater than 8mg/ml. In agreement with our study the previous reports where the crude extracts of leaves of T. catappa and eight other plants were evaluated for MIC against human pathogenic bacteria and reported that pathogens were inhibited at the concentration from 16mg/ml to 0.015mg/ml61. The MIC of leaves of T.catappa was evaluated at concentration ranging from 100mg/ml to 350mg/ml against a panel of bacteria. Bacteria included Staph. aureus, Bacillus subtilis, Bacillus cereus, Pseudomonas aeroginosa, Proteus mirabilis, Salmonella typhi and Shigella dysentriae62. Comparative evaluation of leaf aqueous and methanol extracts of Combretum and Terminalia spp. of southern Africa were evaluated, which reported that both the species had broad spectrum antimicrobial activity inhibiting both bacteria and fungi. The MIC values inhibiting the test strains were less than 1000 μg/ml where in Bacillus subtilis had least MIC ranging from 124-578μg/ml Staphylococcus aureus (395-770 μg/ml), K.pneumoniae (318-531μg/ml) P.aeroginosa (36-512μg/ml)63. Our work reports that the test strains were inhibited at the lowest concentration with special reference to clinical isolate of Staph. aureus and Proteus vulgaris. Members of the Terminalia genus have a long history in traditional system of medicines and are used in several continents for treating numerous diseases including cardiovascular effects, wound healing, abdominal disorders, conjunctivitis, hypertension, pneumonia, gastric ulcers, jaundice, leprosy, edema and skin diseases64. Various species of Terminalia have been utilized in treating infections caused microbes and many current researchers have documented their antibacterial potential. Indian and southern Asian Terminalia species have been particularly studied well. Antibacterial susceptibility of wide panel of organisms has been specifically documented in Terminalia arjuna, Terminalia belllirica Terminalia catappa and Terminalia chebula65.
Qualitative secondary metabolite screening of active extracts (acetone and methanol) exhibited the dominance of terpenoids, phytosterols, flavonoids, flavones and saponins. These compounds have been previously reported by earlier workers66-69. The leaves of Terminalia catappa contain chebulagic acid corilagin, gentisic acid, geranin, granatin B, kaempferol, punicalagin, punicalin, quercetin, terfavin A and terflavin B70. The study is in agreement with the 71 wherein quercetin was identified in the leaves of Terminalia catappa along with other components like flavonoids, carotenoids and other phenolic compounds, which is responsible for the traditional use of the plant.
Hexane extract of N. sativa revealed the presence of alkaloid, which is the major component of the seeds. The results are in accordance with the earlier work72, which reported the occurrence of tannins, alkaloids, flavonoids and sterols.
TLC bioautography helps in locating the antibacterial active spots on the chromatogram. EMW solvent system was capable in eluting the antibacterial compound in acetone extract of with Rf value of 0.59, 0.52, 0.47 and 0.39. Bioautography studies revealed that most of the separated compounds had antibacterial potential, which can be due to the occurrence of polyphenols in the test plant extract, which is also confirmed from the qualitative phytochemical analysis. Time kill studies are important because they provide information about pharmacodynamics of the antibacterial agent73. Extracts showed variable kinetics against test bacteria. Time kill findings displayed different levels of time dependent and concentration dependent inhibition of the organisms. Bacteriostatic and bactericidal activity was displayed by the extract mainly against clinical isolates but regrowth of the test bacteria occurred after specific time interval which could be attributed to the use of lesser concentration of the extracts and also the gram positive and negative bacteria differ in cell wall and the membrane composition which regulate their susceptibilities to plant metabolites. At higher concentration bactericidal activity may be achieved invitro. Time kill kinetics of T.catappa against selected human pathogenic bacteria was carried out for the first time, however there are fewer reports related to time kill kinetics of fungi74 but, reports on bacteria are scarce to the best of our knowledge.
The results achieved in this study indicate that T.catappa and N.sativa are potential sources of antibacterial agents against the bacterial clinical isolates in particular. The acetone extract of T.catappa exhibited wide spectrum activity against (gram-positive and gram-negative) MTCC and clinical isolates. The extracts also inhibited the test bacteria at a very minimal concentration. Bioautography profile of acetone extract resulted in potent antibacterial bands, which exhibited inhibition both MTCC and clinical isolates was observed. Time kill assays indicated time and concentration dependent activity. Both bacteriostatic and bactericidal activity was observed against clinical isolates. Use of higher concentrations could lead to bactericidal activity against the test bacteria. The extracts can be employed in improving human health in the prevention of treatment of infectious diseases and to Fight the emergence and spread of resistant organisms. Further pure compound isolation, structure elucidation and mechanism of action of the isolated molecule followed by invivo toxicity studies may lead to potential drug in the ongoing search for antimicrobial botanicals.
ACKNOWLEDGMENTS
Authors are thankful to Center for Innovative Studies in Herbal Drug Technology, Department Of Studies in Botany, University of Mysore, Manasagangotri, Mysuru, Karnataka for providing the facilities and to the Department of Microbiology, Mysore Medical College and Research Center for providing the clinical bacterial isolates.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors have made substantial, direct and intellectual contribution to the work and approved it for publication.
FUNDING
Authors are thankful to ICMR-New Delhi and VGST-CISEE, Government of Karnataka for financial assistance. funding grant number No.45/19/2018/TM/BMS
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets analyzed during this study are included in the manuscript.
- Mozirandi W, Mukanganyama S. Antibacterial activity and mode of action of Veronia adoensis (Asteraceae) extracts against Staphylococcus aureus and Pseudomonas aeroginosa. Journal of Biologically Active Products form Nature. 2017;7(5):341-357.
Crossref - Tang QL, Kang AR, Lu C X. Phytochemical analysis , antibacterial activity and mode of action of methanolic extract of Scuterllaria barbata against various clinically important bacterial pathogens. Intl J Pharmacol. (IJP). 2016;12(2):116-125.
Crossref - Nijmoh DL, Assob JCN , Mokake SE Nyhalah DJ Yinda CK, Sandjon B. Antimicrobial activity of a Plethora of medicinal plant extract and hydrolates against human pathogenic and their potential to reverse antibiotic resistance. Int J Microbiol. 2015;2015:547156.
Crossref - Saranraj P, Sivasakthi S. Medicinal plants and its antimicrobial properties: A review. Global Journal of Pharmacology. 2014;8(3):316-327.
- Karaman I, Sahin F, Gulluce M, Ogutcu H, Sengul M, Adiguzel A. Antimicrobial activity of aqueous and methanol extracts of Junipers oxycedrus L. J Ethnopharmacol. 2003;85(2-3):231-235.
Crossref - Ghosh A, Das B K, Roy A Mandal B, Chandra G. Antibacterial activity of some medicinal plant extracts. J Nat Med. 2008;62(2):259-262. doi: 10.1007/s11418-007-0216-x
- Orhan DD, Ozcelik B, Ozgen S, Ergun F. Antibacterial, Antifungal and Antiviral activities of some flavonoids. Microbiol Res. 2010;165(6):496-504.
Crossref - Kusuma IW, Murdiyanto, Arung ET, Syafrizal, Kim YU. Antimicrobial and Antioxidant properties of medicinal plats used by the Bentian tribe from Indonasia. Food Science and Human Wellness. 2014;3(3-4):191-196.
Crossref - Khan R, Islam B, Akram M, et al. Antimicrobial activities of five herbal extracts against multidrug reistant (MDR) strains of bacteria and fungus of clinical origin. Molecules. 2009;14(2):580-597.
Crossref - Turker H, Yildirim B. Screening for antibacterial activity of some Turkish plants against fish pathogens: a possible alternative in the treatment of bacterial infections. Biotechnol Biotechnol Equip. 2015;29(2):281-288.
Crossref - Mahida Y, Mohan JSS. Screening of Indian plant extracts for antibacterial activity. Pharm Biol. 2006;44(8):627-631.
Crossref - Zengin G, Uysal A, Gunes E, Aktumsek A. Survery of phytochemical compositions and biological effects of three extracts from wild plant (Cotoneaster nummularia Fisch. Et Mey.): a potential source for functional food ingredients and drug formulations. PLoS One. 2014;9(11):e113527.
Crossref - Gobalakrishnan R, Kulandaivelu M, Bhuvaneshwari R, Kndavel D, Kannan L. Screening of wild plants species for antibacterial activity and phytochemical analysis of Tragia involucrate L. J Pharm Anal. 2013;3(6):460-465.
Crossref - Eddy NO, Ekwumemgbo PA, Mamza PAP. Ethanol extract of Terminalia catappa as a green inhibitor for the corrosion of mild steel in H2SO4. Green Chem Lett Rev. 2009;2(4):223-231.
Crossref - Venkatalakshmi P, Brinda P. Antimicrobial activities of aqueous extracts of different pars of Terminalia catappa L. Int J Curr Microbiol App. Sci. 2016:5(12):493-498.
Crossref - Nair R, Chanda S. Antimicrobial activity of Terminalia catappa, Manilkara zapota and Piper betel leaf extract. Indian J Pharm Sci. 2008;70 (3):390-393.
Crossref - Tanganna JC, Quanico JP, Perono RMG, Armor EC, Rivera WL. Tannin rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS- controlled biofilm maturation and Las A staphylolytic activity in Pseudomonas aeroginosa. J Ethnopharmacol. 2011;134(3):865-871.
Crossref - Fan YM, Xu LZ, Gao J, et al. Phytochemical and antiinflamatory studies on Terminalia catappa. Fitoterapia. 2004;75(3-4):253-260.
Crossref - Anand AV, Divya N, Kotti PP. Pharmacogn Rev. An updated review of Terminalia catappa. Pharmacogn Rev. 2015; 9(18): 93-98.
Crossref - Ravi L, Jindam D, Kumaresan S, Selvaraj VV, Reddy J. Anti-methicillin resistant Staphylococcus aureus potential of phytochemicals in Terminalia catappa and their proposed insilico mechanism of action. Asian J Pharm Clin Res. 2019;12(10):133-137.
Crossref - Packiri swamy V, Vijayalakshi K. Molecular docking studies of the compounds form Pergularia daemia and Terminalia catappa Linn. leaf extracts with CYP2 E1, GST, UDP-Glucuronyl trasnferase and Nrf2 binding site in KEAP1 , IL6. Int J Pharm Sci. 2018;9(5):2037-2045.
- Divya N, Rengarajan RL, Radhakrishnan R, et al. Phytotherapeutic efficacy of medicinal plant Terminalia catappa L. Saudi J Biol Sci. 2019;26(5):985-988.
Crossref - Minninel FS, Junior CSL, Espanha LG, et al. Characterization and quantification of compounds in the hydroalcoholic extract of leaves for Terminalia catappa Linn. (Combretaceae) and their mutagenic activity. J Evid Based Complementary Altern med. 2014;2014:1-11.
Crossref - Nadaf NH, Gawade SS, Muniv AS, Waghmare SR, Jadhav DB, Sonawane KD. Exploring antiyeast activity of Nigella sativa seed extract. Ind Crops Prod. 2015;77:624-630.
Crossref - Butt AS, Nisar N, Ghani N, Altaf I, Mughal TA. Isolation of thymoquinone form Nigella sativa L. and Thymus vulgaris L. and its antiproliferative effect on HeLa cancer cell lines. Trop J Pharm Res. 2019;18(1):37-42.
Crossref - Ramadan MF. nutritional value and applications of Nigella sativa essential oil. A mini review. Journal of Essential Oil Research. 2015;27(4):271-275.
Crossref - Zhao J, Xu F, Huang ZY, et al. evaluation on Anti inflammatory, Analgesic, Antitumor and Antioxidant potential of total saponins from Nigella glandulifera seeds. J Evd Based Complementary Altern Med. 2013;2013:827230.
Crossref - Shahzad M, Yang XD, Aseim MBR, et al. Black seed oil ameliorates allergic airway inflammation by inhibiting T- cell proliferation in rats. Pulm Pharmacol Ther. 2009;22(1):37-43.
Crossref - Andlousi AB, Martinear LC, Vallerand P, et al. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle adipocyte and liver cells. Diabetes Obes Metab. 2010;12(2):148-157.
Crossref - Kaseb AO, Chinnakannu K, Chen D, et al. Androgen receptor and E2F-1-targeted thumoquinone therapy for hormone-refractory prostrate cancer. Cancer Res. 2007;67(16):7782-7788.
Crossref - Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics resistant bacteria. Acta Microbiol Pol. 2000;49(1):63-74.
- Swamy SMK, Tan BKH. Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J Ethnopharmacol. 2000;70(1):1-7.
Crossref - Kanter M, Meral I, Dede S, et al. Effects of Nigella sativa L. and Urtica dioica L. on lipid peroxidation, antioxidant enzyme systems and some liver enzymes in ccl4- treated rats. J Vet Med. 2003;50(5):264-268.
Crossref - Kooti W, Noohi ZH, Ahvazi NS, Samabi MA, Larky DA. Phytochemistry Pharmacology and therapeutic uses of black seed (Nigella sativa). Chi J Nat Med. 2016;14(10):0732-0745.
Crossref - Ameedy TH, Omaran R. Antimicrobial activity of Nigella sativa extract against some bacteria and fungal species. Journal of University of Babylon of Pure and Applied Sciences. 2019:27(1):277-286
- CLSI performance standard for antimicrobial disk susceptibility test, approved standard. 7th ed. CLSI document M02-A 11. Clinical and laboratory standard institute. 950, west valley road, suite 2500, Wayne Pennsylvania 19087, USA. 2012.
- CLSI methods for dilution antimicrobial sucsceptibility tests for bacteria that grow aerobically, approved standard. 2012. 9th ed. CLSI document M07- A9. Clinical and laboratory standard institute. 950, west valley road, suite 2500, wayne Pennsylvania 19087, USA .
- Harbone JB. Phytochemical methods: a guide to modern techniques of plant analysis. Chapman and hall publications, London. 1998.
- Evans WC, Trease GE. 11th ed. Bailliere, Tindall, London. 1989.
- Mahlo SM, Chauke HR, Mc Graw LJ, Eloff JN. Antioxidant and antifungal activity of selected plant spp used in traditional medicine. J Med Plant Res. 2013;7(33):2444-2450.
- Ahmad I, Aquil F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESbetaL- producing multidrug resistant enteric bacteria. Microbiol Res. 2007;162(3):264-275.
Crossref - Joray MB, Rollan MDR Ruiz GM, Palacios SM, Carpinella MC. Antibacterial activity of extracts form plants of central Argentina- isolation of active principle from Achyrcline satureioides. Planta Med. 2011;77(1):95-100.
Crossref - Mambe FT, Igor K, Beng VP, Kuete V. Antibacterial acidity of methanol extracts form Alchornea cordifoloa and four other camaroonian plants against MDR phenotypes. Journal of Taibah University Medical Sciences. 2016;11(2):121-127.
Crossref - Jagessar RC, Alleyne R. Atimicrobial potency of aqueous extracts of leaves of Terminalia catappa. ARInt. 2011;1(3):362-371.
- Sahina N, Ahmad S, Rasool SA. In vitro antibacrtial activity of the extracts derived from Terminalia catappa. Res J Micribiol. 2007;2(2):180-184.
Crossref - Babayi H, Kolo I, Okogun JI, Ijah UJJ. The antimicrobial activities of methanol extracts of Eucalyptus camaldulensis and Terminalia catappa against some pathogenic microorganisms. Biokimistri. 2004;16(2):106-111.
Crossref - Chibane LB, Forquet V, Lanteri D, et al. Antibcaterial properties of polyphenols: characterization and QSAR (Quantitative structure activity relationship models). Front Microbiol. 2019;10:829.
Crossref - Zhang XR, Kaunda JS, Zhu HT, Wang D, Yang CR, Zhang YJ. The Genus Terminalia (Combretaceae): An Ethnopharmacological Phytochemical and Pharamacological review. Nat Prod Bioprospect. 2019;9:357-392.
Crossref - Shinde SL, Junne SB Wadje SS, Baig MMV. Diversity of antibacterial compounds of Terminalia spp.(Combretaceae). Pak J Biol Sci. 2009;12(22):1483-1486.
Crossref - Sanghvi R, Venkatalakshmi P, Brinda P. Antibacterial activity of Terminalia catappa bark against some bacterial pathogens. World J Pharm Sci. 2015;4(9):987-992.
- Neelavathi P, Venkatalakshmi P, Brindha P. Antibacterial activity against aqueous and ethanolic extracs of Terminalia catappa leaves and bark against some pathogenic bacteria. Int J Pharm Pharm Sci. 2013;5(1):114-120
- Khan AR, Kour K. Wide spectrum antibacterial activity of nigella sativa seeds L. IOSR Journal of Pharmacy. 2016;6(7):12-16.
Crossref - Benlafya K , Karrouchi K, Charkaoui Y, Karbane ME, Ramli Y. antimicrobial activity of aqueous, ethanolic, methanolic cyclohexanic extracts and essential oil of Nigella sativa seeds. J Chem Pharm Res. 2014;6(8):9-11.
- Amina B, Rachida A. Moleuclar composition and antibacterial effect of essential oil of Nigella sativa. Afr J Biotechnol. 2013;12(20):3006-3012.
- Singh C, Das SS , Singh G, Schuffc De lampasona MP, Catalan CAN. compositon, in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.). Biomed Res Int. 2014;918209.
Crossref - Sudhir SP, Rohitkumar, S, Verma HN. Study of antimicrobial potential, chemical composition and free radical scavenging property of Nigella sativa seed cold pressed oil and N-hexane extract from different geographies. Int J Pharm Biol Sci. 2017;7(2):131-144.
- Zuridah H, Fairuz ARM, Zakri AZH, Rahim MNA. In vitro antibacterial acitivty of Nigella sativa against Staphylococcus aureus, Pseudomonas aeroginosa, Klebsiella pneumoniae, Escherichia coli, and Bacillus cereus. Asian J Plant Sci. 2008;7(3):331-333.
Crossref - Tpocagic A, Zeljkovi, SC Karalija E, Galijasevic, SC, Sofic E. Evaluation of phenolic profile, enzyme inhibition and antimicrobial activity of Nigella sativa loaded seed extracts. Bosn J Basic Med Sci. 2017;4:286-294. doi: 10.17305/bjbms.2017.2049
- Sahak MKZ, Kabir N, Abbas G, Daraman S, Hashim NH, Adli DSH. Role of Nigella sativa and its active constituents in learning and memory. Evid Based Complementary Altern Med. 2016.
Crossref - Mogana R, Adhikari A, Tzas MN, Ramliza R, Wiart C. Antibacterial activity of the extracts fractions and isolated compounds from Canarium patentinerium Miq. Against bacterial clinical isolates. BMC Complement Altern Med. 2020, 20;55.
Crossref - Kloucek P, Polesny Z, Svobodova B, Vlokova E, Kokosla L. Antibacetrial screening of some Peruvian medicinal plants used in calleria district. J Ethnopharmacol. 2005;99:309-312.
Crossref - Akharaiyi FC, Ilori RM, Adesida JA. Antibacterial effect of Terminalia catappa on some selecte pathogenic bacteria. Int J Pharm Biomed Res. 2011;2(2):64-67.
- Cock IE, Vuuren SFV. A composition of the antimicrobial activity and toxicity of six Combretum and two Terminalia spp from southern Africa. Phcog Mag. 2015;11(41):208-218.
Crossref - Toghueo R, Boyom FF. Enophytic fungi from Terminalia species. A comprehensive review. J Fungi. 2019;5(2):43.
Crossref - Opara FN, Anguforo HU, Okechukwu RI, Mgbemena IC, Akuyobic O, Adjew A. A preliminary phytochemical screening and antibacterial activity of leaf extracts of Terminalia catappa. JETEAS. 2012;3(3):424-428.
- Mbengui RD, Guessennd NK, M’Boh GM, et al. Phytochemical screening and study of comparative antibacterial activity of aqueous and alcoholic extracts of the leaves and barks of Terminalia catappa on multi resistant strains. J Applied Biosci. 2013;66:5040-5048.
Crossref - Muhammad A, Mudi SY. phytochemical screening and antimicrobial activity of Terminalia caatppa leaf extracts. Biokemistri. 2011;23(1):35-39.
- Ahmed MS, VeerabhadraSwamy BM, Dhanpal PGR, Chandrashekara VM. Antidiabetic activity of Terminalia catappa L. leaf extracts in alloxan-induced diabetic rats. IJPT. 2005;4(1):36-39.
- Dukes A . Phytochemical and ethnobotanical database. 2008:11.
- Mndloi S, Mishra R, Varma R, Varugheese B, Tripathi. A study on phytochemical and antifungal activity of leaf extracts of Terminalia catappa. J Int J Pharm Biosci. 2013;4(4):1385-1393.
- Reddy HS, Al-kalbani AS, Al-Rawahi AS. Studies on phytochemical screening and GC-MS characterization, antimicrobial, antioxidant assay of black cumin seeds and Senna alexandria solvent extracts. Int J Pharm Sci. 2018;9(2):490-497.
- Desai SD, Saheb SH, Das KK, Haseena S. Phytochemical analysis of Nigella sativa and its antidiabetic effect. J Pharm Sci Res. 2015;7(8):527-532.
- Yadav A, Yadav M, Kumar S, Yadav JP. Bactericidal effect of Acacia nilotica: In vitro antibacterial and time kill kinetic studies. International Journal of Current Research. 2015;7(11):22289-22294.
- Ngouana TK, Mbouna CDJ, Kuipou RMT, et al. Potent and synergistic extract combination from Terminalia catappa, Terminalia mentaly and Monodora tenuifolia against pathogenic yeasts. Medicines. 2015;2(3):220-235.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.