Drinking water quality monitoring technologies have made significant progress in monitoring water resources and water treatment plants. This paper discusses the adverse effect of microbial contamination and also gives a brief description of the important parameters for drinking water and the technologies currently available used in this field. This paper is focused on studying the requirement for the development of low-cost filter materials that can be suitable as well as economical to be produced on a large-scale for real applications. There are several parameters such as porosity, contact angle, water flux, thickness, microbial activity needed to be focused on in the future to study the transformation of the hydrophilic property on the surface of the water.
Water Filtration, Water Borne Disease, Membrane Filtration
Water is an essential component for the survival of an individual as well as daily essential work. To ensure the availability of water for specific purposes such as drinking water, there is a need to meet some conditions such as its availability, accessibility, and treated as well. In the current scenario with the increasing population, there is also an increase in demand for drinking water that must be fit for consuming. But it is also true that with the increasing population there is an increase in pollution level which ultimately also affects the pollution level in the water. The major cause of water contamination or pollution is human activities that result in decreased quality of water. Out of all, human activities such as improper disposal of sewage, failure of the septic system, animal wastes are a major source for water contamination.1 The discharge of waste material into drinking water not only causes disbalance in nutrient content as well also increases the growth of microbes, such as viruses, bacteria, etc, that can cause various water-borne diseases. From the study of 2012, it is surveyed that about 25% of drinking water is contaminated with fecal or harmful materials which cause the growth of a large number of harmful pathogens.2 Consumption of such contaminated water can cause different severe health issues such as diarrhea, hepatitis, meningitis, encephalitis, polio, etc. According to the World Health Organization (WHO) report issued in 2019, about 844 million people all over the world are underprivileged with drinking water. Out of them, 159 million people are dependent on surface water completely. The contaminated water or polluted water is considered to be a major reason for death. It has been reported that approx. 5,02,000 deaths per year all over the world are caused by drinking contaminated water.3 In India, death due to contaminated drinking water has been reported that 6514 deaths over 5 years until 2017. Similarly, due to virus borne diseases such as hepatitis, 2143 people died and became the second most alarming water-borne disease.4 Some of the water-borne diseases are discussed in Table 1.
Table (1):
Water-borne diseases.
Disease |
Pathogens |
Symptoms |
|---|---|---|
Diarrhea |
virus |
abdominal cramps fever weakness |
Cholera |
Bacteria |
Diarrhea |
Typhoid |
Bacteria |
Fever Diarrhea Vomiting |
Amoebiasis |
Parasite |
Fever Diarrhea Vomiting Loss of appetite |
Hepatitis |
Virus |
Jaundice Diarrhea Vomiting |
Giardia |
Parasite |
Diarrhea Vomiting Fatigue Abdominal pain |
Salmonella |
Bacteria |
Blood in stool Diarrhea Headache |
Hence, it is required to provide provision for safe drinking water as a part of public health protection all over the world. Pretreatment and post-treatment techniques including distillation, treatment with chemical disinfectants, sand filtration, reverse osmosis, and membrane filtration are widely used technologies to purify water. There are many traditional methods to filter water contaminations. It has been observed that many conventional techniques are being used for the filtration and separation of pathogens from water, such as coagulation, deep bed filtration for particle separation, or chlorination. But chlorination is quite inexpensive and takes a long time for operation along with chlorination also causes adverse effects on human health. Further, iodine is used as a substitute for chlorine for purification of drinking water.
One of the simplest methods for water purification is boiling the water before using it for drinking purpose. However, this process consumes more fuels. Then come UV and RO filtration and purification techniques that are gaining popularity nowadays for their efficiency to purify the contaminated water and making it fit for drinking. Ultraviolet (UV) technique destroys the cell structure of pathogens and prevents them to grow. Whereas the reverse osmosis (RO) technique is used across a semi-permeable membrane to separate the contaminants from the drinking water.5 But this technique is quite expensive, so, many researchers focus their work on this membrane filtration technique and researched many polymeric and ceramic membranes.
The membrane filters, made up of polymers have a low cost but due to lower thermal effect as well as the chemical effect with lower mechanical stability limits its usage as purification filter material. As compared to polymers ceramics provide these advantages as well as also protects from pathogens. The ceramic filter membrane provides lower membrane fouling.6
The membrane filtration process shows an advantage over other methods as it separates pathogens from water. This method can be used with a pretreatment process or without a pretreatment process. The conventional membrane water filtration process was successful for large-sized pathogens such as protozoa, bacteria, etc. But these membrane filters are not capable of removal of viruses as their size is very small, up to 20−100 nm. So, to remove viruses, nano-particle material, the adsorbing surface is used to filter the contaminated water surface by applying forces such as van der Waals forces, electrostatic forces, and hydrophobic interactions. The literature reviews that recent research work that contributes to the adsorption process. In the adsorption process, electrostatic forces are applied.7 Many water-borne diseases are caused by a virus. These viruses are negatively charged and it gets attracted by positively charged adsorption surface of the filter. In this way, contaminated water gets filtered.
This paper focuses on the usage of the membrane filtration method for the removal of microbes that contaminates water. Different membrane separation processes are also discussed with their limitations. In the further section of the paper different adsorption methods for the microbial removal process. In this article, a performance comparison is also discussed for the exploration of future scope.
Contaminants in water and its effects
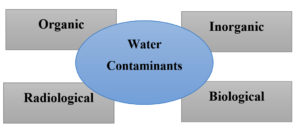
By accessing odor, color, turbidity, or taste are used to access or to determine the contamination level. This may cause severe health issues. However, sometimes contamination cannot be detected easily. In such a situation there is a requirement of the testing process. Types of contaminants are shown in Figure 1.
Chemical parameters of water determine its contamination level or contaminant composition. The hardness of water depends on the geographical location. Due to the presence of calcium or magnesium, water becomes hard and it is classified into two types such as carbonates (containing co3– ions) or non-carbonates. Generally, a hardness level that is considered to be fit is about 300–400 mg/L but its prolonged usage will cause stone formation in the kidney. Some other inorganic compounds such as fluoride, arsenic, lead, copper, chromium, mercury, antimony, cyanide, etc can also contaminate drinking water. Arsenic contamination is the main cause of water contamination all over the world and also in India.8 Another contamination source is organic compounds such as pesticides, domestic waste, industrial waste, etc. Similarly, the presence of living organisms causes biological contamination in water. The sources of radioactive material can also contaminate water as they emit radiations (like a, β). Each of these can cause distinctive problems in water as discussed in Table 2.
Table (2):
Different contaminants and their adverse effect.7-16
Contaminants |
Contaminants classification |
Adverse effect |
|---|---|---|
Cr |
Inorganic |
Toxic in nature Allergic |
Hg |
Inorganic |
Alteration in the immune system Cardiovascular toxicity |
Cd |
Inorganic |
Risk of cancer Damage to kidney |
Pb |
Inorganic |
Hypertension |
As |
Inorganic |
Risk of cancer |
Mg |
Inorganic |
The risk to the brain or neurological disorder The risk to the endocrine system |
Nitrogen |
Inorganic |
Blue-baby syndrome, breathing problem in infants |
Sulfate |
Inorganic |
Diarrhea |
Coliform |
Biological |
Diarrhea |
Radium |
Radiological |
Risk of Cancer |
Uranium |
Radiological |
Risk of Cancer |
Pesticides |
Organic |
Damage liver and nervous system |
Trichloroethylene |
Organic |
Risk of Cancer Nervous system and reproductive disorder Damage to liver or kidney |
Vinyl chloride |
Organic |
Risk of Cancer Nervous system and reproductive disorder Damage to liver or kidney |
Water decontamination methods
Water decontamination is the removal or reduction of contaminants from water sources by physical, chemical, or other methods. There are many approaches developed that aims to decontaminate or disinfect the public water supplies especially for drinking. Contaminated water is a major source of water-borne diseases and also acts transmission medium of such diseases. Some of the processes and techniques in mitigating the contaminations are discussed below and their advantages and disadvantages are discussed in Table 3.
Table (3):
Advantages and disadvantages of different water decontamination methods.17
Methods |
Advantages |
Disadvantages |
|---|---|---|
Precipitation and coagulation |
Simple Easy to implement Suitable for organic contaminants or dissolved organic compounds |
Requires chemicals for this process Effect of by-products Sludge formation and their disposal |
Distillation |
Suitable for removal of inorganic and biological contaminants Doesn’t require post-filtration methods |
Consumes energy Requires maintenance Not much effective for organic contaminants |
Adsorption
(Activated Carbon) |
Effective for removal of carbon impurities and chlorine impurities Cost-effective Durable High capacity |
Filter replacement reduces efficiency Can generate carbon fines |
Adsorption
(Activated alumina) |
Suitable for contaminants such as arsenic, chloride, fluoride |
Not capable to remove other contaminants |
Adsorption
(Silica gel) |
Non-toxic Non-corrosive High adsorption High porosity |
Needed to be precise |
Ion exchange resins |
Simple and low cost Can handle hazardous water contaminants Long-lasting |
Generation of sodium waste Don’t remove organic or biological contaminants |
Membrane Filtration
(Reverse Osmosis) |
Require low energy Environment friendly Don’t produce harmful chemicals Suitable for all types of contaminates No taste or smell alteration |
Require replacement of the membrane |
Membrane Filtration
(Electrodialysis) |
Safe Non-polluting Reliability Effective for ionic particles and heavy metals |
The requirement of a large area for membrane especially for low concentration of contaminants |
UV Treatment |
Easy to use Inexpensive Effective for biological contaminants No adverse effect on mineral contents of water. |
Less effective on bacterial spores |
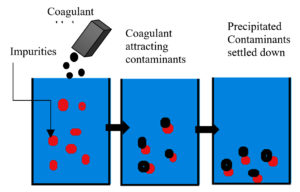
Precipitation and Coagulation
Precipitation and coagulation is a process to remove unwanted substances from the contaminated or polluted water by adding some reagents, as presented in Figure 2. This is based on ‘solubility’ rules and is considered to be one of the simplest methods for decontamination. These techniques are used to remove contaminants like phosphorus, fluoride, arsenic, ferrocyanide and heavy metals, etc.
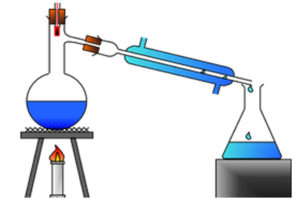
Distillation
In this technique, heat is applied to separate contaminants from water. The concept is based on the difference in boiling points of components of water, as presented in Figure 3. This technology was developed mainly for industrial as well as domestic purposes. This process is not effective for the removal of organic contaminants. As the distillation process is safe but cannot contains any nutrients or minerals. So, such a method cannot be recommended for drinking water filtration.
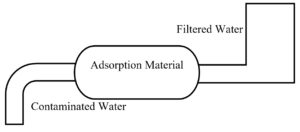
Adsorption
In this process, a porous surface is provided to separate solid contaminants as they adhere to the surface, as presented in Figure 4. In this method, the adsorbent surface is directly added to the supply of water that combines physical and chemical processes to remove contaminants without altering the taste or mineral content of the water. All micro-porous or nano-porous materials can be used as adsorbent. Some of the porous solids such as activated carbon, activated alumina, silica gel, zeolite, etc can be used as adsorbent as they are porous and contains nano-sized surface pores.
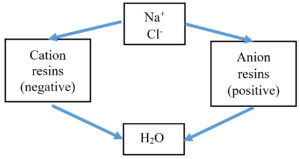
Ion exchange
The ion exchange (as presented in Figure 5) filtration method uses the small polymer matrix having a diameter in millimeter (mm). Its structure is porous and contains gel. The ion exchange technology is based on the attraction of similar ions. Ions present in the water get exchanged with ions present in the polymer matrix. Common methods for the water filtration process are softening as well as deionization. The reduction of hardness level of water is performed by the process of softening which can also be used in the reverse osmosis technique.
Membrane Filtration
Another innovative idea for water filtration is membrane filtration in which a semi-permeable membrane is used for this purpose. Generally, there are two types of water treatment processes i.e. reverse osmosis (RO) and electro-membrane, as presented in Figure 6. Micro-membrane techniques are robust and efficient methods to remove contaminants. In this technique, the pressure-driven separation process is performed that can separate contaminants from water chemically as well as mechanically.
UV Treatment
In the UV filtration process, the ultraviolet (UV) light is allowed to pass through the contaminated/polluted water, as presented in Figure 7. Bypassing UV light through water, it breaks the genetic structure of microbial contaminants present in the water get damaged and, in this way, microbes get killed. But this method is not effective for the treatment of chemicals or other organic/inorganic contaminants.
Membrane based filtration method
The membrane filtration method is considered to be one of the safer and cleaner as compared to other filtration methods in terms of health issues. As the membrane filtration method is best suited for microbes’ filtration of macro to nano size. Bacteria, macro in size, can be completely removed by membrane technology. This technology when implemented in industries can be better in respect of cost as compared to conventional water treatment processes.
The use of ultrafiltration or microfiltration in place of conventional methods reduced the cost of refinement for the same capacity plant. Technological advancement is shown by the fabrication of thin-film nanocomposite material, metal-organic material, carbon nanotubes. This technologically advanced nano-tube offers 2–3 times higher permeability along with salt rejection than conventional membrane technology, especially for industrial usage.18,19 While designing the cost-effective membrane filters following factors must be considered:
- Membrane materials
- Water flux
- Wall thickness
- Water contact angle
- Mechanical/Chemical/Thermal Stability
- Operation cost
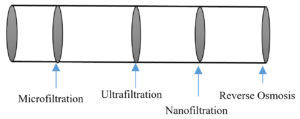
The membrane filtration process is mainly affected by the material and structure of the pores as well as its chemical and physical properties also shows impact. Many research works have been incorporated for exploring new membrane materials or design modifications for increasing its performance level. The most commonly used materials for ultrafiltration (UF), microfiltration (MF), or nanofiltration (NF) are synthetic polymers or ceramics. In the table 4 feature comparison is presented which will help in deciding material for the membrane.
Table (4):
Comparison between ceramic and polymer membrane filters.20-23
Features |
Ceramic |
polymers |
|---|---|---|
Thermal stability |
High |
Low |
Mechanical stability |
High |
Low |
Chemical stability |
High |
Low |
Pathogenic Tolerance |
High |
Low |
Weight |
High |
Low |
Cost |
Low |
High |
Hence, the membrane filtration method shows its supremacy over conventional processes but every technology have some disadvantages. Some of the work contributing membrane technology are discussed in table 5. The preparation material for UF and MF membranes is almost the same but their preparation methods are different which results in different sized pores. As stated earlier these membranes can also be made from inorganic materials such as ceramics or zeolites.24,25 But due to high operational cost and mechanical fragility, these materials are not much effective for large-scale applications. In the below section some of the research improvements are also discussed. The ceramic membranes exhibit high fouling-resistance and chemical stability as compared to current polymeric membranes. Some of the porous ceramics are such as Al2O3, TiO2, ZrO2, ZnO, and SiO2 or their composites. These materials are actively studied due to their widespread applications.26 These materials also exhibit photocatalytic ability for the decomposition of organic substances/biological species which reduces unwanted adsorption on the membrane surface.27 Although inorganic membranes are gaining more attention, the majority of membranes are made of polymeric materials as they provide a wide variety of structures and properties. Some of the common organic and inorganic materials are discussed in table 6 with their key features and drawbacks.
Table (5):
Comparative performance of different membrane filters.
Membrane |
Porosity |
Wall Thickness or Crystal size |
Water contact angle |
Water Flux (L/m2h) |
Ref |
|---|---|---|---|---|---|
Zr-based hollow fiber membrane |
81.51% |
0.18 mm |
62.06o |
50.68 |
[28] |
Green silica based ceramic hollow fiber membrane |
70% |
902 µm |
– |
130 |
[29] |
PP/TiO2 membrane |
– |
2.38 nm |
91.8o |
– |
[30] |
PVDF-PVP-TiO2-FeCl3 ultrafiltration membranes |
43% |
200nm |
16o |
~200 |
[31] |
Gravity-driven membrane |
– |
– |
37o |
52 |
[32] |
3-layer polyacrylonitrile membrane |
– |
~10 µm |
– |
~100 |
[33] |
Polyvinylpyrrolidone membrane |
– |
~0.3mm |
– |
~200 |
[34] |
Table (6):
Membrane materials and their key features.35-41
Materials |
Type |
Features |
|---|---|---|
TiO2 |
Inorganic |
Photocatalytic under UV. Anti-fouling. Applicable to MF and UF. |
Ag/TiO2 |
Inorganic |
Applicable in UF. Bacterial inactivation. |
TiO2–SiO2/ Al2O3 |
Inorganic |
Photocatalytic under UV. Applicable to UF. |
Graphene |
Inorganic |
Applicable to NF. High retention for dyes and ions. |
Polyvinyl Alcohol (PVA)/Polypropylene (PP) |
Organic |
Applicable to MF. Achieve smaller as well as average pore size. High fouling tendency High operation cost and shorter lifetime |
Cellulose Acetate (CA) |
Organic |
Applicable to UF. High flux and lack long term stability |
Polyvinyl chloride (PVC) and polyvinyl formal (PVF) |
Organic |
Applicable to UF. Enhanced anti-fouling property. |
Polysulfone (PSU) |
Organic |
Applicable to NF. Improved hydrophilicity |
Polyisoprene (PI) |
Organic-Inorganic |
Applicable to MF. Improved porosity. |
AgNPs/ polysulfone(PSU) |
Organic-Inorganic |
Applicable to UF Improved anti-bacterial effect and fouling strength. |
ZnO/PES |
Organic-Inorganic |
Applicable to NF Improved permeability and fouling resistance |
Water is the source of life and its safety is fundamental. Since WDS systems are complex systems and the quality of drinking water is influenced by many factors, it is difficult to guarantee the safety of drinking water. The current water treatment & distribution system has a lot of drawbacks. Membrane filtration is considered to be one of the methods that are capable to meet the standards for safe drinking water either in a stand-alone unit or as a hybrid unit. The performance of the membrane-based filtration method is depending on many factors such as the number of pores and thickness of coatings. Nanotechnology has the potential to replace them and increase efficiency to prevent microbial activities. Nano-technology could potentially lead to more effective means of filtration that not only remove more impurities than current methods but do so faster, more economically, and more selectively. So, in the future design of the membrane would direct research work for the development of cost-effective with proven nontoxicity effects on the environment that could revolutionize the water treatment domain and prevention of microbes.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both the authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
Not applicable.
ETHICS STATEMENT
Not applicable.
- Owa FD. Water pollution: sources, effects, control and management. Mediterr J Soc Sci. 2013;4(8):65-65.
Crossref - Gall M, Marinas BJ, Lu Y, Shisler JL. Waterborne viruses: a barrier to safe drinking water. PLoS Pathog. 2015;11:e1004867
Crossref - World Health Organization. Alternative drinking-water disinfectants: bromine, iodine and silver. 2018. https://www.who.int/publications/i/item/9789241513692. Accessed on 24-11-2022.
- Kumar DM, Tortajada C. Assessing Wastewater Management in India. Springer Nature, Singapore. (Book). 2020
- Qasim M, Badrelzaman M, Darwish NN, Darwish NA, Hilal N. Reverse osmosis desalination: a state-of-the-art review. Desalination. 2019;459:59–104.
Crossref - Samael SM, Gato-Trinidad S, Altaee A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters – a review. Sep Purif Technol. 2017;200:198–220.
Crossref - USEPA, Chromium in Drinking Water, United States Environmental Protection Agency. 2018. https://www.epa.gov/dwstandardsregulations/chromium-drinking-water. Accessed on 17 January 2018.
- Khan MMH, Sakauchi F, Sonoda T, Washio M, Mori M. Magnitude of arsenic toxicity in tube-well drinking water in Bangladesh and its adverse effects on human health including cancer: evidence from a review of the literature. Asian Pacific J Cancer Prev. 2003;4(1):7–14. http://journal.waocp.org/article_24154.html
- Baruthio F. Toxic effects of chromium and its compounds. Biol Trace Elem Res. 1992;3:145–153.
Crossref - Passos CJ, Mergler D. Human mercury exposure and adverse health effects in the Amazon: a review. Cad. Saude Publica. 2008;24(suppl 4):S503–S520.
Crossref - Bernard A. Cadmium & its adverse effects on human health. Indian J Med Res. 2008;128(4):557–564. https://journals.lww.com/ijmr/Abstract/2008/28040/Cadmium___its_adverse
_effects_on_human_health.16.aspx - Agusa T, Kunito T, Ramu K, et al. Lead contamination and its human health effects in India, Vietnam, and Cambodia. Biomed Res Trace Elem. 2006;17(4):413–416.
Crossref - Kapaj S, Peterson H, Liber K, Bhattacharya P. Human health effects from chronic arsenic poisoning–a review. J Environ Sci Health A Tox Subst Environ Eng. 2006;41(10):2399–2428.
Crossref - Backer LC. Assessing the acute gastrointestinal effects of ingesting naturally occurring, high levels of sulfate in drinking water. Crit Rev Clin Lab Sci. 2000;37(4):389–400.
Crossref - Fawell J, Nieuwenhuijsen MJ. Contaminants in drinking water. Br Med Bull. 2003;68:199–208.
Crossref - Rahmanian N, Ali SHB, Homayoonfard M, et al. Analysis of physiochemical parameters to evaluate the drinking water quality in the State of Perak, Malaysia. J Chem. 2015;2015:716125.
Crossref - Sharma S, Bhattacharya A. Drinking water contamination and treatment techniques. Appl Water Sci. 2017;7:1043–1067.
Crossref - Tang CY, Yang Z, Guo H, Wen JJ, Nghiem LD, Cornelissen E. Potable water reuse through advanced membrane technology. Environ Sci Technol. 2018;52(18):10215–10223.
Crossref - Le NL, Nunes SP. Materials and membrane technologies for water and energy sustainability. SM & T. 2016;7:1–28.
Crossref - Hofs B, Ogier J, Vries D, Beerendonk EF, Cornelissen ER. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Separ Purif Technol. 2011;79(3):365–374.
Crossref - Verma B, Balomajumder C, Sabapathy M, Gumfekar SP. Pressure-driven membrane process: a review of advanced technique for heavy metals remediation. Processes. 2021;9(5):752.
Crossref - Muntha ST, Kausar A, Siddiq M. Progress in applications of polymer-based membranes in gas separation technology. Polym-Plast Technol. 2016;55(12):1282–1298.
Crossref - Park SH, Park YG, Lim JL, Kim S. Evaluation of ceramic membrane applications for water treatment plants with a life cycle cost analysis. Desalin Water Treat. 2015;54(4-5):973–979.
Crossref - Mohmood CB, Lopes I, Lopes I, Ahmad AC. Duarte and E. Pereira. Nanoscale materials and their use in water contaminants removal-a review. Environ Sci Pollut Res. 2013;20(3):1239- 1260.
Crossref - Koe WS, Lee JW, Chong WC, Pang YL, Sim LC. An overview of photocatalytic degradation: photocatalysts, mechanisms, and development of photocatalytic membrane. Environ Sci Pollut Res. 2020;27(3):2522-2565.
Crossref - Zawodzinski Jr TA, Neeman M, Sillerud LO, Gottesfeld S. Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J Phys Chem.1991;95(15):6040-6044.
Crossref - Leong S, Razmjou A, Wang K, Hapgood K, Zhang X, Wang H. TiO2 based photocatalytic membranes: A review. J. Memb. Sci. 2014;472:167-184.
Crossref - He J, Matsuura T, Chen P. A Novel Zr-based Nanoparticle-Embedded PSF Blend Hollow Fiber Membrane for Treatment of Arsenate Contaminated Water: Material Development, Adsorption and Filtration Studies, and Characterization. J. Memb. Sci. 2013;452:433-445.
Crossref - Hubadillah SK, Othman MHD, Ismail AF, et al. Fabrication of Low Cost, Green Silica Based Ceramic Hollow Fibre Membrane Prepared from Waste Rice Husk for Water Filtration Application. Ceram Int. 2018;44(9):10498-10509.
Crossref - Sievers NV, Pollo LD, Corcao G, Cardozo NSM. In situ synthesis of nanosized TiO2 in polypropylene solution for the production of films with antibacterial activity. Mater Chem Phys. 2020;246:122824.
Crossref - Zhou A, Wang Y, Sun S, et al. Removal of sulfadiazine in a modified ultrafiltration membrane (PVDF-PVP-TiO2-FeCl3) filtration-photocatalysis system: parameters optimizing and interferences of drinking water. Environ Sci Pollut Res. 2020;27(36):45605-45617.
Crossref - Lee D, Lee Y, Choi SS, Lee S-H, Kim K-W, Lee Y. Effect of membrane property and feed water organic matter quality on long-term performance of the gravity-driven membrane filtration process. Environ Sci Pollut Res. 2019;26(2):1152–1162.
Crossref - Nallathambi G, Baskar D, Selvam AK. Preparation and characterization of triple-layer membrane for water filtration. Environ Sci Pollut Res. 2020;27(24):29717–29724.
Crossref - Fan K, Liu E, Zhou G, Li J. Preparation of Filtration Membrane by Grafting of Poly(N-vinylpyrrolidone) onto Polyethersulfone and Its Influence on Pollution Resistance of Membrane. Polym Sci Ser B. 2020;62(5).
- Lee A, Elam JW, Darling SB. Membrane materials for water purification: Design, development, and application. Environ Sci Water Res Technol. 2016;2(1):17–42.
Crossref - Li, Ning, Lu X, He M, et al. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J Hazard Mater. 2021;414:125478.
Crossref - Castro-Munoz R, Garcia-Depraect O. Membrane-based harvesting processes for microalgae and their valuable-related molecules: A review. Membranes. 2021;11(8):585.
Crossref - Chadha U, Selvaraj SK, Thanu SV, et al. A review of the function of using carbon nanomaterials in membrane filtration for contaminant removal from wastewater. Mater Res Express. 2022;9:012003.
Crossref - Manikandan S, Subbaiya R, Saravanan M, Ponraj M, Selvam M, Pugazhendhi A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere. 2022;289:132867.
Crossref - Liu Y, Sun Y, Li H, Ren T, Li C. Co-filtration of bacteria/electrospun oriented carbon nanofibers integrating with carbon nanotubes for microbial fuel cell. J Environ Chem Eng. 2022;10(3):107664.
Crossref - Mahajan-Tatpate P, Dhume S, Chendake Y. Carbon Nanomaterial Embedded Membranes for Heavy Metal Separation. In Carbon Nanotubes for a Green Environment. 1st Edition; Apple Academic Press. 2022:97-122.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.