ISSN: 0973-7510
E-ISSN: 2581-690X
Novel infection control methods are needed to prevent further increases in the morbidity and mortality associated with hospital acquired infections. One method is to minimize bacterial transmission is to prevent bacterial growth on hospital surfaces. Copper has anti-microbial properties but is used sparingly in health care settings due to the cost associated with retrofitting surfaces with copper sheet metal or less durable foil. However, copper nanoparticles (CNPs) embedded in a polymer matrix may be similarly anti-microbial, which would offer a viable alternative to copper metal. In this pilot study, we created films with various densities of CNPs and tested their effectiveness in killing bacteria commonly associated with hospital-acquired infections. We found that films with mass ratios greater than 1:2 of CNP to polymer matrix solution killed Streptococcus pyogenes and Staphylococcus aureus more effectively than a copper plate, while the killing of Pseudomonas aeruginosa was similar when exposed to either surface. We also found that passing an electrical current through the CNP film increased the anti-microbial effect of the films against P. aeruginosa even more so than electrified Cu plates. CNP films, which are relatively inexpensive, could be sprayed directly onto hospital surfaces to reduce the incidence of hospital-acquired infections.
Antimicrobial film, Copper nanoparticles, Antimicrobial copper, MRSA, infection control.
Approximately 1.7 million patients each year acquire a hospital-acquired infection (HAI) in the United States, resulting in 100,000 fatalities and $35 to $45 billion in annual costs.1,2 Medicare has discontinued payments associated with these infections,2,3,4,5 which provides even further incentive for hospitals to reduce the incidence of HAIs. One way HAIs are acquired is by contact with health care providers or directly from other patients;6 as such, current approaches for reducing HAIs often focus on the use of personal protective equipment, aseptic technique, and the prevention of airborne particulate matter.7,8,9 However, HAIs can also be spread by contact with contaminated hospital surfaces.10,11 Some pathogens, including Pseudomonas aeruginosa, can survive for weeks or even months on certain solid surfaces.12-15 It is not known exactly how many HAIs originate from contact with contaminate surfaces, but some estimates have attributed 30% of all HAIs to contact with contaminated surfaces.12,13 Thus, an important step in reducing the incidence of HAIs includes the development of additional methods to decrease the number of viable bacteria present on hospital surfaces. While regular, frequent cleaning and disinfection provides an important line of defense, a complementary approach is to create surfaces that are inherently anti-microbial.
Copper is anti-microbial and numerous studies have confirmed that bacteria are killed quickly on copper surfaces.16-20 The mechanism underlying the anti-microbial nature of copper is unknown, although several mechanisms have been proposed. These include damage to the bacterial cell membrane through lipid peroxidation16,17 and damage to proteins by iron-sulfur protein cluster binding.21,22 While some studies dispute the effect of reactive oxygen species,21,22 recent work by Warnes et al.,33 indicate that reactive oxygen species generated by non-Fenton chemistry play a significant role in the anti-microbial properties of copper. Specifically, they reported that the addition of chelators of Cu to contamination droplets reduced anti-microbial activity. Furthermore, adding Tiron, which protects against superoxide molecules, significantly also reduced antimicrobial activity, while the addition of d-mannitol, which protects against hydroxyl radicals, was only minimally protective. The results suggest that copper’s antimicrobial property is likely due to the formation of reactive oxygen species from non-Fenton chemistry. Despite these well-known antimicrobial properties, copper is sparingly used; in part because it is not practical to overlay all hospital surfaces with copper metal or foils. A potential solution to this problem is the use of copper nanoparticles (CNPs) embedded in a polymer matrix.
CNPs are a broad class of particles ranging in size from a few nanometers to 100 µm and ranging in purity from pure copper to brass. There is a similarly broad range of uses for CNPs. For example, CNPs are used as catalysts and lubricants in chemical industrial applications, provide a lower-cost alternative to expensive noble metals in printed electronics, and are under investigation as an additive to engine coolant systems.30,32 Additionally, CNPs are components in chemical sensors and are used in electro analysis of solutions.31 The increasing ubiquity of CNPs in the manufacturing industry has led to an increase in availability and a decrease in cost. Given what is known about the antimicrobial properties of copper, CNPs may provide a relatively inexpensive and feasible solution for the development of antimicrobial coatings. This is particularly true when CNPs are combined with a flexible, coatable polymer matrix system. It is likely that the CNP-polymer solution could be applied either as an aerosol or as a paint, which would facilitate applications to existing infrastructure, such as faucets, bed rails, door handles, and room dividers without undergoing the expense of refitting surfaces with specifically made copper components. Additionally, surfaces that are not amenable to metallic copper fittings, such as floors or surfaces that require pliability for function, could be coated with a CNP-polymer solution.
While Cu is antimicrobial, it is also highly conductive and exposure of bacteria to an electric field is also lethal. Possible mechanisms underlying this observation include the alteration of ion channels in the bacterial cell membrane, which leads to ion gradient imbalances and cell death, or the generation of free radical species.26-29 Thus it seems likely that applying an electric current to Cu surfaces would enhance the inherent antimicrobial properties of Cu surfaces.
In this pilot study, we determined if spherical CNPs embedded in a polymer matrix could be used as an antimicrobial film. We tested the antimicrobial effect of varying concentrations of CNPs embedded in polymer with pathogens commonly associated with HAIs. The results show that S. pyogenes and S. aureus are killed more effectively when exposed to CNP film than Cu plates and that the films are as effective as plates in killing P. aeruginosa. Moreover, conductivity could be achieved with the CNP films and the electrified films had even greater antimicrobial properties compared to Cu plates. The results provide proof-of-principle that CNP containing films may be useful in decreasing the morbidity and mortality associated with HAIs.
Film construction
We assessed several polymers as candidates for creating CNP films, including polyvinyl butyral, polyvinyl alcohol, polyaniline, polyurethane, and polyacrylate. We eventually selected polyvinyl butyral due to its strong adhesive properties, high viscosity, water resistance, and relatively low cost. We created a 16.7% by mass polymer solution by dissolving 2.0 grams of polyvinyl butyral powder (BUTVAR 98; Sigma-Aldrich) in 10.0 g of glacial acetic acid (Sigma Aldrich). We then added pure copper nanoparticles (10 µm diameter; Alfa Caesar Chemical Company) at varying mass ratios to create copper nanoparticle suspensions. We attempted to make films with greater CNP to polymer solution ratios than 2:1; however, we found that above this concentration, the CNPs settled out of solution too quickly for practical use. We spread the CNP suspensions over plain laminate film with a #10 Meyer rod and allowed the films to dry overnight at room temperature prior to testing.
Bacterial growth conditions
We tested the anti-bacterial properties of CNP films using Streptococcus pyogenes [the group A streptococcus; strain MGAS315 obtained from American Type Culture Collection (ATCC)], methicillin-resistant Staphylococcus aureus (strain WARD470179-158 obtained from VWR), and Pseudomonas aeruginosa (strain [Schroeter] Migula, obtained from ATCC). Bacterial were grown overnight from frozen stocks on either a Todd-Hewitt yeast (THY) agar plates (S. pyogenes) or brain-heart infusion (BHI) agar plates (S. aureus and P. aeruginosa). Working solutions were prepared by suspending colonies obtained from the plates into 2 mL of THY or BHI broth and allowing the culture to grow overnight at 37°C with an atmosphere containing 5% CO2. To estimate the number of bacteria in the working solutions, we diluted the solutions in 1 M pyrophosphate buffer at either 1:10 working solution:buffer (S. pyogenes) or 1:100 working solution:buffer (S. aureus and P. aeruginosa) and measured the absorbance at 600 nm. Dilutions made from the working solutions were used to test the antimicrobial properties of the film.
Assessment of bacterial viability on test surfaces
To distribute bacteria onto the copper nanoparticle film, we spread 20 µL of diluted bacterial solution evenly onto the film over a 2.0 cm diameter circle. We then carefully swabbed the circles and did dilution plating to determine the number of viable bacteria recoverable from the surface. For experiments where bacteria were collected at multiple time points, we used a separate circle for each time point. We spread the swab onto the appropriate agar plates (THY for S. pyogenes; BHI for S. aureus and P. aeruginosa), incubated the plates overnight at 37 °C and 5% CO2, and counted the number of cfu’s.
Statistical analysis
All quantification and statistical analysis of data were performed with GraphPad Prism 6 Software. Statistical analyses used included linear regression, a one way analysis of variance (ANOVA) with a Tukey’s multiple comparisons post-hoc test, or two way ANOVA, as appropriate.
Nanoparticle concentration and antimicrobial activity
To determine if films containing CNP were antimicrobial, and to determine if the activity correlated with the concentration CNPs incorporated into the films, we measured the viability of S. pyogenes exposed to films created with six different concentrations of nanoparticles, ranging from zero nanoparticles (PVB) to films incorporating a 2:1 mass ratio of CNPs to PVB. S. pyogenes (4.5 x 105 cfu’s) were exposed to the films at room temperature and after 2 minutes the number of viable CFUs remaining was determined by dilution plating. In general, as the concentration of copper nanoparticles within the film increased, there was a decrease in the number of viable bacteria recovered from the film surfaces. The range was a maximum of 7 x 103 cfu’s recovered from films make with a 1:10 ratio of CNPs to PVB to an absence of viable S. pyogenes recovered following exposure to films composed with 1:1 and 2:1 mass ratios of CNPs to PVB (Fig. 1). For comparison, we also used a pure copper alloy plate, a plain laminate film, and a film of pure PVB (without CNPs). As expected, we did not detect a decrease in the viability of S. pyogenes following exposure to either the laminate or the PVB films that did not contain CNPs (Fig. 1). The copper alloy plate decreased the number of viable bacteria to 4 x 102 cfu’s. Overall the results showed that the CNP films were antimicrobial and that films with CNP to PVB ratios more than 1:2 had greater antimicrobial activity against S. pyogenes compared to copper metal.
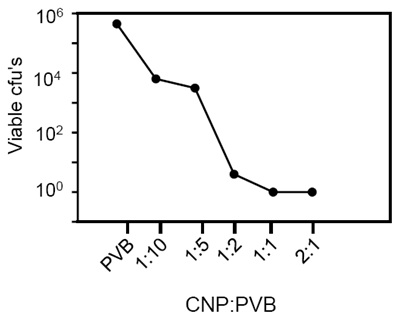
Fig. 1. Films with a ration of CNPs greater than 1:2 had antimicrobial activity. Control films composed of only PVB and no CNPs and films made with increasing amounts of CNPs were tested for the capacity to kill 4.5 X 105cfu’s of S. pyogenes after 2 min exposure. R2 = 0.44.
Assessing the time of exposure of pathogens to the CNP films on decreased viability
To assess the relationship between the time bacteria are exposed to the films and the number of viable bacteria remaining after exposure, we used CNP films made with a mass ratio of 2:1 CNPs to PVB solution based on the results of the previous experiment (Fig. 1). In addition, we tested the activity against two additional pathogens (S. aureus and P. aeruginosa) that are commonly responsible for HAIs. Suspensions of S. pyogenes, S. aureus, and P. aeruginosa were applied to the films and incubated for 0.5, 1, 2, 3, 4, 5, or 10 minutes prior to determining the number of viable cfu’s remaining on the films. As controls, a pure-copper alloy plate and plain laminate films were used. Anti-bacterial properties of the films varied among the three pathogens. S. pyogenes were killed after 2 minutes exposure to CNP films, while three minutes exposure was necessary to similarly kill S. aureus. P. aeruginosa was more refractory to killing and viable bacteria could be detected even after five minutes of exposure to the CNP films (Fig. 2). Overall, the films composed with CNPs killed S. pyogenes and S. aureus more effectively than the pure copper plate, while the antimicrobial activities of the film and plate against P. aeruginosa were similar.
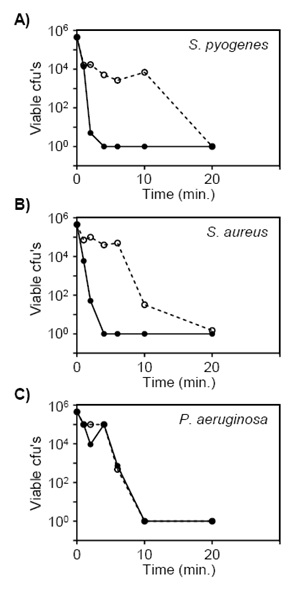
Fig. 2. CNP films were antimicrobial. The relationship between the number of viable cfu’s remaining after being exposed to CNP films was determined using A) S. pyogenes B) S. aureus, and C) P. aeruginosa. ANOVA analyses comparing viability after exposure films versus plates for each bacterial species resulted in P=0.07 for S. pyogenes; P=0.05 for S. aureus; P = 0.36 for P. aeruginosa.
Relationship between film conductivity and nanoparticle concentration
To determine if the antimicrobial CNP films could conduct electricity, which could potentially enhance their antimicrobial properties, we used a simple circuit with a 24 V power source to electrify films of varying CNP:PVB mass ratios. We measured the voltage across a resistor for the various films, as well as a pure copper alloy plate for comparison.
Films created with a mass ratio of 11:9 CNPs to PVB solution were conductive (data not shown). The voltages achieved with films with mass ratios of 11:9, 3:2, 13:7, and 2:1 were similar and similar to that of a pure copper plate (data not shown). Films were tested multiple times, and for several minutes, to ensure conductivity was stable and reproducible.
Applying voltage to the CNP films enhanced antimicrobial activity against P. aeruginosa
Finally, we tested the antimicrobial effect of applying electricity to the CNP films. Because P. aeruginosa was most resistant to the antimicrobial activity of CNP films that we tested (Fig. 2), it was of interest to determine if the application of electricity would enhance microbial killing against this common cause of HAIs. We used films with a 2:1 ratio of CNPs to PVB. Because plain laminate does not conduct electricity, a pure-copper alloy plate was used for comparison. To allow the power source to stabilize, we applied current 2-3 minutes before applying bacteria to the film. To assess the potential effects of different voltages on the antimicrobial property of the film, we exposed P. aeruginosa to CNP films in which approximately 10, 15, or 20 V was applied and determined the number of viable bacteria recovered from the films after either 2 or 5 minutes exposure. Increased voltage correlated with increased antimicrobial activity (Fig. 3). After 5 minutes exposure to films conducting 20 V, we were not able to recover viable bacteria (Fig. 3). In contrast, significantly more bacteria were recovered from electrified copper plates all all voltages tested (Fig. 3).
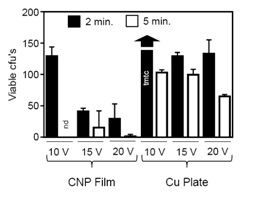
Fig. 3. Conductive CNP films had greater antimicrobial than copper plates. The relationship between the number of viable cfu’s remaining after being exposed for either 2 or 5 min. to electrified CNP films or a Cu plate was determined at 10, 15, and 20 V. ANOVA comparison of film ver-sus plate; P < 0.01.
The goal of this study was to determine if a film containing CNPs would mimic the anti-bacterial properties of a copper alloy plate. For all three bacterial species tested, the film was at least as effective as the plate, and for two gram-positive species, S. pyogenes and S. aureus, the film was more effective in decreasing the number of viable bacteria recovered from the respective surfaces. Several explanations could explain the results. We used spherical copper nanoparticles in the construction of the films. This likely gives the films a surface topography of raised semi-spheres, which could provide a greater surface area in direct contact with the bacteria than a planar copper plate. Additionally, it is likely that during the contruction of the films, a small amount of copper was dissolved into the film solution by the use of acetic acid in the contruction process. As the acid evaporated, residual copper ions likely remained in the polymer matrix. Ionized copper can be taken up by bacteria at a very rapid rate resulting in death.16,17 Thus, the presence of ionized copper might also be a significant advantage of the film, particularly if the film could be “recharged” by spraying the film with acetic acid. Additional experiments are necessary to test these ideas.
Our results indicate that applying current to CNP films increases the antimicrobial activity of the film (Fig. 3). It is likely that the film conducts electricity through chains of adjacent nanoparticles, which requires a critical concentration for there to be enough unbroken chains of nanoparticles in the film to conduct electricity. Discrepancies in the thickness of the film also affect the conductivity by decreasing the number of available unbroken chains of adjacent nanoparticles.
The CNP film, in addition to having superior anti-microbial properties compared to the copper plate, potentially has greater versatility. Although some hospital surfaces, such as door handles and faucets, can be cast in copper metal, it is not feasible or functionally desirable to cast irregular surfaces, such as computer keyboards with copper. For these and similar surfaces, CNP films may provide an ideal solution to create inherently anti-microbial surfaces. In this experiment, we used a Meyer rod to control the thickness of the film for greater experimental accuracy. We were also able to convert the coating solution to a sprayable solution by adding 25% more acetic acid (data not shown). The potential ability to spray surfaces to create CNP films would expand the utility of the film by increasing the range of surfaces suitable for CNP coating. Additionally, for this experiment we used polyvinyl butyral for the polymer matrix, which is an inexpensive, flexible, and water resistant polymer with a superior viscosity profile. There are, however, numerous additives or different polymer systems which could also be used, dependent on the needed application.
There are limitations to our experimental CNP solution; notably, the solution, while water resistant, is susceptible to damage if exposed to the combination of ethanol or acetic acid and vigorous wiping (data not shown). This disadvantages could be overcome by adding a small amount of cross-linking agent to the solution, an experiment which has not yet been done. Another disadvantage of the film is that it is currently unknown how quickly the film loses its antimicrobial activity. Additional experimentation on film weathering is necessary to answer these, and related, questions.
The most obvious application for CNP-containing films is in clinical settings where infection control is paramount, such as burn units. All three of the bacteria used in this study are common infectious agents among patients assigned to burn units.24,25 Coating high contact surfaces, walls, floors, or fabric room dividers with this solution may help to prevent the transmission of microbes.
The results of this study suggest that a copper nanoparticle film could offer a less expensive and logistically simple solution compared to using copper metals or foils as an antimicrobial treatment of surfaces within health care settings. In particular, a film would combine the antimicrobial properties of copper with the ease of spray coating and the ability to treat a variety of surfaces. In addition, film coatings may retain the pliability of such surfaces as curtains used as room dividers or even furniture. The CNP-containg films tested here were more antimicrobial against the gram-positive pathogens S. pyogenes and S. aureus compare to Cu plates. Moreover, specific film preparations could conduct electricity, which further enhanced their antimicrobial properties. The use of copper nanoparticle films on surfaces wihting health care facilities could be an effective adjunct approach to minimize HAIs.
ACKNOWLEDGMENTS
Funding for the project was provided by the Sanford School of Medicine Scholarship Pathways program. We thank A. Herrera for critical review of the manuscript.
- Scott D. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention. Centers for Disease Control and Prevention. 2009.
- Klevens, R. M., Edwards, J. R., Richards, C. L., Horan, T. C., Gaynes, R. P., Pollock, D. A., & Cardo, D. M. Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals. Public Health Reports. 2007: 122(2): 160–166.
- Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin C et al. Health Care–Associated Infections. JAMA Internal Medicine. 2013; 173(22):2039.
- Brown J, Doloresco III F, Mylotte J. “Never Events”: Not Every Hospital Acquired Infection Is Preventable. Clinical Infectious Diseases. 2009; 49(5):743-746.
- Milstein A. Ending Extra Payment for “Never Events” — Stronger Incentives for Patients’ Safety. New England Journal of Medicine. 2009; 360(23):2388-2390
- Donker T, Wallinga J, Slack R, Grundmann H. Hospital Networks and the Dispersal of Hospital-Acquired Pathogens by Patient Transfer. PLoS ONE. 2012; 7(4):e35002.
- Yokoe D, Mermel L, Anderson D, Arias K, Burstin H, Calfee D et al. Executive Summary: A Compendium of Strategies to Prevent Healthcare Associated Infections in Acute Care Hospitals. Infection Control and Hospital Epidemiology. 2008; 29(S1):S12-S21.
- Traub-Dargatz JL, Weese JS, Rousseau JD, Dunowska M, Morley PS, Dargatz DA. Pilot Study to Evaluate 3 Hygiene Protocols on the Reduction of Bacterial Load on the Hands of Veterinary Staff Performing Routine Equine Physical Examinations. The Canadian Veterinary Journal. 2006; 47(7):671-676.
- Leung M, Chan A. Control and Management of Hospital Indoor Air Quality. Medical Science Monitor. 2006; 12(3):17-23
- Weber D, Rutala W, Miller M, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. American Journal of Infection Control. 2010; 38(5):S25-S33.
- Neely A. Persistence of Micro-organisms on Common Hospital Surfaces. Infection Control Resource. 1992: 4(4):1-7
- Kramer A, Schwebke I, Kampf G. BMC Infect Dis. 2006; 6(1):130.
- Hirai Y. Survival of Bacteria Under Dry Conditions; From a Viewpoint of Noscomial Infection. Journal of Hospital Infection. 1991; 19(3):191-200.
- Jawad A, Heritage J, Snelling A, Gascoyne-Binzi D, Hawkey, P. Influence of Relative Humidity and Suspending Menstrua on Survival of Acinetobacter spp. on Dry Surfaces. Journal of Clinical Microbiology. 1996: 34(12): 2881–2887.
- Aitken C, Jeffries D. Nosocomial Spread of Viral Disease. Clinical Microbiology Reviews. 2001; 14(3):528-546.
- Grass G, Rensing C, Solioz M. Metallic Copper as an Antimicrobial Surface. Applied and Environmental Microbiology. 2010; 77(5):1541-1547.
- Santo C, Taudte N, Nies D, Grass G. Contribution of Copper Ion Resistance to Survival of Escherichia coli on Metallic Copper Surfaces. Applied and Environmental Microbiology. 2007; 74(4):977-986.
- Santo C, Lam E, Elowsky C, Quaranta D, Domaille D, Chang C et al. Bacterial Killing by Dry Metallic Copper Surfaces. Applied and Environmental Microbiology. 2010; 77(3):794-802.
- Elguindi J, Wagner J, Rensing C. Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. Journal of Applied Microbiology. 2009; 106(5):1448-1455.
- Santo C, Quaranta D, Grass G. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. MicrobiologyOpen. 2012; 1(1):46-52.
- Karpanen T, Casey A, Lambert P, Cookson B, Nightingale P, Miruszenko L et al. The Antimicrobial Efficacy of Copper Alloy Furnishing in the Clinical Environment: A Crossover Study. Infect Control Hosp Epidemiol. 2012; 33(01):3-9.
- Macomber L, Imlay J. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proceedings of the National Academy of Sciences. 2009; 106(20):8344-8349.
- Weber D, Rutala W. Self-disinfecting surfaces: Review of current methodologies and future prospects. American Journal of Infection Control. 2013; 41(5):S31-S35.
- Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn Wound Infections. Clinical Microbiology Reviews. 2006; 19(2):403-434.
- Mayhall C. The Epidemiology of Burn Wounds: Then and Now. Healthcare Epidemiology. 2003; 37: 543-550
- Patel R. Biofilms and antimicrobial resistance. Clinical orthopaedics and related research. 2005; 437:41-7.
- Jass J, Lappin-Scott HM. The efficacy of antibiotics enhanced by electrical currents against Pseudomonas aeruginosa biofilms. Journal of Antimicrobial Chemotherapy. 1996; 38(6):987-1000.
- del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrobial agents and chemotherapy. 2009; 53(1):41-5.
- Shimada K, Shimahara K. Leakage of cellular contents and morphological changes in resting Escherichia coli B cells exposed to an alternating current. Agricultural and biological chemistry. 1985; 49(12):3605-7.
- Lee Y, Choi JR, Lee KJ, Stott NE, Kim D. Large-scale synthesis of copper nanoparticles by chemically controlled reduction for applications of inkjet-printed electronics. Nanotechnology. 2008; 19(41):415604.
- Welch CM, Compton RG. The use of nanoparticles in electroanalysis: a review. Analytical and bioanalytical chemistry. 2006; 384(3):601-19.
- Leong KY, Saidur R, Kazi SN, Mamun AH. Performance investigation of an automotive car radiator operated with nanofluid-based coolants (nanofluid as a coolant in a radiator). Applied Thermal Engineering. 2010; 30(17):2685-92.
- Warnes S, Keevil C. Lack of Involvement of Fenton Chemistry in Death of Methicillin-Resistant and Methicillin-Sensitive Strains of Staphylococcus aureus and Destruction of Their Genomes on Wet or Dry Copper Alloy Surfaces. Applied and Environmental Microbiology. 2016; 82(7):2132-2136.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


