Rutin is one of important bioflavonoids and biomarker that helps to increase the quality of the herbal product. It has a wide variety of pharmacological applications such as neuroinflammation, anti-hypercholesterolemic, neuroprotective, cardioprotective, wound healing, radioprotective, nephroprotective, hepatoprotective, antiplasmodial, antiarthritic, antiviral, antihypertensive, Antinociceptive, antimicrobial, gastroprotective, antiosteoporotic, anticancer, diuretic and anticonvulsant effect. The current review article helps to identify the current and future prospects of rutin. Most of the previous studies were more focus on their pharmacological activities and to understand their mechanism of action but less focus on its clinical trial, commercial potential and formulation development. This review article documents the pharmacological activities, standardization methods and formulation development over the last six years.
Rutin, Standardization, Pharmacology, Formulation development.
The role of flavonoids can never be ignored in our daily life. They had a wide variety of application in our daily health care such as antioxidant 1,2, anticancer3, neurodegeneration4 and cardiovascular disease5,6. They are commonly available in our food such as fruits, beverages, and vegetables. There is a different class of flavonoids such as flavones, flavonols, and flavanones. Quercetin, rutin, kaempferol belongs to most common available flavonoids in our diet. This review article is based on updated information about the pharmacological potential, standardization method and recent knowledge about formulation development of rutin. Google scholar is used as a search engine to find the information from 2012 till to date. The keywords used to find the information was rutin, standardization method of rutin, pharmacological activities of rutin and formulation development of rutin in recent years (2012-2017).
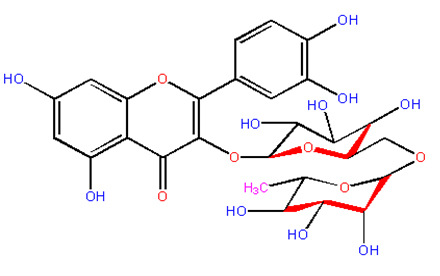
Fig. 1. Structure of Rutin
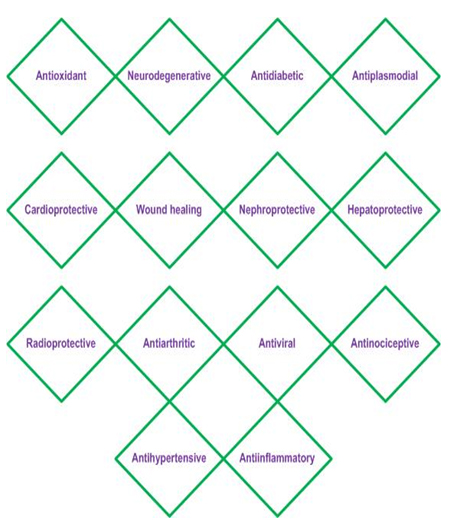
Fig. 2. Pharmacological activities of Rutin
Pharmacological activities
Rutin possesses several pharmacological activities such as reducing oxidative stress, prevent neuroinflammation7, anti-diabetic8, aiding to reduce neurodegeneration9, cardioprotective activity10, wound healing activity11, radioprotective activity12,13, nephroprotective activity14, hepatoprotective activity15, antiplasmodial activity16, antiarthritic activity17, antiviral activity18, improved endothelial functions19 and antinociceptive activities20.
Antioxidant
The recent research on rutin was based on enhancing the activity and optimization of antioxidant potential. The formation of supramolecular inclusion complexes between rutin and four cyclodextrins, namely b-cyclodextrin (b-CD), (2-hydroxypropyl)-a-cyclodextrin (HP-a-CD), (2-hydroxypropyl)-b-cyclodextrin (HP-b-CD) and (2-hydroxypropyl)-g-cyclodextrin (HP-g-CD), and the effects of the complexation on the stability and antioxidant activity of rutin were investigated. Formation of such an inclusion complex conferred moderate degrees of protection to rutin from degradation by heat and UV radiation during storage, and significantly enhanced its antioxidant capacity21. Enzymatic de-glycosylation enhance the antioxidant potential of rutin22. Encapsulation of rutin with lipid-based onion-type multilamellar vesicles (MLVs) optimized the antioxidant activity23. It considerably decreased lipid peroxidation in 6-hydroxydopamine (6-OHDA) toxicity induced in pheochromocytoma (PC-12) cells24. Rutin has a protective effect against hydrogen peroxide-induced oxidative stress in human lens epithelial cells25.
Neurodegenerative effect
Rutin can help to treat peripheral neuropathy in mice24. Rutin showed neuroprotective effects in streptozotocin-induced diabetic rat’s retina26. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease27. Rutin is an effective bioflavonoid against neurotoxicity in rats by activating the mitogen-activated protein kinase (MAPK) pathway and brain-derived neurotrophic factor (BDNF) gene expression 28. Rutin decreased the oxidative stress in seizure-induced mice by kainic acid29. Rutin is an effective bioactive compound against neurotoxicity caused by acrylamide30
Antidiabetic effect
Rutin possesses the antidiabetic activity by stimulating b-cells to release more insulin31. It showed anti-hyperglycemic activity in streptozotocin-induced diabetic rats32.
Cardioprotective effect
Rutin possesses the cardioprotective effect but recent studies found that the effect was less effective than quercetin on isoproterenol-induced cardiac fibrosis in the rat10. It was found to be an effective as protective agent in hypercholesterolemic male rat33.
Wound healing activity
Rutin containing hydrogel was found to be an effective bio compound in healing the wound11. The roots and leaves of Tephrosia purpurea possess 2.5% of rutin and was found as an effective wound healing agent in streptozotocin-induced diabetic rats34. Rutin was also identified as bioactive compounds in the polyherbal formulation of Clinacanthus nutans and Elephantopus scaber in healing the wound using high-performance liquid chromatography35.
Nephroprotective activity
Rutin has a protective effect on hexachlorobutadiene-induced nephrotoxicity36.
Hepatoprotective effect
Rutin was found to be an effective flavonoid in reducing hepatotoxicity caused by CCl415 and liver injury induced by biliary obstruction in rats 37. A Recent study reveals that it alters the expression of genes in the IL-6/STAT3 pathway in reducing hepatotoxicity caused by CCl438.
Radioprotective effect
Rutin plays an important role as a radio modulator effect in Swiss albino mice when exposed to gamma radiations13. Monoglucosyl rutin is an enzymatically modified form of rutin that is found be an effect radioprotector in mammalian cells39.
Miscellaneous activities
The antiproliferation of rutin in human neuroblastoma cells LAN-5 were detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay through inducing G2/M cell cycle arrest and promoting apoptosis40. The quercetin analog rutin was the most active flavonoid against Plasmodium falciparum parasites16.
Standardization of Rutin
Rutin in the polyherbal formulation of Azadirachta indica and Gynura procumbens ethanolic extracts was standardized by TLC-densitometry 41. High-performance liquid chromatography was found be the most used method of standardization using C18 column. It was identified and quantified in the polyherbal formulation of Clinacanthus nutans and Elephanopus scaber using high-performance liquid chromatography. The mobile phase was a binary mixture of methanol-water, 1:1 (v/v), adjusted to pH 3.0 with glacial acetic acid was used42. Erythroxylum suberosum extract possesses rutin. Aqueous phosphoric acid (1%) (solvent A) and acetonitrile (solvent B) was used as a solvent system in HPLC43. Rutin was identified from Strychnos nux-vomica extract using High-performance thin layer chromatography (HPTLC)44. It was quantified from Lepidagathis prostrate using silica gel plate as a stationary phase and ethyl acetate, n-butanol, formic acid, water in the ratio (5:3:1:1) as mobile phase in HPTLC45.
Formulation Development of Rutin
Rutin has been used in different formulation nowadays. Rutin hydrogel was effective in healing the wound11. Rutin is poorly soluble in water. Liposome hydrogel can function as potential drug delivery systems to enhance transdermal permeation of the water-insoluble antioxidants such as quercetin and rutin46. Rutin loaded nanoemulsions were prepared by spontaneous emulsification method and high-pressure homogenization (HPH) technique using sefsol 218 and tocopheryl polyethylene glycol 1000 succinate (TPGS) (1:1), solutol HS15 and transcutol P as oil phase, surfactant and co-surfactant will increase the solubility and permeability of rutin47. Rutin loaded nanophytosomal formulation was found to be the useful carrier to improve the bioavailability and antioxidant properties48. Elastic liposome containing rutin helps to improve the skin permeation effect49. Self-emulsifying drug delivery systems of rutin were prepared by polyethylene glycol (PEG 6000), polyvinylpyrrolidone (PVP K30 and PVP K17) or sodium desoxycholate by co-precipitation method to improve the bioavailability of bioactive compound50. Rutin-encapsulated chitosan nanoparticles can be helpful as neuroprotective and readily available to the brain in the treatment of Cerebral Ischemia51. Rutin nanocrystals produced by the smart crystal help in enhancing skin penetration52. Nanostructured lipid carriers (NLCs) contains rutin can be useful as photoprotective cream. Apifil/TiO2 rutin NLC is found be effective as sun protective cream53.
Rutin is found to be one of a member of an essential flavonoid used in our daily routine. The current research is more focus on optimization of its pharmacological activities and formulation development. There is a lot of effort going on to enhance its pharmacokinetic profile. The commercial potential of rutin is still yet to be explored. There are limited studies on a clinical trial. Most of the preclinical studies are limited on animal models. It is need of time to make use of its pharmacological activity by developing different pharmaceutical dosage form and standardization of new rutin based formulation.
- P. G. Pietta, “Flavonoids as antioxidants,” Journal of Natural Products, 2000; 63, no. 7. pp. 1035–1042.
- D. Pal and P. Dubey, “Flavonoids: A powerful and abundant source of antioxidants,” International Journal of Pharmacy and Pharmaceutical Sciences, 2013; 5(3). pp. 95–98.
- W. Ren, Z. Qiao, H. Wang, L. Zhu, and L. Zhang, “Flavonoids: Promising anticancer agents,” Medicinal Research Reviews, 2003; 23(4). pp. 519–534.
- F. Dajas et al., “Neuroprotection by flavonoids,” Brazilian Journal of Medical and Biological Research, 2003; 36(12): 1613–1620.
- S. E. Rasmussen, “Flavonoids and cardiovascular disease,” in Functional Foods, Cardiovascular Disease and Diabetes, 2004, pp. 157–186.
- J. A. Lovegrove, A. Stainer, and D. A. Hobbs, “Role of flavonoids and nitrates in cardiovascular health,” Proc. Nutr. Soc., 2016, pp. 1–13, 2017.
- H. Javed et al., “Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type,” Neuroscience, 2012; 210: pp. 340–352.
- A. Hunyadi, A. Martins, T.-J. Hsieh, A. Seres, and I. Zupkó, “Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats.,” PLoS One, 2012; 7(11), p. e50619.
- P. xin Xu et al., “Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aâ oligomer level and attenuating oxidative stress and neuroinflammation,” Behav. Brain Res., 2014; 264: pp. 173–180.
- M. Li, Y. Jiang, W. Jing, B. Sun, C. Miao, and L. Ren, “Quercetin provides greater cardioprotective effect than its glycoside derivative rutin on isoproterenol-induced cardiac fibrosis in the rat.,” Can. J. Physiol. Pharmacol., 2013; 91(11), pp. 951–959.
- J. S. Almeida, D. M. Benvegnú, N. Boufleur, P. Reckziegel, R. C. S. Barcelos, and et al. Coradini, K., “Hydrogels containing rutin intended for cutaneous administration: efficacy in wound healing in rats.,” Drug Dev. Ind. Pharm., 2012; 38(7), pp. 792–9.
- S. L. Patil, S. H. Mallaiah, and R. K. Patil, “Antioxidative and radioprotective potential of rutin and quercetin in Swiss albino mice exposed to gamma radiation.,” J. Med. Phys., 2013; 38(2): pp. 87–92.
- S. L. Patil, H. Somashekarappa, and K. Rajashekhar, “Radiomodulatory role of Rutin and Quercetin in Swiss Albino mice exposed to the whole body gamma radiation.,” Indian J. Nucl. Med., 2012; 27(4): pp. 237–42.
- K. M. Kamel, O. M. Abd El-Raouf, S. A. Metwally, H. A. Abd El-Latif, and M. E. El-sayed, “Hesperidin and rutin, antioxidant citrus flavonoids, attenuate cisplatin-induced nephrotoxicity in rats,” J. Biochem. Mol. Toxicol, 2014; 28(7): pp. 312–319.
- R. a Khan, M. R. Khan, and S. Sahreen, “CCl4-induced hepatotoxicity: protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat.,” BMC Complement. Altern. Med., 2012; 12: p. 178.
- D. Ganesh et al., “Antiplasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: Evidence from clinical isolates in Bangladesh and standardized parasite clones,” Parasitol. Res., 2012; 110(6): pp. 2289–2295.
- M. N. Horcajada et al., “Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig,” Osteoarthr. Cartil., 2015; 23(1): pp. 94–102.
- O. V. Carvalho et al., “In vitro inhibition of canine distemper virus by flavonoids and phenolic acids: Implications of structural differences for antiviral design,” Res. Vet. Sci., 2013; 95(2): pp. 717–724.
- A. Ugusman, Z. Zakaria, K. H. Chua, N. A. M. M. Nordin, and Z. Abdullah Mahdy, “Role of Rutin on Nitric Oxide Synthesis in Human Umbilical Vein Endothelial Cells.,” Sci. World J., 2014; 2014, pp. 1–9.
- G. Selvaraj, S. Kaliamurthi, R. Thirungnasambandam, L. Vivekanandan, and T. Balasubramanian, “Anti-nociceptive effect in mice of thillai flavonoid rutin.,” Biomed. Environ. Sci., 2014; 27(4): pp. 295–9.
- T. A. Nguyen, B. Liu, J. Zhao, D. S. Thomas, and J. M. Hook, “An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex,” Food Chem., 2013; 136(1): pp. 186–192.
- M. E. M. . de Araújo et al., “Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities,” Food Chem., 2013; 141(1): pp. 266–273.
- A. Kerdudo, A. Dingas, X. Fernandez, and C. Faure, “Encapsulation of rutin and naringenin in multilamellar vesicles for optimum antioxidant activity,” Food Chem, 2014; 159: pp. 12–19.
- N. Haleagrahara, “Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity,” Int. J. Mol. Med., May 2013.
- Y. F. Zhou, B. Guo, M. J. Ye, R. F. Liao, and S. L. Li, “Protective Effect of Rutin Against HO-Induced Oxidative Stress and Apoptosis in Human Lens Epithelial Cells,” Curr Eye Res, 2015; 3683, no. November, pp. 1–10.
- M. S. Ola, M. M. Ahmed, R. Ahmad, H. M. Abuohashish, S. S. Al-Rejaie, and A. S. Alhomida, “Neuroprotective Effects of Rutin in Streptozotocin-Induced Diabetic Rat Retina,” J. Mol. Neurosci., 2015; 56(2), pp. 440–448.
- M. Moshahid Khan et al., “Rutin Protects Dopaminergic Neurons from Oxidative Stress in an Animal Model of Parkinson’s Disease,” Neurotox. Res., 2012; 22(1): pp. 1–15.
- S. Moghbelinejad et al., “Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats,” Toxicol. Lett., 2014; 224(1); pp. 108–113.
- M. Nassiri-Asl et al., “Effects of rutin on oxidative stress in mice with kainic acid-induced seizure,” J. Integr. Med., 2013; 11(5), pp. 337–342.
- V. S. Motamedshariaty, S. Amel Farzad, M. Nassiri-Asl, and H. Hosseinzadeh, “Effects of rutin on acrylamide-induced neurotoxicity,” Daru, 2014; 22(1): p. 27.
- R. Jadhav and G. Puchchakayala, “Hypoglycemic and antidiabetic activity of flavonoids: Boswellic acid, Ellagic acid, Quercetin, Rutin on streptozotocin-nicotinamide induced type 2 diabetic rats,” Int. J. Pharm. Pharm. Sci., 2012; 4(2): pp. 251–256.
- N. T. Niture, A. A. Ansari, and S. R. Naik, “Anti-hyperglycemic activity of Rutin in streptozotocin-induced diabetic rats: An effect mediated through cytokines, antioxidants and lipid biomarkers,” Indian J. Exp. Biol., 2014; 52(7): pp. 720–727.
- S. S. Al-Rejaie et al., “Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat.,” BMC Complement. Altern. Med., 2013; 13(1): p. 136.
- S. Lodhi, A. P. Jain, V. K. Sharma, and A. K. Singhai, “Wound-Healing Effect of Flavonoid-Rich Fraction from Tephrosia purpurea Linn. on Streptozotocin-Induced Diabetic Rats,” J. Herbs. Spices Med. Plants, 2013; 19(2): pp. 191–205.
- M. S. Aslam, M. S. Ahmad, A. S. Mamat, M. Z. Ahmad, and F. Salam, “Reverse phase-high performance liquid chromatography method for estimation of bioactive compounds from different fractions of new polyherbal formulation of Clinacanthus Nutans and Elephantopus Scaber,” Int. J. Pharm. Sci. Res., 7(12).
- H. R. Sadeghnia, B. S. Yousefsani, M. Rashidfar, M. T. Boroushaki, E. Asadpour, and A. Ghorbani, “Protective effect of rutin on hexachlorobutadiene-induced nephrotoxicity,” Ren. Fail, 2013; 35: pp. 1151–1155.
- P. H. Pan et al., “Protective effects of rutin on liver injury induced by biliary obstruction in rats,” Free Radic. Biol. Med., 2014; 73: pp. 106–116.
- M. M. Hafez et al., “Hepato-protective effect of rutin via IL-6/STAT3 pathway in CCl4-induced hepatotoxicity in rats,” Biol. Res., 2015; 48: p. 30.
- S. Sunada et al., “Monoglucosyl-rutin as a potential radioprotector in mammalian cells,” Mol. Med. Rep., 2014; 10(1): pp. 10–14.
- H. Chen et al., “Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis.,” ScientificWorldJournal., 2013; 2013, p. 269165.
- A. L. Sunarwidhi, S. Sudarsono, and A. E. Nugroho, “Hypoglycemic Effect of Combination of Azadirachta indica A. Juss. and Gynura procumbens (Lour.) Merr. Ethanolic Extracts Standardized by Rutin and Quercetin in Alloxan-induced Hyperglycemic Rats.,” Adv. Pharm. Bull., 2014; 4(Suppl 2), pp. 613–8.
- M. S. Aslam, M. S. Ahmad, A. S. Mamat, and M. Z. Ahmad, “Fast development of RP-HPLC method for estimation of rutin from aqueous fraction of new polyherbal formulation containing Clinacanthus nutans and Elephantopus scaber,” Int. J. Res. Ayurveda Pharm., 2016; 7(5).
- D. Silveira and Y. M. Fonseca, “Determination of rutin in Erythroxylum suberosum extract by liquid chromatography: applicability in standardization of herbs and stability studies,” Bol. Latinoam. y del Caribe Plantas Med. y Aromáticas, 2014; 13(2): pp. 135–143.
- D. K. Patel, K. Patel, B. Duraiswamy, and S. P. Dhanabal, “Phytochemical analysis and standardization of Strychnos nux-vomica extract through HPTLC techniques,” Asian Pacific J. Trop. Dis., 2012; 2, no. SUPPL.1.
- R. A. Devkar, S. Chaudhary, H. Makwana, K. S. Chandrashekar, and M. M. Setty, “Quantification of Rutin by High Performance Thin Layer Chromatography in Lepidagathis prostrata Dalz.,” Adv. Sci. Lett., 23(3), pp. 1811–1813.
- S. N. Park, M. H. Lee, S. J. Kim, and E. R. Yu, “Preparation of quercetin and rutin-loaded ceramide liposomes and drug-releasing effect in liposome-in-hydrogel complex system,” Biochem. Biophys. Res. Commun., 2013; 435(3): pp. 361–366.
- S. Sharma, J. K. Sahni, J. Ali, and S. Baboota, “Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin – pharmacodynamic and antioxidant studies.,” Drug Deliv., 2014; 7544, no. August 2016, pp. 1–11.
- Z. Hooresfand, S. Ghanbarzadeh, and H. Hamishehkar, “Preparation and Characterization of Rutin-loaded Nanophytosomes,” Pharm. Sci., 2015; 21(3); pp. 145–151.
- M.-S. Lim, S.-B. Han, S.-S. Kwon, M.-A. Park, and S.-N. Park, “Elastic Liposome Formulation for Transdermal Delivery of Rutin,” J. Soc. Cosmet. Sci. Korea, 2012; 38(2): pp. 147–154.
- P. V. Kumar and A. K. P. Bhopal, “Formulation design and evalution of rutin loaded selfemulsifying drug delivary system (SEDDs) using edible oil,” Asian J. Pharm. Clin. Res., 2012; 5(1), pp. 76–78.
- N. Ahmad et al., “Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia,” Int. J. Biol. Macromol., 2016; 91: pp. 640–655.
- S. M. Pyo, M. Meinke, and C. M. Keck, “Rutin – Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology,” Cosmetics, 2016; 3(1).
- R. Kamel and D. M. Mostafa, “Rutin nanostructured lipid cosmeceutical preparation with sun protective potential,” J. Photochem. Photobiol. B Biol., 2015; 153: pp. 59–66.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


