ISSN: 0973-7510
E-ISSN: 2581-690X
In fermented foods, lactic acid bacteria are important for flavor enhancement, preservation, and pathogen control via the production of antimicrobial compounds, such as bacteriocins. This study aimed to isolate and characterize lactic acid bacteria from cheese that can produce bacteriocin-like inhibitory substances and to evaluate their antibacterial activity against foodborne pathogens. Lactic acid bacteria were isolated from two cheese samples using de Man, Rogosa and Sharpe agar and were preliminarily identified through phenotypic and biochemical tests, including Gram staining and carbohydrate fermentation. Antibacterial activity was tested using live cells (cell culture), cell-free supernatants (CFS), and neutralized CFS against Escherichia coli, Salmonella Typhimurium, Klebsiella pneumoniae, and Listeria monocytogenes using the agar well diffusion method. Seven lactic acid bacterial strains were gram-positive, rod-shaped, catalase-negative, and showed a positive reaction to the methyl red test. The results showed strong inhibition of E. coli and S. Typhimurium, moderate inhibition of K. pneumoniae, and no inhibition of L. monocytogenes. The strongest antibacterial activity was observed with lactic acid bacteria cultures, followed by CFS and neutralized CFS. Bacteriocin-like inhibitory substances-producing strains were identified as Lacticaseibacillus rhamnosus, Lactiplantibacillus pentosus, and Lentilactobacillus parabuchneri. These findings emphasize the potential of lactic acid bacteria as natural antimicrobial agents and bio-preservatives, providing a promising strategy for enhancing safety and reducing foodborne pathogens in foods.
Lactic Acid Bacteria, Bacteriocin-like Inhibitory Substances (BLIS), Lacticaseibacillus rhamnosus, Lactiplantibacillus pentosus, Lentilactobacillus parabuchneri, Antagonistic LAB effects
Lactic acid bacteria (LAB) represent various microorganisms vital to food fermentation practices and the broader field of microbiology. For thousands of years, humans have harnessed the fermentative ability of these bacteria to preserve food and enhance its flavor. LAB are a diverse group of gram-positive, non-spore-forming microorganisms known for producing lactic acid as the main byproduct during the fermentation of carbohydrates.1 These bacteria are common, particularly in fermented dairy products, including kefir, yogurt, and cheeses.2 Because of their beneficial properties, many LAB strains, such as Lactobacillus, Streptococcus spp., and Enterococcus, are classified and sold as probiotics.3 Probiotics are live microorganisms that, when administered in sufficient quantities, confer health benefits to the host.4 Both the United States Food and Drug Administration and European Food Safety Authority classify LAB as Generally Recognized as Safe, making them safe for human consumption.5,6 They can be utilized in food processing either as starter cultures or as part of the natural microbiota. Their significance in food preservation lies in their capacity to generate various antimicrobial metabolites, including organic acids, hydrogen peroxide, and bacteriocins, during the fermentation process.7-9 Moreover, their antagonistic effects against bacterial pathogens are attributed to stimulation of the immune system and modulation of the intestinal microbiota. LAB can prevent pathogen adhesion by competing for binding sites on intestinal epithelial cells, thereby reducing pathogen colonization and delaying the onset of infection.10,11 Bacteriocins produced by LAB have become the focus of extensive research because of their potential applications in food safety, medicine, and biotechnology. These antimicrobial peptides inhibit or kill related or unrelated microorganisms and exhibit variability in their structure, size, and mechanisms of action. Bacteriocin-producing LAB have also been categorized as Generally Recognized as Safe and are considered safe food preservation additives. Bacteriocins from LAB have been shown in numerous investigations to possess antibacterial properties against foodborne pathogens, including Salmonella Paratyphi, Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Listeria monocytogenes.12-14 Cheeses, as rich sources of LAB, offer an excellent opportunity to investigate the antimicrobial capabilities of these microorganisms. The isolation of LAB from cheese and evaluation of their effectiveness against common foodborne pathogens can provide valuable insights into their potential use in food preservation strategies. Such studies may help to identify specific strains of LAB that can be used as natural preservatives to enhance food safety. This study aimed to isolate and characterize LAB from cheese samples and assess their capacity to generate bacteriocin-like inhibitory substances (BLIS) targeting prevalent foodborne pathogens.
Collection of cheese samples
Two types of cheese (one sample of cheddar and one sample of feta) were obtained from different stores in Jeddah, Saudi Arabia. After being immediately placed in sterilized containers, the samples were transported to the Microbiology Department Laboratory at King Abdulaziz University and stored in a refrigerator until analysis.
Isolation of LAB
LAB cultivation was conducted following the procedure described by Mohammed and Con,15 with slight alterations. Ten grams of the sample was homogenized with 40 mL of de Man, Rogosa and Sharpe (MRS) broth in a sterile Falcon tube and incubated under anaerobic conditions at 37 °C for 24 h. A sterile cotton swab was inserted into each tube, streaked onto a new MRS agar plate, and left to dry. Subsequently, the plates were covered and incubated anaerobically using a candle jar at 37 °C for 24-48 h. Colonies with different morphological features were sub-cultured (purified) at least three times using the streak plate method on new MRS agar plates. Pure colonies were transferred to MRS agar slants for short-term preservation at 4 °C.
Preliminary characterization of LAB
Initial characterization was conducted based on phenotypic characteristics, including cell morphology and Gram staining, as well as biochemical properties, such as the catalase, carbohydrate fermentation, and methyl red tests. For additional identification, isolates that were positive for Gram staining and negative for the catalase test were chosen as presumed LAB.16-18 The chosen isolates were preserved using MRS broth (Biolab, Hungary) with 30% (1 v/v) glycerol at -80 °C.
Evaluation of the antibacterial properties of LAB against foodborne pathogens
Collection of bacterial pathogens
This study utilized four prevalent foodborne pathogens: Escherichia coli ATCC 11775, Listeria monocytogenes ATCC 13932, Klebsiella pneumoniae ATCC 700613, and Salmonella Typhimurium ATCC 14028. These bacterial strains were obtained from the Department of Microbiology at the King Fahad Medical Research Center in Jeddah, Saudi Arabia. The test bacteria were transferred to nutrient agar slants for short-term preservation at 4 °C.
Antibacterial effects via LAB cell cultures as preliminary assessment
The antimicrobial potential of the isolates was initially evaluated using an agar well diffusion assay against E. coli, L. monocytogenes, K. pneumoniae, and S. Typhimurium. The bacterial pathogens were incubated in nutrient broth at 37 °C for 24 h. Mueller-Hinton agar plates (Oxoid, USA) were streaked overnight on bacterial cultures (1 × 108 CFU/mL). Wells with a diameter of 6 mm were created in the agar and filled with 100 µL of LAB cultures, previously grown anaerobically in MRS broth for 24 h at 37 °C. Uninoculated MRS broth was used as a negative control. To ensure proper diffusion, the plates were left for 2 h before being incubated anaerobically at 37 °C for 24 h. Following incubation, inhibition zones were observed and measured to evaluate antibacterial effects.19,20
Antibacterial effects of cell-free supernatants (CFS) and neutralized CFS (NCFS)
Preparation of CFS
To exclude the influence of LAB live cells through competitive exclusion, the CFS was prepared following a modified method presented by Rzepkowska et al.21 LAB cultures were grown in MRS broth anaerobically at 37 °C for 24 h. Subsequently, they were centrifuged at 4,500 rpm for 30 min at 4 °C to separate the bacterial cells from the supernatant. The obtained CFS was then sterilized using a 0.22 µm filter to ensure the elimination of any residual cells. Finally, the filtered supernatant was stored at 4 °C for subsequent analysis.
Preparation of NCFS
The NCFS was obtained to detect BLIS while eliminating the influence of other inhibitory agents, including organic acids and H2O2. It was prepared according to the same method provided by Rzepkowska et al.21 To control the impact of organic acids, the pH of CFS was neutralized to 6.5 using 1 M NaOH, after which it was filtered using a 0.22 µm pore filter. The presence of H2O2 was excluded by incubating the samples under anaerobic conditions.
Evaluation of the antibacterial effects of CFS and NCFS
The evaluation of the antibacterial activity for all treatments was achieved using the agar well diffusion test.22 The pathogenic bacteria were grown in nutrient broth, then incubated at 37 °C for 24 h. Subsequently, the pathogenic bacterial strains (1 × 108 CFU/mL) were evenly distributed on Mueller-Hinton agar plates. Wells measuring 6 mm in diameter were then created using a cork borer, and 100 µL of the CFS from each strain was added into the wells. The plates were placed to ensure proper diffusion of the supernatant before being incubated anaerobically at 37 °C for 24 h. The same procedure was applied to the NCFS, and the zones of inhibition were observed.
Molecular identification of BLIS-producing strains via 16S rRNA gene analysis
Total LAB DNA was isolated following the procedure described by Azcarate-Peril and Raya,23 with some modifications. Microbial cells (5 × 10⁹ CFU) were collected from overnight cultures, and the resulting pellets were suspended in 200 µL of Tris-EDTA-Saline TES solution. To facilitate cell lysis, 20 µL of lysozyme (10 mg/mL) was incorporated, and the mixture was maintained in a 37 °C water bath for 20 min. Subsequently, 20 µL of proteinase K (10 mg/mL) was added, followed by further incubation at the same temperature for another 20 min. The sample was then cooled in an ice bath for 5 min before adding 250 µL of 4 M sodium acetate and 250 µL of chloroform:isoamyl (24:1). The mixture was gently stirred and centrifuged at 13,000 rpm for 2 min (G-force = 30285). The upper aqueous layer was carefully transferred to a new microtube, and an equivalent volume of isopropanol was added for DNA precipitation. The sample was stored at -20 °C overnight. The following day, centrifugation at 13,000 rpm was performed again for 2 min to collect the DNA pellet, which was then air-dried at room temperature (approximately 18-20 °C) and rehydrated in 50 µL of distilled water. The quality of the isolated DNA was verified by gel electrophoresis. To amplify the 16S rRNA gene, primers 27F (5′-AGAGTTTGA-TCCTGGCTCAG-3′) and 1492R (5′-AAGGAGGT-GATCCAGCCGCA-3′) were utilized in a Polymerase Chain Reaction (PCR). DNA amplification was performed using a PCR master mix (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. A thermocycler (Mastercycler Gradient, Eppendorf, Germany) was used to start the amplification process at 94 °C for 5 min, followed by 32 cycles of 45 s at 94 °C, 45 s at 60 °C, and 90 s at 72 °C, with a final extension at 72 °C for 10 min. A transilluminator (BioDoc-IT System, Japan) was used for electrophoresis, and a suitable fraction of each PCR amplicon was visualized under UV light. Macrogen (Seoul, Korea) sequenced the PCR products. NCBI BLAST was used to analyze the sequences. MEGA-X software and the maximum likelihood approach were used to construct a phylogenetic tree.
Statistical analysis
Data analysis was conducted using Excel and GraphPad Prism 9.5.1 (528) software (GraphPad, San Diego, CA, USA). The results are expressed as the mean ± standard deviation of three replicates, with statistical significance set at p <0.05.
Study duration
The study was conducted from May 2023 to January 2024.
Isolation and preliminary characterization of LAB
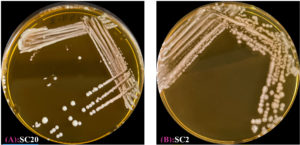
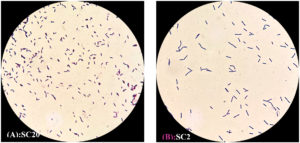
These results indicate the detection and preliminary characterization of the seven LAB isolates in accordance with their morphological and biochemical traits. All isolates appeared as gram-positive rods (Figures 1 and 2) and were catalase-negative and methyl red-positive (Table 1). These characteristics confirmed their classification as LAB.
Table (1):
The morphological and biochemical properties of LAB
| Isolate No. | Gram reaction | Cell shape | Catalase activity | MR test | Carbohydrates fermentation | ||
|---|---|---|---|---|---|---|---|
| Glucose | Lactose | Sucrose | |||||
| SC18 | + | Rod | – | + | + | + | + |
| SC19 | + | Rod | – | + | + | + | + |
| SC20 | + | Rod | – | + | + | + | + |
| SC21 | + | Rod | – | + | + | + | + |
| SC22 | + | Rod | – | + | + | + | + |
| SC23 | + | Rod | – | + | + | + | + |
| SC24 | + | Rod | – | + | + | + | + |
Key: + = Positive reaction, – = negative reaction; SC: Strain of cheese
Figure 1. Some LAB isolates colony on MRS agar following 48 h of incubation at 37 °C. (A) Strain of cheese No. 20. (B) Strain of cheese No. 2
Figure 2. Microscopic appearance of LAB isolates using the light microscope (100X) (Rod-shaped). (A) Strain of cheese No. 20. (B) Strain of cheese No. 2
Evaluation of antibacterial effects of LAB against foodborne pathogens
The antibacterial activities of LAB isolates were evaluated against E. coli, L. monocytogenes, K. pneumoniae, and S. Typhimurium. The inhibitory effects were assessed by observing the inhibition zones around the wells, with some variability among the tested pathogens.
Antibacterial effects of LAB cell cultures
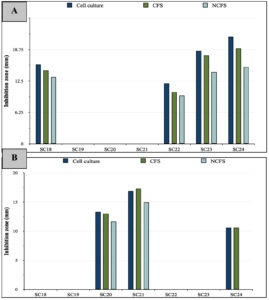
The antibacterial potentials of the LAB cell cultures are shown in Table 2. Four LAB strains displayed antimicrobial effects against E. coli (Figure 3A), with SC24 exhibiting the largest inhibition zone of 21.3 ± 3.05 mm. S. Typhimurium was susceptible to three LAB strains, with SC21 showing the most significant inhibition zone of 16.9 ± 0.85 mm (Figure 3B). Moreover, the growth of K. pneumoniae was affected by one strain, SC24, with an inhibition zone of 10.46 ± 0.5 mm (Figure 3C). In the case of L. monocytogenes, none of the isolates exhibited inhibitory effects (Figure 4).
Table (2):
Inhibition zone diameter (mm) of LAB cell culture against tested pathogens
Isolate No. |
E. coli |
S. typhimurium |
K. pneumonia |
L. monocytogenes |
|---|---|---|---|---|
SC18 |
15.8 ± 0.76 |
– |
– |
– |
SC19 |
– |
– |
– |
– |
SC20 |
– |
13.33 ± 0.57 |
– |
– |
SC21 |
– |
16.9 ± 0.85 |
– |
– |
SC22 |
12 ± 3 |
– |
– |
– |
SC23 |
18.5 ± 1.3 |
– |
– |
– |
SC24 |
21.3 ± 3.05 |
10.6 ± 1.15 |
10.46 ± 0.5 |
– |
The values show the three determinations’ mean ± standard deviation. No significant variances were noted between means within the same column (p >0.05). Diameter of inhibition zone. – No inhibition activity
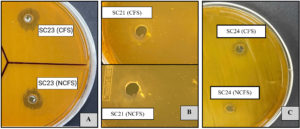
Figure 3. Evaluation of antibacterial effects of CFS and NCFS from LAB isolates toward E. coli (A), S. typhimurium (B), and K. pneumonia (C), showing distinct inhibition zones
Antibacterial effects of LAB CFS
CFS was used to exclude the inhibitory effects of live LAB cells. As presented in Table 3 and Figure 4A, E. coli exhibited the highest susceptibility among all tested pathogens. It was inhibited by the CFS of four LAB strains, reaching a maximum inhibition zone of 19 ± 0 mm by SC24. The growth of S. Typhimurium was suppressed by the CFS of three LAB strains, with the highest inhibition zone of 17.3 ± 0.57 mm observed for SC21 (Table 3 and Figure 4B). Moreover, only the CFS of strain SC24 showed antagonistic effects against K. pneumoniae. By contrast, L. monocytogenes was resistant to all LAB treatments (Figures 3 and 4).
Table (3):
Inhibition zone diameter (mm) of LAB CFS against tested pathogens
Isolate No. |
E. coli |
S. typhimurium |
K. pneumonia |
L. monocytogenes |
|---|---|---|---|---|
SC18 |
14.6 ± 1.15 |
– |
– |
– |
SC19 |
– |
– |
– |
– |
SC20 |
– |
13 ± 1 |
– |
– |
SC21 |
– |
17.3 ± 0.57 |
– |
– |
SC22 |
10.3 ± 2.3 |
– |
– |
– |
SC23 |
17.6 ± 0.57 |
– |
– |
– |
SC24 |
19 ± 0 |
10.6 ± 0.57 |
10 ± 1.73 |
– |
The values show the three determinations’ mean ± standard deviation. No significant variances were noted between means within the same column (p >0.05). Diameter of inhibition zone. – No inhibition activity.
Figure 4. Diameter of inhibition zones formed by LAB cell culture, CFS, and NCFS against (A) E. coli, and (B) S. typhimurium
Antibacterial effects of LAB NCFS
Bacteriocin activity was evaluated using NCFS of LAB, which was prepared by eliminating the impact of organic acids and H2O2‚ from the CFS. As shown in Table 4, the NCFS of the LAB strains showed reduced or no inhibitory action compared with that of both cell cultures and CFS. E. coli growth was inhibited by the NCFS of four LAB strains, with the largest inhibition zone of 15.3 ± 1.15 mm produced by SC24. The growth of S. Typhimurium was suppressed by the NCFS of two LAB strains, with SC21 producing the largest inhibition zone of 14.96 ± 0.95 mm. For K. pneumoniae, only the NCFS of SC24 exhibited inhibitory effects, with a zone measuring 7.6 ± 1.03 mm, whereas L. monocytogenes showed resistance to all treatments (Figures 3A-C and 4). Further identification at the species level was applied to the strains that exhibited antagonistic activity through NCFS.
Table (4):
Inhibition zone diameter (mm) of LAB NCFS against tested pathogens
Isolate No. |
E. coli |
S. typhimurium |
K. pneumonia |
L. monocytogenes |
|---|---|---|---|---|
SC18 |
13.3 ± 0.57 |
– |
– |
– |
SC19 |
– |
– |
– |
– |
SC20 |
– |
11.66 ± 1.5 |
– |
– |
SC21 |
– |
14.96 ± 0.95 |
– |
– |
SC22 |
9.6 ± 1.5 |
– |
– |
– |
SC23 |
14.3 ± 1.15 |
– |
– |
– |
SC24 |
15.3 ± 1.15 |
– |
7.6 ± 1.03 |
– |
The values show the three determinations’ mean ± standard deviation. No significant variances were noted between means within the same column (p >0.05). Diameter of inhibition zone. – No inhibition activity.
Molecular identification of BLIS-producing strains via 16S rRNA gene analysis
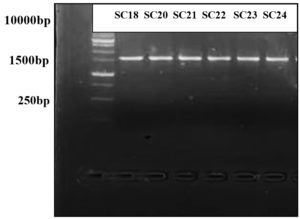
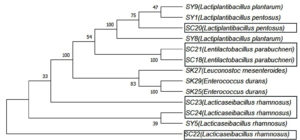
Six isolates of LAB were selected for molecular identification, as they exhibited inhibitory effects through their NCFS, suggesting their potential as BLIS producers. PCR amplification was performed using a universal bacterial primer pair. All isolates produced clear, intense bands on an agarose gel, corresponding to an anticipated product size of 1500 bp. As shown in Figure 5, the PCR products appeared as single bands representing the 16S rRNA region amplified from the DNA of each isolate. The sequencing data were compared with those of closely related strains in GenBank (Table 5). Identification at the species level was determined based on 98%-100% similarity to previously published sequences in the NCBI database. A dendrogram showing the phylogenetic analysis of the sequenced bacterial strains is shown in Figure 6. The BLIS-producing isolates were identified as three isolates of Lacticaseibacillus rhamnosus, two of Lentilactobacillus parabuchneri, and one of Lactiplantibacillus pentosus (Table 5).
Table (5):
Molecular identification of LAB isolates
Isolate No. |
Bacterial strain |
Accession number |
Similarity (%) |
|---|---|---|---|
SC18 |
Lentilactobacillus parabuchneri |
NR_041294.1 |
100% |
SC20 |
Lactiplantibacillus pentosus |
NR_029133.1 |
99.90% |
SC21 |
Lentilactobacillus parabuchneri |
NR_041294.1 |
100% |
SC22 |
Lacticaseibacillus rhamnosus |
NR_113332.1 |
100% |
SC23 |
Lacticaseibacillus rhamnosus |
NR_113332.1 |
100% |
SC24 |
Lacticaseibacillus rhamnosus |
NR_113332.1 |
100% |
Figure 5. Gel electrophoresis of PCR product for all isolates that produced a BLIS showing the bands that obtained by using 16S rRNA primer
Study of LAB derived from cheese provides critical scientific insights into microbial diversity and functional properties to confirm product safety and quality. LAB are known for their strong antimicrobial activities, which arise from their capacity to produce organic acids, bacteriocins, and H2O2, as well as through competitive exclusion of pathogens by competing for nutrients and adhesion sites. These mechanisms make LAB highly effective against various pathogens.24-26 The initial screening of the isolates led to identification of promising defensive cultures with antibacterial activity against the selected pathogens. Six LAB isolates demonstrated antagonistic effects against different pathogenic bacteria: four from cheddar cheese (SC20, SC21, SC23, and SC24) and two from feta cheese (SC18 and SC22).
As shown in Table 2, LAB cell cultures demonstrated suppressive effects against E. coli and S. Typhimurium, displaying significantly larger inhibition zones than those of the other treatments. However, the inhibition of K. pneumoniae was weak, indicating variability in LAB effectiveness depending on the pathogen. Unexpectedly, LAB cell culture was ineffective against L. monocytogenes. Previous studies have explained the defense mechanisms of L. monocytogenes, as it can develop various stress response mechanisms, including acid tolerance response pathways, resistance to oxidative stress, and resistance to bacteriocins.27,28 According to Awaisheh and Ibrahim,29 who isolated LAB from meat products, LAB isolates have antimicrobial potential against E. coli, S. Typhimurium, and L. monocytogenes. Our findings corroborated the inhibitory effects on E. coli and S. Typhimurium; however, they differed for L. monocytogenes, for which no significant inhibition was observed. The observed inhibitory effects of LAB cell cultures can be attributed to competitive exclusion, along with the synthesis of antimicrobial metabolic compounds, such as lactic acid, H2O2, and bacteriocins. These compounds lower the pH and disrupt pathogen cell membranes, leading to the observed inhibition.25 The weak inhibition of K. pneumoniae, compared to E. coli and S. Typhimurium, might be related to the complex structure of the external layer in the gram-negative bacterial cell wall, which serves as a protective barrier that restricts the entry of specific bacteriocins.30,31 Moreover, probiotics exert lower efficacy compared with that of antibiotics in inhibiting K. pneumoniae strains, which have been documented to exhibit resistance to various antibiotics. These findings explain the limited efficacy against this bacterium.30,32,33
CFS from LAB exhibited inhibitory effects, particularly toward E. coli and S. Typhimurium, but the efficacy was lower than that of cell culture. Moreover, the inhibition of K. pneumoniae was minimal, whereas no inhibition was observed for L. monocytogenes (Table 3). The CFS of LAB contains various metabolic byproducts, including organic acids, H2O2, and bacteriocins, which are generated during fermentation. These metabolites play key roles in inhibiting pathogenic microorganisms. However, unlike cell cultures, CFS lacks active competition and continuous synthesis of these antimicrobial compounds, which explains its reduced effectiveness.24,25 According to Techaoei et al.,34 CFS obtained from various Lactobacillus isolates exhibited suppressive effects against E. coli and S. aureus, indicating their promising use as biopreservatives in food products. Yazgan et al.35 indicated that the CFS of LAB isolates effectively antagonized various foodborne pathogens, including K. pneumoniae and L. monocytogenes. Campana et al.36 also confirmed that the CFS of LAB strains suppress the growth of various pathogenic bacteria, including E. coli and L. monocytogenes. Although our findings align with these results, they differ in that L. monocytogenes exhibited resistance to all treatments in the present study.
The antibacterial effects of NCFS provide strong evidence of the presence and production of bacteriocins. Referring to the data of this study, it is noticeable that there is variability between the effects of NCFS and those of the other treatments, which can be explained by the absence of several factors, including competitive exclusion, organic acids, and hydrogen peroxide, after neutralizing the extracts.25,37 As shown in Table 4, the NCFS of the six LAB strains was effective against gram-negative bacterial pathogens (E. coli, S. Typhimurium, and K. pneumoniae) but showed no effects on gram-positive bacteria (L. monocytogenes). These isolates were classified as BLIS producers and were subjected to molecular identification. The isolates were identified as Lacticaseibacillus rhamnosus (three isolates), Lentilactobacillus parabuchneri (two isolates), and Lactiplantibacillus pentosus (one isolate). Previous studies on bacteriocins from LAB strains have demonstrated their ability to suppress various bacterial pathogens. According to Kamal et al.,38 NCFS of L. rhamnosus demonstrated inhibitory effects against various pathogens, including E. coli, but did not affect S. Typhimurium, suggesting that the inhibitory activity of L. rhamnosus is group-specific. The current findings concur with these results, as all L. rhamnosus isolates (SC22, SC23, and SC24) exhibited antimicrobial activity against E. coli but did not affect S. Typhimurium. Chen et al.14 also discovered that L. rhamnosus produces a bacteriocin (CLK_01) with various antimicrobial effects against foodborne pathogens. CLK_01 suppresses the growth of several bacterial pathogens, including K. pneumoniae and E. coli. These findings were consistent with our results. Wayah and Philip39 found that Lactobacillus pentosus produces Pentocin MQ1, which inhibits several pathogens, such as L. monocytogenes and E. coli. Our findings are consistent with the inhibition of E. coli but differ regarding L. monocytogenes, which demonstrated resistance to all treatments. Other studies have also shown that L. parabuchneri strains were able to eliminate the growth of several pathogens, such as E. coli and S. Typhimurium.40,41 Importantly, it should be noted that the antimicrobial activities of bacteriocins have been documented to be species- and strain-specific.42
Limitations
The discovery and isolation of specific molecules responsible for antibacterial activity remain limitations of the current investigation.
In this study, we successfully isolated and identified LAB from cheese samples and demonstrated their potential as natural antimicrobial agents against common foodborne pathogens. These findings revealed that the LAB strains, particularly the BLIS producers, exhibited strong inhibitory effects on E. coli and S. Typhimurium. Additionally, moderate inhibition was observed against K. pneumoniae, whereas L. monocytogenes remained resistant to all treatments. The identified LAB strains-L. rhamnosus, L. pentosus, and L. parabuchneri-have the potential to enhance food safety and preservation via natural means. Further studies are needed to explore the application of these strains in various food products and efficacy of their bacteriocins in food preservation.
Recommendations
Further investigations aimed at the extraction, purification, and structural characterization of the antimicrobial metabolites produced by LAB isolates that exhibit potent inhibitory effects against pathogenic microorganisms are necessary. Given the growing concerns surrounding antibiotic overuse and its disruptive impact on the indigenous gut microbiota, the inclusion of LAB-rich fermented dairy products, such as cheese, in the human diet may serve as a complementary strategy to modulate microbial dysbiosis and support intestinal homeostasis.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MO developed the main research idea. MO and RH designed experiments. RH conducted experiments, collected and analyzed data, wrote and revised the manuscript. MO supervised the work, reviewed and revised the manuscript, and supported funding. NM provided general support and contributed to funding. All authors read and approved the final manuscript.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Akpoghelie PO, Edo GI, Ali ABM, et al. Lactic acid bacteria: Nature, characterization, mode of action, products and applications. Process Biochem. 2025;152:1-28.
Crossref - Ayivi RD, Gyawali R, Krastanov A, et al. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy. 2020;1(3):202-232.
Crossref - Roobab U, Batool Z, Manzoor MF, Shabbir MA, Khan MR, Aadil RM. Sources, formulations, advanced delivery and health benefits of probiotics. Curr Opin Food Sci. 2020;32:17-28.
Crossref - Ji J, Jin W, Liu SJ, Jiao Z, Li X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm. 2023;4(6):e420.
Crossref - Leuschner RGK, Robinson TP, Hugas M, et al. Qualified presumption of safety (QPS): a generic risk assessment approach for biological agents notified to the European Food Safety Authority (EFSA). Trends Food Sci Technol. 2010;21(9):425-435.
Crossref - Jovanovic JN, Nikolic B, Seatovic S, et al. Characterization of some potentially probiotic Lactobacillus strains of human origin. Food Sci Biotechnol. 2015;24(5):1781-1788.
Crossref - Diop MB, Dubois DR, Tine E, Ngom A, Destain J, Thonart P. Bacteriocin producers from traditional food products. Biotechnologie, Agronomie, Societe et Environnement. 2007;11(4). https://orbi.uliege.be/handle/2268/40541. Accessed May 28, 2023.
- Miranda C, Contente D, Igrejas G, Camara SPA, Dapkevicius M de LE, Poeta P. Role of Exposure to Lactic Acid Bacteria from Foods of Animal Origin in Human Health. Foods. 2021;10(9):2092.
Crossref - Yang H, Hao L, Jin Y, Huang J, Zhou R, Wu C. Functional roles and engineering strategies to improve the industrial functionalities of lactic acid bacteria during food fermentation. Biotechnol Adv. 2024;74:108397.
Crossref - Ashaolu TJ. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed Pharmacother. 2020;130:110625.
Crossref - Latif A, Shehzad A, Niazi S, et al. Probiotics: mechanism of action, health benefits and their application in food industries. Front Microbiol. 2023;14:1216674.
Crossref - Hassan MU, Nayab H, Rehman TU, et al. Characterisation of Bacteriocins Produced by Lactobacillus spp. Isolated from the Traditional Pakistani Yoghurt and Their Antimicrobial Activity against Common Foodborne Pathogens. BioMed Res Int. 2020;2020(1):e8281623.
Crossref - Zadeh RG, Asgharzadeh S, Darbandi A, Aliramezani A, Jazi FM. Characterization of bacteriocins produced by Lactobacillus species against adhesion and invasion of Listeria monocytogenes isolated from different samples. Microb Pathog. 2022;162:105307.
Crossref - Chen SY, Yang RS, Ci BQ, et al. A novel bacteriocin against multiple foodborne pathogens from Lacticaseibacillus rhamnosus isolated from juice ferments: ATF perfusion-based preparation of viable cells, characterization, antibacterial and antibiofilm activity. Curr Res Food Sci. 2023;6:100484.
Crossref - Mohammed S, Con AH. Isolation and characterization of potential probiotic lactic acid bacteria from traditional cheese. 2021;152:112319.
Crossref - Coico R. Gram Staining. Current Protocols in Microbiology. 2006;00(1):A.3C.1-A.3C.2.
Crossref - Kusale SP, Attar YC, Sayyed RZ, et al. Inoculation of Klebsiella variicola Alleviated Salt Stress and Improved Growth and Nutrients in Wheat and Maize. Agronomy. 2021;11(5):927.
Crossref - Bhutia MO, Thapa N, Tamang JP. Molecular Characterization of Bacteria, Detection of Enterotoxin Genes, and Screening of Antibiotic Susceptibility Patterns in Traditionally Processed Meat Products of Sikkim, India. Front Microbiol. 2021;11:599606.
Crossref - Udhayashree N, Senbagam D, Senthilkumar B, Nithya K, Gurusamy R. Production of bacteriocin and their application in food products. Asian Pac J Trop Biomed. 2012;2(1, Suppl):S406-S410.
Crossref - Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71-79.
Crossref - Rzepkowska A, Zielinska D, Oldak A, Kolozyn-Krajewska D. Safety assessment and antimicrobial properties of the lactic acid bacteria strains isolated from polish raw fermented meat products. Int J Food Prop. 2017;20(11):2736-2747.
Crossref - Dejene F, Dadi RB, Tadesse D. In Vitro Antagonistic Effect of Lactic Acid Bacteria Isolated from Fermented Beverage and Finfish on Pathogenic and Foodborne Pathogenic Microorganism in Ethiopia. Int J Microbiol. 2021;2021(1):1-10.
Crossref - Azcarate-Peril MA, Raya RR. Methods for Plasmid and Genomic DNA Isolation from Lactobacilli. In: Spencer JFT, de Ragout Spencer AL, eds. Food Microbiology Protocols. Methods in Biotechnology. Humana Press. 2001:135-139.
Crossref - Martin I, Rodriguez A, Delgado J, Cordoba JJ. Strategies for Biocontrol of Listeria monocytogenes Using Lactic Acid Bacteria and Their Metabolites in Ready-to-Eat Meat- and Dairy-Ripened Products. Foods. 2022;11(4):542.
Crossref - Vieco-Saiz N, Belguesmia Y, Raspoet R, et al. Benefits and Inputs From Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters During Food-Animal Production. Front Microbiol. 2019;10:57.
Crossref - Rachwal K, Gustaw K. Lactic Acid Bacteria in Sustainable Food Production. Sustainability. 2024;16(8):3362.
Crossref - Bucur FI, Grigore-Gurgu L, Crauwels P, Riedel CU, Nicolau AI. Resistance of Listeria monocytogenes to Stress Conditions Encountered in Food and Food Processing Environments. Front Microbiol. 2018;9:02700.
Crossref - Liu Y, Zhu L, Dong P, et al. Acid Tolerance Response of Listeria monocytogenes in Various External pHs with Different Concentrations of Lactic Acid. Foodborne Pathogens and Disease. 2020;17(4):253-261.
Crossref - Awaisheh SS, Ibrahim SA. Screening of Antibacterial Activity of Lactic Acid Bacteria Against Different Pathogens Found in Vacuum-Packaged Meat Products. Foodborne Pathog Dis. 2009;6(9):1125-1132.
Crossref - Riley MA, Wertz JE. Bacteriocins: Evolution, Ecology, and Application. Annu Rev Microbiol. 2002;56(1):117-137.
Crossref - Loforte Y, Fernandes N, de Almeida AM, Cadavez V, Gonzales-Barron U. A Meta-Analysis on the In Vitro Antagonistic Effects of Lactic Acid Bacteria from Dairy Products on Foodborne Pathogens. Foods. 2025;14(6):907.
Crossref - Cotter PD, Ross RP, Hill C. Bacteriocins – a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11(2):95-105.
Crossref - Quraishi F, Fatima G, Shaheen S, et al. In-Vitro Comparison of Antimicrobial Actions of Probiotics (Lactobacilli Species and Saccharomyces boulardii) with Standard Antibiotics for the Treatment of Diarrhea in Pediatric Population. Int J Clin Med. 2018;9(12):827-840.
Crossref - Techaoei S, Jarmkom K, Dumrongphuttidecha T, Khobjai W. Bioactive compound and chemical characterization of lactic acid bacteria from fermented food as bio-preservative agents to control food-borne pathogens. J Pharm Pharmacogn Res. 2023;11(6):1044-1055.
Crossref - Yazgan H, Kuley E, Gokmen TG, Regenstein JM, Ozogul F. The antimicrobial properties and biogenic amine production of lactic acid bacteria isolated from various fermented food products. J Food Process Preserv. 2021;45(1):e15085.
Crossref - Campana R, van Hemert S, Baffone W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017;9(1):12.
Crossref - Imade EE, Omonigho SE, Babalola OO, Enagbonma BJ. Lactic acid bacterial bacteriocins and their bioactive properties against food-associated antibiotic-resistant bacteria. Ann Microbiol. 2021;71(1):44.
Crossref - Kamal RM, Alnakip ME, Aal SFAE, Bayoumi MA. Bio-controlling capability of probiotic strain Lactobacillus rhamnosus against some common foodborne pathogens in yoghurt. Int Dairy J. 2018;85:1-7.
Crossref - Wayah SB, Philip K. Pentocin MQ1: A Novel, Broad-Spectrum, Pore-Forming Bacteriocin From Lactobacillus pentosus CS2 With Quorum Sensing Regulatory Mechanism and Biopreservative Potential. Front Microbiol. 2018;9:564.
Crossref - Hussein NA, Luti KJK. In Vitro Antimicrobial Activity of Lactobacillus parabuchneri NU14 as a probiotic. Iraqi J Agric Sci. 2023;54(6):1647-1658.
Crossref - Agostini C, Eckert C, Vincenzi A, et al. Characterization of technological and probiotic properties of indigenous Lactobacillus spp. from south Brazil. 3 Biotech. 2018;8(11):451.
Crossref - Corsetti A, Gobbetti M, Smacchi E. Antibacterial activity of sourdough lactic acid bacteria: isolation of a bacteriocin-like inhibitory substance from Lactobacillus sanfranciscoC57. Food Microbiol. 1996;13(6):447-456.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.