ISSN: 0973-7510
E-ISSN: 2581-690X
Non healing ulcers constitute a potentially serious medical and as well as surgical illness. Wounds that contain necrotizing and less perfused (low oxygen content) tissue take longer to close and heal without proper clearing of dead or non viable tissue. The Siddha formulation “Oon Poochu Thailam” (OPT) is indicated for curing sloughing ulcers, sinuses and fistulae, supposedly by acting as a chemical debriding agent (like topical debriding agent). In this present study it was aimed for screening the antimicrobial activities of the ingredients of Oon Poochu Thailam and to analyze the elemental constituents of the ingredients [salt of Achyranthes aspera (AA), and salt of Sesamum indicum (SI) plant] by using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). The antimicrobial activity of the ingredients of OPT were studied in the concentration of 100mg/ml against Escherichia coli, Klebsiella pneumonia, Proteus mirabilis, Staphylococcus aureus, Pseudomonas aeruginosa, Shigella sonnei, Aeromonas hydrophila, Salmonella typhimurium, Vibrio cholera, Bacillus cereus and Candida albicans. Antibacterial and antifungal potential of the OPT ingredients were assessed both individually and together in terms of zone of inhibition of bacterial and fungal growths. The test items 1(AA), 2(SI) and 4(Mixture of AA, SI & SA) have shown a broad spectrum of anti-microbial activity against bacteria and fungi.
Oon Poochu Thailam, Sinus, Fistula, Debridement, Wound.
Debridement is the medical terminology for removal of dead (necrotic), damaged or infected tissue which is least viable so as to improve the healing potential of the remaining healthy tissue, thereby facilitating the wound healing process. Thus wounds that contain less perfused (low oxygen content), infected and hence necrotizing tissue take longer to close and heal, without proper clearing of infected and non-viable tissue. Non healing ulcers constitute a potentially serious medical and surgical illness.
Sometimes, debridement is executed naturally on the body’s own ability to shed off dead tissue termed as Apoptosis. However, more often, debridement warrants a medical procedure which unfolds into different types such as surgical, mechanical and chemical debridement.
The Siddha formulation “Oon Poochu Thailam”, which is mentioned in the literature Agathiar Rana Vaithyam1 is indicated for curing sloughing ulcers, sinuses and fistulae, supposedly by acting as a chemical debriding agent; either solely perfecting the debriding process or complementing the surgical or parasurgical debridement carried out in conjunction. Sangu (Turbinella rapa, Conch shell), Salt precipitated from the filtered solution in which ash of whole plant Achyranthes aspera (Naayuruvi samoolam) is a solute and in the same way herbal salt prepared from capsules of Sesamum indicum fruit (Ellukai Thol) are used as ingredients of which are homogenously mixed with sesame oil as a liquid vehicle to be applied externally. This homogenous physical mixture of the above ingredients with the Sesame oil is termed as Oon Poochu Thailam (OPT).
All the ingredients of OPT are of alkaline nature that leads to the lysis and liquefaction of necrotic tissues. Sangu parpam (Calcinate of Conch shell) is said to have a pH range of (9.12– 9.33)2 and antiulcer property3; Achyranthes aspera and Sesamum indicum herbal ash extract salts possess anti-inflammatory, anti-microbial, anti-oxidant and wound healing properties4,5; The above mentioned salt prepared of Achyranthes aspera (Kshar, pH:10.61-11.12) is used in Karanool (Kshar sutra) for treating fistula-in-ano, haemorrhoids and fissure-in-ano conditions.6
In this present study it was aimed to study the antimicrobial activities of the ingredients of Oon Poochu Thailam and to analyze the elemental constituents of the plant salts.
A. Preparation of Oon poochu thailam
Ingredients
Turbinella rapa (Conch shell, Sangu), Whole plant of Achyranthes aspera (Naayuruvi samoolam), Capsule of Sesamum indicum fruit (Ellukai Thol).
Preparation of salts
Each ingredient was prepared as salt form as follows. First, the ingredients were charred each separately to convert into ash, and the ash of each ingredient was put into separate mud pot. Four parts of water were added for one part of ash, stirred with oar like spoon 3 hours once for 3 days. On the 4th day, the supernatant was put into another mud pot, boiled until it became semisolid and the effervescence was settled. Then the semisolid end product was crystallized by drying it in the Sun light.
Usage
Powdered salt forms of above 3 Ingredients, were taken in the 1:1:1 ratio and mixed with sesame oil to apply on wound for healing.7
B. ICP-MS
The elemental analysis of salt of Achyranthes aspera (AA), and salt of Capsule of Sesamum indicum (SI) fruit were done by using Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
The study was conducted at Centre for Laboratory Animal Technology and Research, Sathyabama University, Chennai, Tamil Nadu, India.
Machine Model: Agilent 7700 ICPMS
Samples are decomposed to neutral elements in high temperature argon plasma and analyzed based on their mass to charge ratios. Digestion of sample was carried out by transforming 0.5gm of the sample into a closed beaker and 5 ml of concentrated HNO3 was added and digested to near dryness. 16 M nitric acid was further added each time to the sample and digested until the clear solution was obtained. 5ml of 12 M Hydrochloric acid was added to ensure complete digestion.The digested solution was cooled to room temperature and made to the final volume of 100 ml with deionized water. Sample solutions were then filtered through membrane (0.45micron) filter. Finally, the digested samples were used for metal analysis using inductively coupled plasma Mass Spectrometry. Each sample was digested in triplicate. A blank solution was also prepared in a similar manner.8
C. Antimicrobial activity of Oon Poochu Thailam
Test Procedure
The antimicrobial study was conducted at Centre for Laboratory Animal Technology and Research, Sathyabama University, Chennai, Tamil Nadu, India.
Cleaning and Sterilization
The glass-wares used in the present study were cleaned with cleaning solution and sterilized in hot air oven to 1800C for 3 hours. All nutrient media were sterilized by autoclave (1210C, 15psi for 15-20 minutes).
Culture of Pathogens
The Microbial strains used in the sensitivity assay were Escherichia coli (MTCC 1687), Klebsiella pneumoniae (MTCC 432), Proteus mirabilis (MTCC 3310), Staphylococcus aureus (MTCC 737), Pseudomonas aeruginosa (MTCC 424), Shigella sonnei (MTCC 646), Aeromonas hydrophila (MTCC 1739), Salmonella typhimurium (MTCC 733), Vibrio cholera (MTCC 3906), Bacillus cereus (MTCC 430) and Candida albicans (Diploid fungus) (MTCC 854) were purchased from MTCC, Chandigarh , India and they were sub cultured as per the guideline and standard protocol laid down by National committee for clinical Laboratory standards. Microbial Stock cultures were maintained at 4°C on slopes of nutrient agar (Hi Media, Mumbai).
Preparation of Test samples
Test samples (Ingredient 1- Salt of Achyranthes aspera (AA), Ingredient 2- Salt of Capsule of Sesamum indicum fruit (SI), Ingredient 3- Sangu parpam (SA), OPT formulation 4- mixture of formulations 1,2 and 3) provided for evaluation were powder in nature and in order to make the sample less viscous and suitable for handling through pipette about 2 ml of sterile distilled water was added to make up to the volume and triturated for nearly 30 minutes to make it a homogenous liquid (Fig 1).
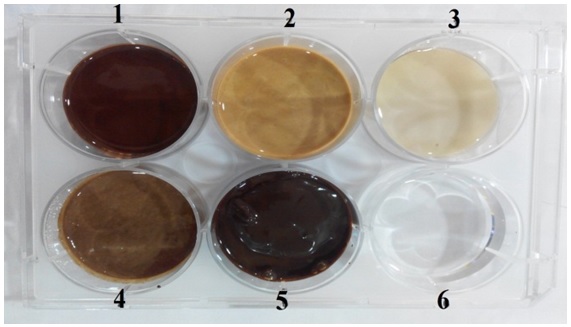
Sample Well Details
Well 1: Test formulation 1(AA- Salt of Achyranthesaspera)
Well 2: Test formulation 2 (SI-Sesamumindicum fruit)
Well 3: Test formulation 3 (SA-Sanguparpam)
Well 4: Test formulation 4 (Combination of Formulation 1 to 3 mixed at equal proportions)
Well 6: Standard Ciprofloxacin (20 mug/ml)
Fig. 1. Sample well
Standard control
Standard drug was used as control in this study. Ciprofloxacin was used as a standard for anti-bacterial study and the marketed formulation CandidTM cream (Clotrimazole) was used as a standard in the anti-fungal screening.
Sterility Test for Test formulations
The test substances were subjected to the preliminary sterility evaluation by disc plate method. Freshly prepared nutrient agar medium was loaded on the sterile disc and the same was used for enumeration of sterility of the test substances. As the test substances were colloidal in nature, streaking or swabbing was not possible. Diluted form of test substances at the concentration of 100µl and 200µl were loaded on to the top of the disc and incubated for the period of 48 hours with timely observations in between. Incubated plate was observed for 12, 24 and 48 hours after incubation and no growth of organism either as an isolated or as a colony was found in the incubated test substances. (Fig. 2).
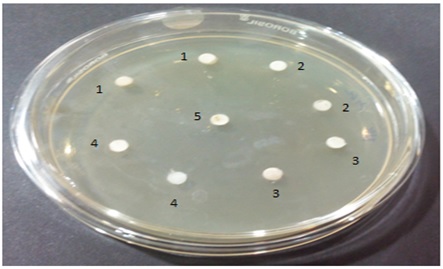
Disc marking
Well Marking 1: Test ingredient 1 (AA- Salt of Achyranthesaspera)
Well Marking 2: Test ingredient 2 (SI- Salt of Capsule of Sesamumindicum fruit)
Well marking 3: Test ingredient 3 (SP-Sanguparpam)
Well Marking 4: Test formulation 4 (Combination of Formulation 1 to 3 mixed at equal proportion)
Well Making C: Standard Ciprofloxacin (20 mug/ml) – For Anti-bacterial activity;
Standard Clotrimazole- For Anti-fungal activity
Formulation 1 to 4 – Evaluated at two different concentrations (100µl and 200 µl)
Fig. 2. Disc plates of Sterility test
Preparation of inoculums
Active cultures for experiments were prepared by transferring a loopful of cells from the stock cultures to test tubes of Mueller-Hinton broth (MHB) for bacteria and Sabouraud dextrose broth (SDB) for fungi that were incubated without agitation for 24 hrs at 37°C and 25°C respectively. The cultures were diluted with fresh Mueller-Hinton and Sabouraud dextrose broth to achieve optical densities corresponding to 2.0·106 colony forming units (CFU/ml) for bacteria and 2.0·105 spore/ml for fungal strains.9
Hole-plate diffusion method
Equidistant holes of 6mm were made in the agar using sterile cork borers. A 100µL volume of sample solution was added to the holes using a pipette or (Eppendorf). The compound was allowed to diffuse for 5 minutes and the plates were kept for incubation at 37°C for 24 hrs. At the end of incubation, inhibition zones formed around the disc were measured with transparent ruler in millimeter. The same procedure was followed for the fungus also. These studies were performed in triplicate.10,11,12
Inductively Coupled Plasma Mass Spectrometry (ICP-MS): ICP-MS is a type of mass spectrometry that is highly sensitive and capable of the determination of a range of metals and several non-metals at concentration below one part in 1012 (parts per trillion). It is an automated, simple and unique quantitative and qualitative analysis. It measures elemental isotopes ratio. The result of elemental analysis of salt of Achyranthes aspera (Naayuruvi samoolam), and salt of Capsule of Sesamum indicum fruit (Ellukai Thol) are furnished in Table 1.
Table (1):
Result of ICP-MS Analysis.
Elements |
Elemental Symbol |
Concentration of Elements present in salt of Achyranthes aspera |
Concentration of Elements present in salt of Capsule of Sesamum indicum fruit |
|---|---|---|---|
Copper |
Cu |
BDL |
BDL |
Arsenic |
As |
BDL |
BDL |
Calcium |
Ca |
0.27 mg/L |
0.32 mg/L |
Iron |
Fe |
24.11 mg/L |
4.425 mg/L |
Mercury |
Hg |
0.91 mg/L |
0.63 mg/L |
Manganese |
Mn |
1.18 mg/L |
0.415 mg/L |
Lead |
Pb |
1.09mg/L |
BDL |
Sodium |
Na |
410.3 mg/L |
BDL |
Sulfur |
S |
14.12 mg/L |
8.51 mg/L |
Zinc |
Zn |
12.16 mg/L |
6.72 mg/L |
Phosphorous |
P |
2.82 mg/L |
1.54 mg/L |
Chromium |
Cr |
1.45 mg/L |
0.21 mg/L |
Cadmium |
Cd |
0.451 mg/L |
0.18 mg/L |
The antimicrobial activity of the ingredients of Oon poochu thailam were studied in the concentration of 100mg/ml against Escherichia coli (MTCC 1687), Klebsiella pneumoniae (MTCC 432), Proteus mirabilis (MTCC 3310), Staphylococcus aureus (MTCC 737), Pseudomonas aeruginosa (MTCC 424), Shigella sonnei (MTCC 646), Aeromonas hydrophila (MTCC 1739), Salmonella typhimurium (MTCC 733), Vibrio cholerae (MTCC 3906), Bacillus cereus (MTCC 430) and Candida albicans. (Diploid fungus) (MTCC 854). Antibacterial and antifungal potential of the ingredients and formulation were assessed in terms of zone of inhibition of bacterial and fungal growths (Fig 3). The results of the antibacterial and antifungal activities are presented in Table 2. Percentages of antimicrobial activity of each test substance as compared to standard drugs Ciprofloxacin and Clotrimazole are given in Table 3.
Table (2):
Antimicrobial activity of OPT ingredients in terms of Zone of Inhibition.
| S. No | Test Organism | Inhibition Zone Diameter in mm | ||||
|---|---|---|---|---|---|---|
| Standard Control | TF-1(AA) | TF-2(SI) | TF-3(SP) | TF- 4 (Mixture of AA, SI & SP) | ||
| 1 | Escherichia coli | 29-32 | 22-25 | 22-26 | 0 | 20-24 |
| 2 | Pseudomonas aeruginosa | 29-33 | 23-26 | 23-25 | 0 | 21-24 |
| 3 | Proteus mirabilis | 19-22 | 22-25 | 26-31 | 0 | 22-25 |
| 4 | Staphylococcus aureus | 28-31 | 23-27 | 19-22 | 0 | 21-25 |
| 5 | Shigella sonnei | 25-29 | 26-31 | 27-31 | 0 | 24-28 |
| 6 | Aeromonas hydrophila | 25-27 | 24-28 | 24-27 | 0 | 20-23 |
| 7 | Klebsiella pneumonia | 26-30 | 25-28 | 24-29 | 0 | 23 -26 |
| 8 | Salmonella typhimurium | 29-33 | 24-27 | 26-31 | 0 | 24-27 |
| 9 | Vibrio cholerae | 30-34 | 24-29 | 25-28 | 0 | 23-27 |
| 10 | Bacillus cereus | 24-28 | 25-30 | 23-26 | 0 | 22-23 |
| 11 | Candida albicans | 14-17 | 24-26 | 26-31 | 0 | 22-26 |
The value represented in the table is a triplicate performed for each formulation against each organism.The zone diameter mentioned as a range of minimum to maximum values in mm;
TF- Test Formulation, .AA- Salt of AA – Achyranthesaspera; SI-Sesamumindicum fruit; SP-Sanguparpam.
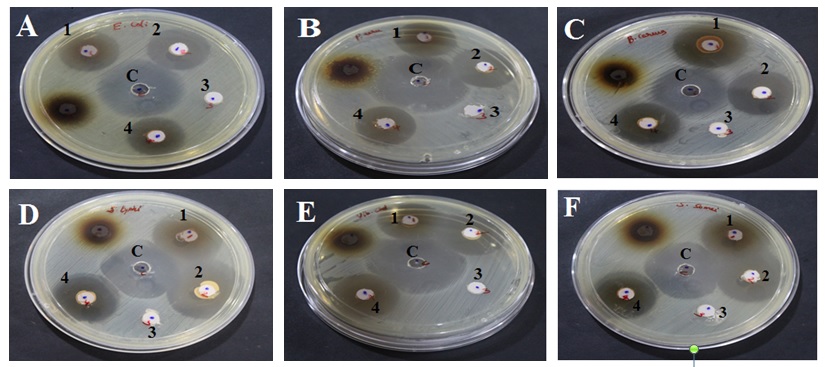
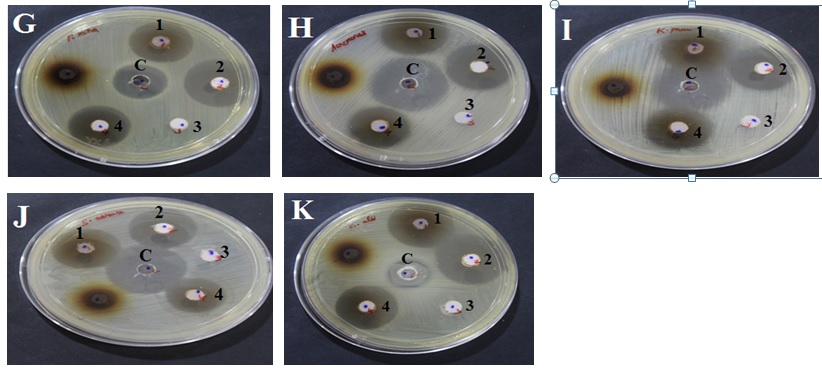
A-Escherichia coli, B-Pseudomonas aeruginosa, C-Bacillus cereus, D-Salmonella typhimurium, E-Vibrio cholera, F-Shigella sonnei, G-Proteus mirabilis, H-Aeromonas hydrophila, I-Klebsiella pneumonia, J-Staphylococcus aureus, K-Candida albicans (Diploid fungus).
Well marking ‘c’- Standard control; 1- Test ingredient 1( AA- Salt of Achyranthes aspera); 2- Test ingredient 2(SI- Salt of Capsule of Sesamum indicum fruit); 3- Test formulation 3(SA-Sangu parpam); 4- Test formulation 4 (Combination of Formulation 1 to 3 mixed at equal proportion).
Fig. 3. Anti-microbial effect of Test formulations (Oon Poochu thailam ingredients) against selected Pathogens
Table (3):
Percentage of Zone of inhibition compared with standard.
| S. No | Test Organism | Corresponding % of inhibition zone with Standard | |||
|---|---|---|---|---|---|
| TF- 1(AA) | TF- 2(SI) | TF- 3(SP) | TF-4(Mixture of AA, SI &SP) | ||
| 1 | Escherichia coli | 76-78 | 76-81 | 0 | 69-85 |
| 2 | Pseudomonas aeruginosa | 78-79 | 76-79 | 0 | 72-73 |
| 3 | Proteus mirabilis | 113-115 | 136-140 | 0 | 113-115 |
| 4 | Staphylococcus aureus | 82-87 | 68-71 | 0 | 75-81 |
| 5 | Shigella sonnei | 104-106 | 106-108 | 0 | 96-97 |
| 6 | Aeromonas hydrophila | 96-103 | 96-100 | 0 | 80-85 |
| 7 | Klebsiella pneumonia | 93-96 | 92-97 | 0 | 86-88 |
| 8 | Salmonella typhimurium | 81-83 | 90-94 | 0 | 82-83 |
| 9 | Vibrio cholerae | 80-85 | 82-83 | 0 | 77-79 |
| 10 | Bacillus cereus | 104-107 | 93-96 | 0 | 82-92 |
| 11 | Candida albicans | 153-171 | 182-185 | 0 | 153-157 |
TF- Test Formulation; AA- Salt of Achyranthesaspera; SI-Salt of Sesamumindicum fruit; SP-Sanguparpam.
Test substance 1 (Salt of Achyranthes aspera -AA) showed maximum zone of inhibition against Proteus mirabilis, Shigella sonnei, Aeromonas hydrophila and Bacillus cereus which were higher than the standard drug (Ciprofloxacin). Antibacterial activity of AA was nearly equal to standard against Klebsiella pneumonia, Staphylococcus aureus, Vibrio cholera and Salmonella typhimurium.
Test substance 2 (Salt of Capsule of Sesamum indicum fruit -SI) showed maximum zone of inhibition against Proteus mirabilis and Shigella sonnei which were higher than the standard drug. Antibacterial activity of SI is nearly equal to standard against Aeromonas hydrophila, Klebsiella pneumonia, Salmonella typhimurium. Vibrio cholerae and Bacillus cereus.
No measurable activity was observed with respect to test substance 3 (Sangu parpam- SA) against test pathogens.
Test substance 4 (Mixture of AA, SI & SA- OPT formulation) showed maximum zone of inhibition against Proteus mirabilis which was higher than that of the standard drug. Antibacterial activity of Test substance 4 was nearly equal to standard against Aeromonas hydrophila, Klebsiella pneumoniae, Salmonella typhimurium, Bacillus cereus and Vibrio cholera.
The test formulations 1(AA), 2(SI) and 4(Mixture of AA, SI & SA) have shown a broad spectrum of anti-fungal activity against Candida albicans which is significantly higher when compared to the standard clotrimazole (CandidTM). The data obtained from the present study clearly indicates that the test formulations 1(AA), 2(SI) and 4(Mixture of AA, SI & SA) have shown a broad spectrum of anti-microbial activity against bacteria and fungi. Regarding antibacterial assay, test formulations exerted promising activity against both gram positive and gram negative organisms.
- Ramachandhiran SP, Agathiyar Rana vaidhiyam, 2nd edition, July 2000, Thamarai Noolagam, pg-84.
- Madhavan R et al, Standardization of Sangu Parpam – a Herbo Marine Siddha Drug, Int. J. Curr. Res. Chem. Pharm. Sci. 2016; 3(6): 77-84.
- Thanga Thirupathi A et al, Pharmacological validation of two Siddha drugs (parpams) for antiulcer effect in albinorats: a preliminary study, Ancient science of life, XXII (1), 7/2002, 48-54.
- Abhijit Dey, AchyranthesasperaL: phytochemical and pharmacological aspects, 2011; 9(2); Article-013.
- Shasmitha R, Health benefits of Sesamum indicum: a short review, Asian J Pharm Clin Res, 2015; 8(6): 1-3.
- Hasmukh R. Jadav, Pharmaceutical standardization of Apamargakshara, Journal of Ayurveda & Integrative Medicine, 2015; 6(4): 290-294.
- Uthamarayan K S, AruvaiMaruthuvam, 4th edition, 2005, Dept. of Indian medicine and Homoeopathy, Pg48.
- Fairuz Liyana Mohd Rasdi et al, A Comparative Study of Selected Trace Element Content in Malay and Chinese Traditional Herbal Medicine (THM) Using an Inductively Coupled Plasma-Mass Spectrometer (ICP-MS), Int J Mol Sci, 2013; 14(2): 3078–3093.
- Varaprasad Bobbarala, Prasanth Kumar Katikala , K. Chandrasekhar Naidu and Somasekhar Penumajji. Antifungal activity of selected plant extracts against phytopathogenic fungi Aspergillus niger F2723. Indian Journal of Science and Technology. 2009; 2(4):87-90.
- Sahin F M, Daferera D, SO kmen, A., SO kmen M, Polissiou M. Biological activities of the essential oils and methanol extract of Origanumvulgare ssp. vulgare in the EasternAnatolia region of Turkey. Food Control. 2004; 15: 549–557.
- F. Sharififar , M.H Moshafi . In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic ZatariamultifloraBoissFood Control. 2007; 18: 800–805.
- Perez C and Paul M and Bezique P (1990) An Antibiotic assay by the agar well diffusion method. Alta Biomed. Group Experiences. 1990; 15: 113-125.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


