Phenolic compounds (PCs) are a prominent class of secondary metabolites produced by plants and are essential for the natural role of the entire plant life cycle. PCs are formed in plants under both favorable and unfavorable conditions and have essential functions in signaling pathways, such as cell division, nutrient mineralization, hormone control, and reproduction. Under abiotic stress conditions, plants produce more polyphenols, which aid them in adapting to their environment. The phenylpropanoid biosynthetic pathway is activated under various environmental stress conditions, such as drought, heavy metal toxicity, salinity, and high/low temperatures, resulting in the deposition of compounds. These compounds can neutralize reactive oxygen species (ROS) produced in excessive amounts in crops under stressful conditions and adversely affect plants. It is imperative to investigate the functions of PCs in response to several abiotic stresses, as the phenylpropanoid pathway plays a crucial role in the metabolic pathway in crop plants, leading to the biosynthesis of a wide range of PCs. These compounds play various roles in plant growth, development, and response to environmental stress. Therefore, this review provides a comprehensive understanding of PCs and their exchanges with other cellular components, which is crucial for harnessing their potential to improve crop resilience to environmental stresses.
Environment, Abiotic Stress, Phenolic, Phenylpropanoid Pathways, ROS, Plant Defense
Climate change plays a crucial role in agriculture and decreases agricultural productivity and crop quality owing to salinity, heat waves, flooding, and drought.1-4 Based on the yearly report of Weather, Climate, and Catastrophe Insight, the economic loss due to natural calamities spiked from USD 200 billion to USD 225 billion per year between 2016 and 2018 worldwide.1 Owing to temperature changes and atmospheric CO2 levels triggered by unprecedented climatic events, multiple abiotic stresses are the ultimate consequences of global.5 According to some forecast reports, agriculture is thought to be the most vulnerable industry that is negatively impacted by climate change. Currently, the two issues that worry people the most about the world are food security and ecological resilience. Before climate variations have a significant impact on global crop production, climate-smart agriculture is the only way to reduce their negative effects on crop adaptation.4,6 Climate change has also altered the precipitation patterns, contributing to erratic drought/flood stress. The emission of greenhouse gases from various sources is one of the factors that contribute to the gradual rise in temperature. Rising temperatures, heat waves, and rainfall variations directly affect changing weather patterns. Elevated atmospheric CO2 levels affect the quality and nutrient content of foods.7 The loss of farmland due to sea level rise is an unintended consequence of climate change. Floods, water logging, extreme temperatures, and salinity are abiotic aspects that confine crop quality and quantity. Environmental factors significantly influence plant growth and productivity. This almost certainly results in higher food availability, disproportionately affecting poor communities disproportionately.8 Current climate prediction models project that, as the climate deteriorates, the recurrence of heat waves, drought, and salinization will rise; therefore, agricultural output will struggle to withstand environmental challenges.
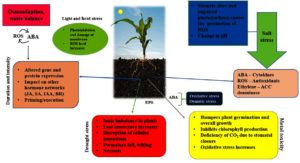
Increasing temperature and rainfall variability have affected crop yields. By 2050, it is predicted that without effective management strategies, approximately half of agricultural land will be salinized.9 In the current scenario, soil salinity has affected one-fifth of the arable land, and each year, excessive salinity levels render 1.5 million hectares of land unfit for agricultural use. By 2050, 50% of arable land will disappear if soil salinization continues to increase.10 Approximately 30 crops produce 90% of plant-centered human sustenance, and the bulk of these crops are salt-sensitive, if not salt-intolerant.11 Environmental repercussions, such as salt, heat/cold, water deficit, toxic metals, and floods/waters, have grown as a result of drastic and severe climate change (Figure 1).12 Upon the onset of abiotic stress, plants generate reactive oxygen species (ROS) owing to oxidative damage as a defense mechanism.13 Hence, studies on plant antioxidant defence mechanisms can be a comprehensive predictor of plant damage.14 According to previous studies, these stresses alter the overall plant growth and development and cause a 70% decrease in plant production.15
Phenolics are a diverse set of bioactive secondary metabolites.16 Phenolic compounds (PCs) are present in roots, shoots, fruits, seeds, and most of the other parts of the plant.17 In plants, phenolics are usually categorized into two types: (1) performed phenolics formed during normal plant tissue development and (2) induced phenolics produced when exposed to different environmental stress situations.18 These compounds are often made and accrued in the subcutaneous layers of crops that are sensitive to biotic and abiotic stress. The quantity of specific PCs in tissues depends upon season as well as growth stage of the crop. Subjection to light promotes the formation of PCs in different cell organelles. Phenolics consist of variations of compounds such as stilbene, arylpyrones, flavonoids, lignans, lignins, etc.20 The number of carbon atoms present in the molecule determines the classification of phenols.21 Phenols are produced by three different biosynthetic pathways (i) the shikimate chorismate or succinyl benzoate pathway (ii) the acetate malonate or polyketide pathway and (iii) dehydrogenation reactions of the acetate/mevalonate pathway.22 Understanding the contribution of phenolics to plants under abiotic stress tolerance will offer valuable insights for adopting sustainable environments and this implies an investigation into the significance of phenolics in aiding plants to withstand environmental stresses.
Biosynthesis of phenolic compounds and its effect on plants
Phenolic acid
Phenolic acids are classified into two groups based on their structure: hydroxybenzoic acids (gallic acid, vanillic acid, etc.) and hydroxycinnamic acids (ferulic acid). Lignin is an organic polymer that is important for plants to tolerate abiotic stresses. Lignin protects tobacco from the detrimental effects of cold temperatures.23 Drought stress increases the levels of vanillic acid in Cucumis sativus (Table).24 Vanillic acid has potent antioxidant properties that aid plants in dealing with a variety of stressors.25 Application of exogenous vanillic acid to tomatoes improves salt tolerance by increasing osmolyte formation, ion regulation, and antioxidant activity of antioxidants.26 Similarly, El-Soud et al.27 found that ellagic acid alleviated salt-induced osmotic stress (Table). Salicylic acid protects plants from biotic and abiotic stressors.28 Salicylic acid concentrations are known to change in response to various stressors.29
Table:
Synthesis and regulation of different phenolic compounds in different crops upon exposure to various abiotic stress
Abiotic stress |
Phenolic acids |
Crops |
Reference |
|---|---|---|---|
Cold stress |
Lignin |
Tobacco |
23 |
Drought stress |
vanillic acid |
Cucumis sativus |
24 |
Salt stress |
Gallic, p-hydroxybenzoic, syringic. |
Amaranthus tricolor |
35 |
Salt tolerance |
vanillic acid |
Tomatoes |
26 |
Salt-induced osmotic stress |
Ellagic acid |
Chickpea |
27 |
Abiotic stress |
Flavonoids |
Crops |
Citations |
Drought stress |
Naringin |
Citrus unshiu |
30 |
Drought stress |
Hesperidin and aglycone naringenin |
Peppermint |
31 |
Drought stress |
Naringenin, especially naringin |
Tobacco |
32 |
Salt stress |
Naringenin |
Chickpea |
34 |
Water-deficit stress |
Luteolin |
Chrysanthemum |
36 |
Flooding and excess salinity |
Luteolin |
Artichoke and cardoon |
37 |
Salinity |
Apigenin |
Nigella sativa |
38 |
Drought stress |
Luteolin |
Achillea pachycephala |
39 |
Cold stress, osmotic stress |
Isoflavones |
Soybean |
40 |
Water-deficit stress |
Isoflavones |
Glycine max |
41 |
Salt stress |
Isoflavone |
Soybean |
42 |
Cold stress |
Catechin |
Date palm |
43 |
Cold stress |
Catechin |
Arabidopsis |
44 |
Salt stress |
Epicatechin |
Wheat sprout |
45 |
Cold stress |
Flavanols |
Prunus persica |
46 |
Drought stress |
Flavanols |
Camelia sinensis |
47 |
Salt stress |
Quercetin-3-rutinoside |
Tomatoes, Ocimum basilicum |
48,49 |
Salt stress |
Quercetin-3-beta-glucoside |
Solanum nigrum |
50 |
Salt stress |
Quercetin-3-beta-glucoside |
Amaranthus tricolor and Solanum villosum |
35,51 |
Abiotic stress |
Lignans |
Crop |
|
Drought stress |
Lignans |
Sesamum indicum L. |
52 |
Salt and drought stress |
Lariciresinol |
Isatis indigotica |
19 |
Flavanoids
Flavonoids are classified into flavones, flavonols, and isoflavones based on their structures. Water deprivation had no effect on hesperidin levels but significantly increased naringin levels in Citrus unshiu et al. leaves (Table).30 Drought stress causes a significant increase in hesperidin levels in peppermint, whereas aglycone naringenin levels increase only marginally.31 Tobacco plants with elevated CHS gene activity have higher levels of naringenin, particularly naringin, and are drought tolerant.32 Naringenin is a powerful antioxidant and radical scavenger, making it an excellent compound for tolerating stress.33 When chickpeas are subjected to high sodium concentrations in nodules, the naringenin concentration decreases (Table).34 Naringenin mitigates the effects of salt and osmotic stresses on photosynthesis and the chloroplast antioxidant system in bean (Phaseolus vulgaris) plants.35 Drought increases the luteolin content of chrysanthemum cultivar leaves while decreasing or maintaining unchanged apigenin levels.36 Similarly, flooding combined with high salinity increased luteolin content in artichoke and cardoon leaves (Table).37 Salinity promotes apigenin synthesis in black cumin.38 Drought decreases the content of apigenin-7-O-glucosides and increases that of luteolin-7-O-glucosides in Achillea pachycephala.39 Low temperature, osmotic stress, and combined stress all increase isoflavone levels in soybeans, according to Swigonska et al.40 Long-term drought minimizes the overall isoflavone content of soybean seeds throughout seed developmental stages.41 Flavonols play an important role in the response of plants to temperature change. The level of flavonol in Prunus persica increases when it is chilled.46 Catechin build-up has been observed in date palms (Du et al.,43 and Leyva et al.,44) at low temperatures (Table). Under drought conditions, flavanol accumulation is observed in Camelia sinensis which helps it tolerate stressful conditions.47 The buildup of isoflavones is an indication of an increase in the salt-sensitive cultivar and a decrease in the salt-tolerant cultivator of soybeans (Table).42 Under salt stress conditions, epicatechin levels increase in wheat is observed.45 There has been an upsurge in quercetin-3-rutinoside levels in tomatoes and Ocimum basilicum under salt stress.48,49 NaCl significantly increased quercetin-3-beta-glucoside levels in Solanum nigrum,50 Amaranthus tricolor,35 and Solanum villosum (Table).51
Lignans
Lignans are involved in lignin production and cell wall synthesis. Drought stress may affect lignan content in Sesamum indicum, depending on the genotype (Table).52 The elevation of lariciresinol in Isatis indigotica has been shown to improve root development and tolerance to salt and drought stress (Table).19,53
Biosynthesis pathway
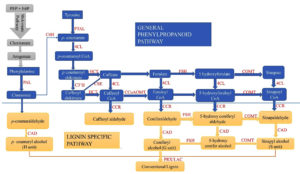
The general phenylpropanoid pathway, which includes numerous steps to convert phenylamine to feruloyl-CoA, and the monolignol-specific pathway, including the conversion of feruloyl-CoA to a variety of monolignols, are the two primary categories of the lignin biosynthesis pathway.54 All species except grasses use phenylalanine (Phe) as their starting substrate.55 The pathway includes processes such as deamination and methylation, and the 11 enzymes involved in this pathway are mentioned above (Figure 2). The major phenylpropanoid route is initiated by the ammonia lyase of Phe and conversion activity of PAL.56 Coniferyl and sinapyl alcohols are produced by methylation of the aromatic ring, whereas hydrocyanic alcohols are produced by carboxylic acid reduction (Figure 2). This segment, referred to as the standard phenylpropanoid route, frequently uses PAL, C4H, and 4CL. In subsequent steps, p-coumaric acid, ferulic acid, and sinapic acid were stimulated to produce their respective CoA thioesters. Following reduction by CCR and CAD, these thioesters are converted into p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol, respectively.57 The aromatic rings of the precursors were hydroxylated and O-methylated at hydroxycinnamic acid concentrations (Figure 2). Overall, owing to their effects on caffeoyl shikimate, the enzymes HCT, CCR, C3H, and CAD produce lignin G units and caffeoyl alcohol; F5H, COMT, and CAD generate sinapyl alcohol and S units through a unique branching pathway. Most recently, the CSE enzyme has been integrated into the wet chemical process. The tyrosine route skips tyrosine catalysis, resulting in a faster monolignol synthesis. During this process, polyfunctional phenylalanine and tyrosine ammonia-lyase (PTAL) transform tyrosine into p-coumarate (Figure 2).
Figure 2. Genes related to the lignin and phenylpropanoid pathway in plant defence.62 The following enzymes are involved in phenylalanine metabolism: PAL (phenylalanine ammonia-lyase), PTAL (phenylalanine tyrosine ammonia-lyase), C4H (cinnamic acid 4 hydroxylases), CAD (hydroxy) cinnamyl alcohol dehydrogenase, HCT (hydroxycinnamoyl-CoA: shikimate/quinate hydroxycin hydroxycinnamoyltransferase; C3’H, p-coumaroyl shikimate32 hydroxylase; 4CL, 4-hydroxycinnamoyl-CoA ligase; CSE, caffeoyl shikimate esterase; CCR, cinnamoyl-CoA reductase; F5H, coniferaldehyde/ferulate5 hydroxylase; COMT, caffeic acid/5-hydroxyferulic acid O-methyltransferase; CCoAOMT, caffeoyl-CoAO methyltransferase
Regulatory genes involved in lignin and phenylpropanoid pathway for plant defense
Understanding the genes involved in lignin and phenylpropanoid pathways will improve our knowledge of plant defense mechanisms and may lead to the development of crops with increased resistance to various stressors (Figure 2). The phenylpropanoid pathway produces lignin along with other phenolic polymers, which contributes to a number of resistance mechanisms of resistance.58 The process of producing lignin results in the synthesis of secondary compounds and phenylpropanoid chemicals, which have antibacterial properties and serve a purpose in plant defenses. It is known which genes are associated with shikimate pathways, and some studies (Bonawitz et al.,56) have shown, for the first time, how they react when given access to cell walls. Under biotic and abiotic stress conditions, many phenylpropanoid pathway genes have been reported to be strongly expressed, which increases both the production and accumulation of associated enzymes.59 The two primary components of lignin were sinapyl alcohol and coniferyl monolignol. Lignins perform several functions under various stress conditions. The phenylpropanoid pathway also produces a variety of additional chemicals (phenolic phytoalexins, stilbenes, and coumarins) along with monolignols, which were originally linked to plant defense.60 This disruption of flux in the system may be the cause of some resistance mechanisms. The quantity of lignin can affect its physicochemical properties, and mutations within families of genes in the lignin cascade may cause unexpected responses to disease resistance. For instance, a change in the S/G lignin element ratio has a significant effect on the transcriptional function of genes that control stress responses, although it has no effect on morphological changes. Instead, it affects how chemicals involved in protective signals are stored within the cell wall matrices.61 According to previous research, some alterations can be effectively controlled along the path of opposition. Arabidopsis thaliana has been used to study illness responses by silencing or removing functional genetic variations in regulatory networks. These investigations have mainly focused on PAL, F5H, C4H, COMT, 4CL, CAD, CSE, and CCoAOMT gene families.
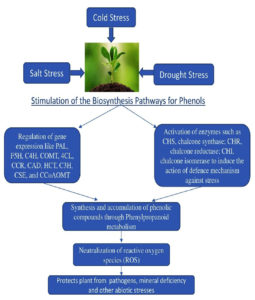
Control of phenylpropanoid pathways in transcription
It is well known that, in response to infection, transcriptionally active genes involved in a variety of metabolic pathways produce phenylpropanoid metabolites (Figure 3). However, it is important to emphasize that steady transcript levels are often measured to obtain this result. According to Dixon et al.,60 it is impossible to distinguish between increased transcription and mRNA stability using steady-state transcript level measurements. However, several studies using nuclear transcript run-on assays have demonstrated a strong correlation between the onset of illness and higher levels of phenylpropanoid pathway gene transcription.63 The cis-regulatory elements included in the monolignol biosynthesis pathway are used in several prediction methods to elucidate the enigmas around the phenylpropanoid pathways. The predicted and observed results for complicated transcriptional systems were validated by promoter and electrophoretic mobility shift assays (EMSA). The transcription factors MYB, LIM, ERF, and KNOX have unique binding sites in the promoters of genes implicated in pathways that create lignin.64 It is well accepted that the main conclusions of many investigations indicate the presence of AC components in enhancers. The promoter regions of the phenylpropanoid pathway genes were subjected to detailed functional investigation that revealed their modular design and allowed the primary coordinator elements to be recognized as AC elements. It has been discovered that the majority of the genes involved, including PAL, 4CL, C3H, and CAD, have promoter regions that are sufficient to demonstrate the presence of these elements. However, neither the AC-rich element nor lignin-specific transcriptome factors were discovered in the S-lignin-producing genes.
Figure 3. Expression of biosynthesis pathways involved in the formation of phenolics upon onset of various abiotic stresses.
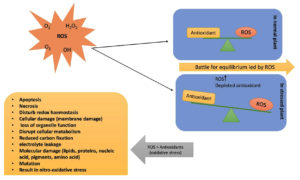
Reactive oxygen species (ROS) and antioxidant defense mechanism
ROS are byproducts of the normal cellular metabolism in crops. However, their extremely active nature causes substantial harm to DNA, proteins, carbohydrates, lipids, and other essential cellular components (Figure 4). In the long run, many unfavorable effects result from oxidative stress, which is generated by an abundance of ROS, such as free radicals (hydroperoxyl radical HO2; hydroxyl radical OH), and non-radicals, such as O2 and H2O2.65 The principal spots of ROS formation include the chloroplasts, peroxisomes, mitochondria, and cell membranes.66,67 Chloroplasts are the primary site of ROS production. The ROS produced by chloroplast are 30-100 times higher than those produced by mitochondria.68 Crops mainly deal with oxidative damage through defensive procedures consisting of enzymatic antioxidants, such as monodehydroascorbate reductase and ascorbate peroxidase, as well as non-enzymatic antioxidants, such as phenolic acids, flavonoids, and tocopherol.69 These enzymes are known to be ROS-scavenging enzymes. The antioxidant defense system and the buildup of ROS in plant cells maintain a constant equilibrium, maintaining an adequate quantity of ROS inside the cell, allowing for proper redox-biological interactions, and governing various plant functions, such as growth and maturation balance between ROS formation and ROS degradation, maintain this intermediate level.65 However, undue ROS generation breaks the equilibrium and causes oxidative stress, damaging cells and resulting in apoptosis and decreased plant yield.70
Oxidative stress under salinity
Salinity affects plants by creating osmotic stress, ionic toxicity, nutritional inadequacy, and genotoxicity, which causes ROS overproduction and oxidative damage.6,71,72 Under sodium chloride (NaCl) stress, H2O2 production increases by three times, and the concentration of thiobarbituric acid-reactive particles (TBARS) increases by two to three times (Rehman et al.,2 & Cheng et al.73) found that under salt stress, the total ROS level, electrolyte leakage (EL), and lipid peroxidation in rice root tissues were two-fold greater than those in control. Ahanger et al.74 found an increase in EL and malondialdehyde (MDA) as well as an increase in O2 and H2O2, indicating the oxidative impact of salinity in tomatoes. Salt stress increases MDA, EL, and O2 in pepper by four-fold.75 Lalarukh and Shahbaz76 exposed two sunflower genotypes (FH-572 and FH-621) to high concentrations of saline NaCl and found that FH-572 elevated H2O2 whereas FH-621 reduced H2O2, indicating that FH-621 is more salt tolerant.
The accumulation of Na+ ions in plant roots is associated with the overexpression of oxidative stress symptoms, such as MDA, EL, and H2O2 as well as lowered ASC and GSH redox status. Numerous plant cells and tissues suffer oxidative damage owing to various abiotic stresses.77 Salt stress causes an increase in the quantity of ROS in plant tissues owing to changes in the ETS reaction in plants. This extra electrochemical energy can be released by the Mehler reaction, which leads to the generation of ROS, primarily H2O2, and membrane damage, as shown by greater levels of EL and MDA.77
Chemical elicitors improve the ability of plants to respond to salinity, which lowers the production of excess ROS (Wang et al.46, Hernandez JA,78 Yu et al.79). Furthermore, it has been demonstrated that the upregulation of a number of transgenes enhances ion homeostasis, hormone signaling, abscisic acid (ABA) synthesis, photosynthetic capacity, and antioxidant components, all of which increase stress tolerance and ROS metabolism.78 Under high-salinity conditions, maize protects itself from oxidative stress through both non-enzymatic and enzymatic processes. Although many organs respond similarly to salt stress, there are behaviors specific to each organ. An increase in polyphenol content and ASC, both of which function as non-enzymatic antioxidants, results from older leaves and roots, and this response is correlated with greater deposition of Na+ in these tissues. Hichem et al.80 found that despite newly formed leaves proliferating more than older leaves, maize plants accumulate greater amounts of polyphenols under high salinity. Tocopherol concentrations have been shown to increase to shield tomato leaves from oxidative damage.
Oxidative stress under water deficit/drought
Catalase (CAT), ascorbate peroxidase (APX), and other antioxidant defence mechanisms have been reported to be utilized by crops under water scarcity to minimize reactive oxidative stress and oxidative stress caused by drought in general.81 Glutathione, an important signaling molecule in an array of metabolic processes, regulates the relationships between seed germination, stomatal pore closure, and drought.82 Additionally, glutathione peroxidase (GPX) supports ROS scavenging by catalyzing the reduction of H2O2 and other organic hydroperoxides to protect cells from any harm caused by oxidative damage and ultimately enhance resilience to drought.83
Proteins that frequently appear within the ROS system, carbon metabolism, photosynthesis, signal transmission, and amino acid metabolic activity have been demonstrated to be involved in a number of stress responses in soybeans.84,85 These proteins are thought to play genetic and molecular roles in the regulation of stress in soybean.86 Moreover, the most sensitive reaction to water deficit is fluctuations in protein quantity.87 Roots begin several modifications in the face of drought because they are the primary sites for collecting stress signals, and the water intake of the roots is essential for causing the strain.87
Drought causes stomatal closure, decreases CO2 input, diminishes photosynthetic rate, disrupts light harvesting and use, and alters chloroplast photochemistry, all of which cause excess ROS production.65,88 ROS overproduction is also linked to photorespiration-induced dissolution of proteins and membranes, inactivity of TCA cycle enzymes, and decreased carboxylate efficiency caused by dryness.89 It has been found that high ROS generation in many plant species is caused by oxidative and water stress. Phragmites escape was grown under dry conditions by Abideen et al.,90 who discovered a substantial increase in MDA concentrations, and Coffea arabica recorded considerably higher MDA concentrations under the same circumstances.91 Saha et al.92 removed irrigation from rice for a few days to simulate drought and observed a large increase in Oxygen, Hydrogen peroxide, and MDA due to the lack of water. Similarly, finger millets have higher H2O2 and EL concentrations.93
Oxidative stress under cold/low temperatures
ROS formation is noted in crucial biochemical processes such as photosynthesis and respiration, and is a significant biological change caused by cold stress. The function of ROS-scavenging systems in plants is linked to low temperatures. SOD converts O2• into H2O2, a molecule with dual functions that acts in both the signaling process and in toxifying cell activities before being rummaged by APX and CAT.94 ROS and peroxidescause severe oxidative damage in rice when exposed to low-temperature stress, and the antioxidant defense system mitigates their negative effects.95 Proline accumulation stimulates membrane stability and subcellular structure stability, and protects cells from oxidative stress under abiotic conditions.96 In addition to its role in reducing ROS damage, the AOX pathway also reduces ROS production in plants that can withstand abiotic stress. The electrolyte leakage index is a key measure of the permeability of the cytoplasmic membrane.97
Plant chloroplasts play a crucial role in sensing environmental temperature. At low temperatures, an imbalance occurs in the leaves because of their ability to collect energy from the sun and use it for metabolism. This imbalance can produce ROS, which can destroy the photosynthetic apparatus and harm healthy cells by acting as a secondary messenger. Under cold stress, there is an elevation in the concentrations of MDA and EL in rice seeds under cold stress.98 Low-temperature stress, when applied to the cold-sensitive S. lycopersicum genotype, resulted in considerably greater MDA and H2O2 content than plants grown under normal temperature conditions.99
Genomic advances in abiotic stress
Plant’s response to salt stress
Jasmonic acid (JA), cytokinins, gibberellic acid (GA), ethylene, and other phytohormones are crucial for plant responses to salt stress. ABI5 expression is suppressed and ABA signaling is interfered with by type-A response regulator proteins that are activated by cytokinins due to salt stress.100 A complex is formed when GA and gibberellin-insensitive dwarf 1 (GID1) bind, causing DELLA proteolysis.101 When combined with XERICO, DELLA proteins function as regulators of the pro-salt stress response. By inhibiting the negative salt stress regulators ETR1/ETR2/EIN4, the ethylene act as a beneficial regulator in the response of plants to salinity stress by inhibiting the negative salt stress. EIN2 is activated by ethylene receptor inactivation and positively controls salinity tolerance reactions.102
High salinity-induced osmotic stress promotes ABA production. The mechanism by which rice produces ABA via the terpenoid route begins with isopentenyl pyrophosphate (IPP). Within a few hours of salt exposure, OsNCED5, OsNCED3, OsNCED4, and OsPSY3 are activated in rice, and their expression is closely related to ABA levels in rice roots. Both jasmonate deactivation and increased CYP94C2b expression have been linked to salinity.103 Two MAPKs, OsMSRMK2 and OsMSRMK3, are expressed in response to numerous climatic stimuli, indicating their potential association with stress-response pathways.104,105
The thioredoxin protein-encoding gene MsTRX boosts the salt tolerance capacity of transgenic tobacco by preserving the osmotic balance (Nicotiana tabacum L.).106 Abscisic acid-sensitive (ABI) regulates ROS metabolism by adhering to vitamin C insufficiency 2 (VTC2) and RbohD during salt stress regulation of Arabidopsis seed germination. ABI is an essential component of ABA signaling and is associated with ROS production and scavenging.107
Plant’s response to drought
Drought is a major concern in the study of how climate change affects agricultural productivity.108,109 Owing to natural selection and long-term evolution, plants have developed many drought-coping strategies, including drought escape via developmental flexibility and drought resistance by stomatal adjustment, antioxidant capabilities, osmotic adaptation, and water absorption.110 Several studies have shown that genes that affect how species adapt to dry conditions are also activated. Several interrelated molecular systems support various metabolic functions, including stress signal reception, DNA and protein expression signal transduction, and cellular metabolic processes.111
The RD29A gene and its product keep cells hydrated. The dehydration-responsive element (DRE) is required for cis-acting RD29A initiation in an ABA-independent reaction under dehydration and cold stress conditions.112 Three CBF/DREB1 genes found in Arabidopsis plants have a crucial function not only in water deficit conditions but also in cold stress. These genes were DREB1B/CBF1, DREB1A/CBF3, DREB1C/CBF2. DREB2A and DREB2B are DREB2 proteins. DREB2 is induced under water-deficient and cold stress.113
Numerous genes are induced under water deficit conditions during plant reproductive development. Examples include OsMID1, ANAC019, and OsERF101.114,115,116 These genes improve anther (pollen) fertility and seed production by mitigating anther defects under drought conditions. ANAC019 is an early drought response regulator.117 ANAC019 not only promotes reproductive development but also protects crops under water deficit stress conditions.116 AtMYB37 plays a significant role in seed production regulation and drought stress response in Arabidopsis. OsMID1 (Guo et al.,114) and OsERF101 are found in rice flowers and are activated under stress conditions. Upregulation of OsMID1 enhances pollen fertility and reduces anther defects, resulting in increased grain production.114 Upregulation of OsERF101 elevates peroxidase activity and proline content, which in turn enhances pollen fertility and drought tolerance.
Plant’s response to cold
Plants growing at extremely low temperatures require unique systems to handle such climatic conditions. Temperate plants face two types of cold stress: chilling stress (0-15°C) and freezing (0°C). Temperate plants can tolerate extreme temperatures118 but tropical plants are vulnerable to cooling shock and do not possess a cold adaptation mechanism. The reproductive development of plants is also slowed by exposure to low temperature.
Plants detect cold via the plasmalemma receptor, and a signal is sent to activate genes that respond to cold and transcription factors that mediate stress tolerance.119 Cold stress is mediated by salicylic acid (SA), abscisic acid (ABA), brassinosteroids (BRs), and other phytohormones.120 Phytohormones, such as GAI and JAZ7, regulate the expression of low-temperature-inducible genes in cassava.121 Cold stress inhibits gibberellins and cytokinins, which promote the expression of CBF1 and CBF3. The upregulation of these genes protects plants from freezing temperatures.122 This causes an increase in the expression of the cold-responsive genes. JA also boosts cold tolerance by interacting with other hormone signaling pathways.86 ESK1, GIGANTEA (GI), HOS9, AtHAP5A, and AtXTH21 genes found in Arabidopsis provide tolerance to low temperatures without using the CBF pathway.123
LEA proteins are important for membrane stabilization. These proteins also prevent protein aggregation.124 CuCOR19, a citrus LEA gene, improves cold tolerance in transgenic tobacco.125 Other genes, such as wheat dehydrin WCS19 and Arabidopsis COR15a (Artus et al.)126 enhance the freezing resistance of transgenic Arabidopsis plants. Dehydrins such as wheat WCOR410,127 barley DHN5,128 have highly resistant to cold stress. These heat shock proteins (HSPs) are induced by low temperatures and function to prevent membrane refolding, denaturing protein, and hindering protein aggregation.129
Phenolic chemicals are abundant in plants and provide a variety of activities such as protection against biotic and abiotic stresses. Phenolics are found in various plants and fruits. They are categorized into several classes based on their structure and are classified as phenolics, stilbenoids, flavonoids, and lignans based on their chemical structures. In general, phenolics can be seen as free forms in plants, but they are mostly conjugated to sugar residues linked to a hydroxyl group (O-glycosides) or an aromatic ring carbon atom (C-glycosides) by a glycosidic bond. Plants experience both biotic and abiotic stresses, and phenolic chemicals play a crucial role in plant growth and defence mechanisms, allowing them to endure different stresses. The potential role of phenolics in abiotic stress tolerance is promising, and holds great potential for shaping the future of agriculture and environmental sustainability. To enhance the resistance of crops to environmental stressors, such as drought, extreme temperatures, and nutrient deficiencies, crops with increased phenolic production can be developed through genetic engineering or biotechnological approaches. Utilize phenolic-rich plant extracts or compounds for eco-friendly stress management strategies in agriculture to reduce reliance on chemical interventions. Integrate the role of phenolics in sustainable agricultural practices, promoting a balance between high crop yields and environmental conservation. To investigate the potential of phenolic-rich plants for phytoremediation, they were used to detoxify and rehabilitate soil and water contaminated by pollutants. Phenolic compounds play a crucial role in enhancing plant performance under adverse conditions by synthesizing pigments, maintaining structural integrity, producing secondary metabolites, acting as antioxidants, and contributing to biochemical defense. Plants have a range of genes that are activated when exposed to various stressors, enabling them to endure stressful situations.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Arora NK. Impact of climate change on agriculture production and its sustainable solutions. Environ Sustain. 2019;2:95-96.
Crossref - Rehman S, Abbas G, Shahid M, et al. Effect of salinity on cadmium tolerance, ionic homeostasis, and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicol Environ Saf. 2019;171:146-153.
Crossref - Shukla P, Kumar A, Kumar R, Pandey MK. Molecular Response and Genetic Engineering for Stress in Plants. IOP Publishing. 2022;1.
Crossref - Shukla P, Kumar A, Kumar R. Climate change and stress mitigation strategy in plants. Front Plant Sci. 2023;14: 1291905.
Crossref - Porter J R, Xie L, Challinor AJ, et al. Food Sec and Food Production Systems. 2014. https://hdl.handle.net/10568/68162
- Gupta A, Bano A, Rai S, et al. ACC deaminase producing plant growth promoting rhizobacteria enhance salinity stress tolerance in Pisum sativum. 3 Biotech. 2021;11(12):514.
Crossref - Loladze I. Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends Ecol Evol. 2002;17(10):457-461.
Crossref - Mbow C, Rosenzweig C, Barioni et al. Food security. In Shukla J, Skea E, Calvo Buendia V, et al (Eds.), IPCC, 2019: Climate change and land: An IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. 2019, 1.
- Butcher K, Wick AF, DeSutter T, Chatterjee A, Harmon J. Soil salinity: A threat to global food security. J Agron. 2016;108(6):2189-2200.
Crossref - Hossain MS. Present Scenario of Global Salt Affected Soils, its Management and Importance of Salinity Research. Int Res J Biol Sci. 2019;1(1):1-3.
- Zorb C, Geilfus CM, Dietz KJ. Salinity, and crop yield. Plant Biol. 2019;21(S1):31-38.
Crossref - Raza A, Razzaq A, Mehmood S, et al. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants. 2019;8(2):34.
Crossref - Benhiba L, Fouad MO, Essahibi A, Ghoulam C, Qaddoury A. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees. 2015;29:1725-1733.
Crossref - Bhattacharjee S. ROS and oxidative stress: origin and implication. Reactive Oxygen species in Plant Biology. 2019:1-31.
Crossref - Samota MK, Sasi M, Singh, A. Impact of seed priming on proline content and antioxidant enzymes to mitigate drought stress in rice genotype. Int J Curr Microbiol App Sci. 2017;6(5):2459-2466.
Crossref - Albuquerque BR, Heleno SA, Oliveira MBP, Barros L, Ferreira IC. Phenolic compounds: Current industrial applications, limitations, and future challenges. Food Funct. 2021;21:14.
Crossref - Ruiz-Ruiz JC, Aldana GDCE, Cruz AIC, Segura-Campos MR. Antioxidant activity of polyphenols extracted from hop used in craft beer. Biotechnological Progress and Beverage Consumption. 2020;19:283-310.
Crossref - Xiao L, Carrillo J, Siemann E, Ding J. Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. AoB Plants. 2019;11(1):plz003.
Crossref - Xiao Y, Feng J, Li Q, et al. IiWRKY34 positively regulates yield, lignan biosynthesis and stress tolerance in Isatis indigotica. Acta Pharma Sin B. 2020;10(12):2417-2432.
Crossref - Khaleghnezhad V, Yousefi AR, Tavakoli A, Farajmand B. Interactive effects of abscisic acid and temperature on rosmarinic acid, total phenolic compounds, anthocyanin, carotenoid and flavonoid content of dragonhead (Dracocephalum moldavica L.). Sci Hortic. 2019;250:302-309.
Crossref - Harborne JB. Phenolic Compounds. In: Phytochemical Methods. Springer, Dordrecht; 1973.
Crossref - Fraga CG. Plant phenolics and human health:biochemistry, nutrition, and pharmacology. John Wiley & Sons. 2009.
Crossref - Zhou P, Li Q, Liu G, et al. Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacum under chilling stress. Funct Plant Biol. 2018;46(1):30-43.
Crossref - Li M, Li Y, Zhang W, et al. Metabolomics analysis reveals that elevated atmospheric CO2 alleviates drought stress in cucumber seedling leaves. Anal Biochem. 2018;559:71-85.
Crossref - Kiokias S, Proestos C, Oreopoulou V. Phenolic acids of plant origin-A review on their antioxidant activity in vitro (o/w emulsion systems) along with their in vivo health biochemical properties. Foods. 2020;9(4):534.
Crossref - Parvin K, Nahar K, Hasanuzzaman M, Bhuyan MB, Mohsin SM, Fujita M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol Biochem. 2020;150:109-120.
Crossref - El-Soud WA, Hegab MM, AbdElgawad H, Zinta G, Asard H. Ability of ellagic acid to alleviate osmotic stress on chickpea seedlings. Plant Physiol Biochem. 2013;71:173-183.
Crossref - Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015;6:462.
Crossref - Pavlovi’c I, Petrik I, Tarkowska D, et al. Correlations between phytohormones and drought tolerance in selected Brassica crops: Chinese cabbage, white cabbage and kale. Int J Mol Sci. 2018;19(10):2866.
Crossref - Malik NS, Perez JL, Kunta M, Olanya M. Changes in Polyphenol Levels in Satsuma (Citrus unshiu) Leaves in Response to Asian Citrus Psyllid Infestation and Water Stress. The Open Agric J. 2015;9:1-5.
Crossref - Abdi G, Shokrpour M, Karami L, Salami SA. Prolonged water deficit stress and methyl jasmonate-mediated changes in metabolite profile, flavonoid concentrations, and antioxidant activity in peppermint (Mentha ׳ piperita L.). Notulae Botanicae Horti Agrobotanici Cluj- Napoca. 2018;47(1):70.
Crossref - Hu B, Yao H, Gao Y, et al. Overexpression of chalcone synthase gene improves flavonoid accumulation and drought tolerance in tobacco. 2019.
Crossref - Cavia-Saiz M, Busto MD, Pilar Izquierdo MC, Ortega N, Perez Mateos M, Muniz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric. 2010;90(7):1238-1244.
Crossref - Garg N, Singla P. Stimulation of nitrogen fixation and trehalose biosynthesis by naringenin (Nar) and arbuscular mycorrhiza (AM) in chickpea under salinity stress. Plant Growth Regul. 2016;80:5-22.
Crossref - Sarker U, Oba S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids, and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci Rep. 2018;8(1):12349.
Crossref - Hodaei M, Rahimmalek M, Arzani A, Talebi M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind Crops Prod. 2018;120:295-304.
Crossref - Colla G, Rouphael Y, Cardarelli M, Svecova E, Rea E, Lucini L. Effects of saline stress on mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon genotypes grown in floating system. J Sci Food Agric. 2013;93(5):1119-1127.
Crossref - Bourgou S, Pichette A, Marzouk B, Legault J. Antioxidant, anti-inflammatory, anticancer and antibacterial activities of extracts from Nigella sativa (black cumin) plant parts. J Food Biochem. 2012;36(5):539-546.
Crossref - Gharibi S, Tabatabaei BES, Saeidi G, Talebi M, Matkowski A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis-related genes in Achillea pachycephala Rech. f. Phytochemistry. 2019;162:90-98.
Crossref - Swigonska S, Amarowicz R, Krol A, Mostek A, Badowiec A, Weidner, S. Influence of abiotic stress during soybean germination followed by recovery on the phenolic compounds of radicles and their antioxidant capacity. Acta Soc Bot Pol. 2014;83(3):209-218.
Crossref - Gutierrez-Gonzalez J, Guttikonda SK, Tran LSP, et al. Differential expression of isoflavone biosynthetic genes in soybean during water deficits. Plant Cell Physiol. 2010;51(6):936-948.
Crossref - Meng N, Yu BJ, Guo JS. Ameliorative effects of inoculation with Bradyrhizobium japonicum on Glycine max and Glycine soja seedlings under salt stress. Plant Growth Regul. 2016;80(2):137-147.
Crossref - Du B, Kruse J, Winkler JB, et al. Climate, and development modulate the metabolome and antioxidative system of date palm leaves. J Exp Bot. 2019;70(20):5959-5969.
Crossref - Leyva A, Jarillo JA, Salinas J, Martinez-Zapater JM. Low temperature induces the accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiol. 1995;108(1):39-46.
Crossref - Cuong DM, Kwon SJ, Nguyen BV, Chun SW, Kim JK, Park SU. Effect of salinity stress on phenylpropanoid genes expression and related gene expression in wheat sprout. J Agron. 2020;10(3):390.
Crossref - Wang LI, Shan T, Xie B, et al. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019;272:530-538.
Crossref - Gai Z, Wang YU, Ding Y, et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci Rep. 2020;10(1):12275.
Crossref - Scagel CF, Lee J, Mitchell JN. Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind Crops Prod. 2019;127:119-128.
Crossref - Martinez V, Mestre TC, Rubio F, et al. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front Plant Sci. 2016;7:838.
Crossref - Agati G, Brunetti C, Ferdinando MD, Ferrini F, Pollastri S, Tattini M. Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol Biochem. 2013;72:35-45.
Crossref - Ben-Abdallah S, Aung B, Amyot L, et al. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol Plant. 2016;38(3):1.
Crossref - Kermani SG, Saeidi G, Sabzalian MR, Gianinetti A. Drought stress influenced sesamin and sesamolin content and polyphenolic components in sesame (Sesamum indicum L.) populations with contrasting seed coat colors. Food Chem. 2019;289:360-368.
Crossref - Ozfidan-Konakci C, Yildiztugay E, Alp FN, Kucukoduk M, Turkan I. Naringenin induces tolerance to salt/osmotic stress through the regulation of nitrogen metabolism, cellular redox and ROS scavenging capacity in bean plants. Plant Physiol Biochem. 2020;157:264-275.
Crossref - Miedes E, Vanholme R, Boerjan W, Molina A. The role of the secondary cell wall in plant resistance to pathogens. Front Plant Sci. 2014;5:358.
Crossref - Barros J, Serk H, Granlund I, Pesquet E. The cell biology of lignification in higher plants. Ann Bot. 2015;115(7):1053-1074.
Crossref - Bonawitz ND, Chapple C. The genetics of lignin biosynthesis: connecting genotype to phenotype. Annu Rev Gennet. 2010;44:337-363.
Crossref - Vanholme R, De Meester B, Ralph J, Boerjan W. Lignin biosynthesis and its integration into metabolism. Curr Opin Biotechnol. 2019;56:230-239.
Crossref - Lozovaya VV, Lygin AV, Zernova OV, et al. Modification of phenolic metabolism in soybean hairy roots through down-regulation of chalcone synthase or isoflavone synthase. Planta. 2007;225(3):665-679.
Crossref - Zhao J, Buchwaldt L, Rimmer SR, et al. Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum. Mol Plant Pathol. 2009;10(5):635-649.
Crossref - Dixon L J, Castlebury LA, Aime MC, Glynn NC, Comstock J C. Phylogenetic relationships of sugarcane rust fungi. Mycol Prog. 2010;9(4):459-468.
Crossref - Gallego-Giraldo L, Pose S, Pattathil S, et al. Elicitor and defense gene induction in plants with altered lignin compositions. New Phytol. 2018;219(4):1235-1251.
Crossref - Yadav V, Wang Z, Wei C, et al. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens. 2020;9(4):312.
Crossref - Zhao Q, Dixon RA. Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci. 2011;16(4):227-233.
Crossref - Ohtani M, Demura T. The quest for transcriptional hubs of lignin biosynthesis: beyond the NAC-MYBgene regulatory network model. Curr Opin Biotechnol. 2019;56:82-87.
Crossref - Hasanuzzaman M, Bhuyan MB, Anee TI, et al. Regulation of an ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8(9):384.
Crossref - Singh A, Kumar A, Yadav S, Singh IK. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene. 2019;18:100173.
- Kohli SK, Khanna K, Bhardwaj R, Abd Allah EF, Ahmad P, Corpas FJ. Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants. 2019;8(12):641.
Crossref - Dogra V, Kim C. Singlet oxygen metabolism: from genesis to signaling. Front Plant Sci, 2020;10:1640.
Crossref - Kaur N, Kaur J, Grewal SK, Singh I. Effect of heat stress on antioxidative defense system and its amelioration by heat acclimation and salicylic acid pre-treatments in three pigeonpea genotypes. Ind J Agric Biochem. 2019;32(1):106-110.
Crossref - Raja V, Majeed U, Kang H, Andrabi KI, John R. Abiotic stress: Interplay between ROS, hormones, and MAPKs. Environ Exp Bot. 2017;137:142-157.
Crossref - Gupta A, Mishra R, Rai S, et al. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int J Mol Sci. 2022;23(7):3741.
Crossref - Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2002;59:651-681.
Crossref - Cheng YW, Kong XW, Wang N, Wang T, Chen J, Shi ZQ. Thymol confers tolerance to salt stress by activating anti-oxidative defense and modulating Na+ homeostasis in rice root. Ecotoxicol Environ Saf. 2020;188:109894.
Crossref - Ahanger MA, Mir RA, Alyemeni MN, Ahmad P. Combined effects of brassinosteroid and kinetin mitigate salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol Biochem. 2020;147:31-42.
Crossref - Abdelaal KA, EL-Maghraby LM, Elansary H, et al. Treatment of sweet pepper with stress toleranceinducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. J Agron. 2019;10(1):26.
Crossref - Lalarukh I, Shahbaz M. Response of antioxidants and lipid peroxidation to exogenous application of alphatocopherol in sunflower (Helianthus annuus L.) under salt stress. Pak J Bot. 2020;52(1):75.
Crossref - Chawla S, Jain S, Jain V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J Plant Biochem and Biotechnol. 2013;22(1):27-34.
Crossref - Hernandez JA. Salinity tolerance in plants: Trends and perspectives. Int J Mol Sci. 2019;20(10):2408.
Crossref - Yu Z, Duan X, Luo L, Dai S, Ding Z, Xia. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020;25(11):1117-1130.
Crossref - H Hichem, D Mounir, El-Ayeb N. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Industrial Crops and Products. 2009;30(1):144-151.
Crossref - Fan HF, Ding L, Xu YL, Du CX. Antioxidant system and photosynthetic characteristics responses to short-term PEG-induced drought stress in cucumber seedling leaves. Russ J Plant Physiol. 2017;64:162-173.
Crossref - Koramutla MK, Negi M, Ayele BT. Roles of glutathione in mediating abscisic acid signaling and its regulation of seed dormancy and drought tolerance. Genes. 2021;12(10):1620.
Crossref - Zhang L, Wu M, Teng Y, et al. Overexpression of the glutathione peroxidase 5 (RcGPX5) gene from rhodiola crenulata increases drought tolerance in Salvia miltiorrhiza. Front Plant Sci. 2019;9:1950.
Crossref - Du Y, Zhao Q, Chen L, et al. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem. 2020;146:1-12.
Crossref - Dumont S, Rivoal J. Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front Plant Sci, 2019;10:166.
Crossref - Wang J, Song L, Gong X, Xu J, Li M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int J Mol Sci. 2020;21(4):1446.
Crossref - Wang X, Komatsu S. Proteomic analysis of calcium effects on soybean root tip under flooding and drought stresses. Plant Cell Physiol. 2017;58(8):1405-1420.
Crossref - Mirza H, Kamrun N, Mahabub AM, Masayuki F. Regulation of reactive oxygen species metabolism and glyoxalase systems by exogenous osmolytes confers thermotolerance in Brassica napus. Gesunde Pflanzen. 2020;72(1):3-16.
Crossref - Hasanuzzaman M, Nahar K, Gill S, Fujita M. Drought stress responses in plants, oxidative stress, and antioxidant defense. Climate Change and Plant Abiotic Stress Tolerance. 2014:209.
Crossref - Abideen Z, Koyro HW, Huchzermeyer B, Ansari R, Zulfiqar F, Gul BJ. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defense of Phragmites karka under drought stress. Plant Biol. 2020;22(2):259-266.
Crossref - Campos CN, Avila RG, de Souza KRD, Azevedo LM, Alves JD. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric Water Manag. 2019;211:37-47.
Crossref - Saha I, De AK, Sarkar B, Ghosh A, Dey N, Adak MK. Cellular response of oxidative stress when sub1A QTL of rice receives water deficit stress. Plant Sci Today. 2018;5(3):84-89.
Crossref - Satish L, Rency AS, Ramesh M. Spermidine sprays alleviate the water deficit-induced oxidative stress in finger millet (Eleusine coracana L. Gaertn.) plants. 3 Biotech. 2018;8(1):63.
Crossref - Das K, Roychoudhury A. Reactive oxygen species (ROS) and the response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci Eng. 2014;2:53.
Crossref - Rezaie R, Mandoulakani BA, Fattahi M. Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci Rep. 2020;10(1):5290.
Crossref - Liu Z, Li L, Luo Z, Zeng F, Jiang L, Tang K. Effect of brassinolide on energy status and proline metabolism in a postharvest bamboo shoot during chilling stress. Postharvest Biol Technol. 2016;111:240-246.
Crossref - Panda SK, Sahoo L, Katsuhara M, Matsumoto H. Overexpression of alternative oxidase gene confers aluminum tolerance by altering the respiratory capacity and the response to oxidative stress in tobacco cells. Mol Biotechnol. 2013;54(2):551-563.
Crossref - Han QH, Huang B, Ding CB, et al. Effects of melatonin on anti-oxidative systems and photosystem II in coldstressed rice seedlings. Front Plant Sci. 2017;8:785.
Crossref - Liu T, Ye X, Li M, Li J, Qi H, Hu X. H2O2 and NO are involved in trehalose-regulated oxidative stress tolerance in cold-stressed tomato plants. Environ Exp Bot. 2020;171:103961.
Crossref - O’Brien JA, Benkova E. Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci. 2013;4:451.
Crossref - Lan Z, Krosse S, Achard P, van Dam NM, Bede JC. DELLA proteins modulate Arabidopsis defenses induced in response to caterpillar herbivory. J Exp Bot. 2014;65(2):571-583.
Crossref - Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20(4):219-229.
Crossref - Kurotani KI, Hayashi K, Hatanaka S, et al. Elevated levels of CYP94 family gene expression alleviate the jasmonate response and enhance salt tolerance in rice. Plant Cell Physiol. 2015;56(4):779-789.
Crossref - Agrawal GK, Agrawal SK, Shibato J, Iwahashi H, Rakwal R. Novel rice MAP kinases OsMSRMK3 and OsWJUMK1 involved in encountering diverse environmental stresses and developmental regulation. Biochem Biophys Res Commun. 2003;300(3):775-783.
Crossref - Agrawal GK, Rakwal R, Iwahashi H. Isolation of novel rice (Oryza sativa L.) multiple stress-responsive MAP kinase gene, OsMSRMK2, whose mRNA accumulates rapidly in response to environmental cues. Biochem Biophys Res Commun. 2002;294(5):1009-1016.
Crossref - Duan X, Wang Z, Zhang Y, et al. Overexpression of a Thioredoxin-Protein-Encoding Gene, MsTRX, from Medicago, sativa Enhances Salt Tolerance to Transgenic Tobacco. J Agron. 2022;12(6):467.
Crossref - Luo X, Dai Y, Zheng C, et al. The ABI4 RbohD/VTC2 regulatory module promotes Reactive Oxygen Species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2021;229(2):950-962.
Crossref - Zhang SH, Xu XF, Sun YM, Zhang JL, Li CZ. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J Integr Agric. 2018;17(2):336-347.
Crossref - Niu J, Zhang S, Liu S, et al. The compensation effects of physiology and yield in cotton after drought stress. J Plant Physiol. 2018;224:30-48.
Crossref - Agurla S, Gahir S, Munemasa S, Murata Y, Raghavendra AS. Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv Exp Med Biol. 2018;1081:215-232.
Crossref - Zeng W, Peng Y, Zhao X, et al. Comparative proteomics analysis of the seedling root response of drought sensitive and drought-tolerant maize varieties to drought stress. Int J Mol Sci. 2019;20(11):2793.
Crossref - Yamaguchi-Shinozaki K, Shinozaki K. A novel cisacting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. The Plant Cell. 1994;6(2):251-264.
Crossref - Sakuma Y, Maruyama K, Osakabe Y, et al. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. The Plant Cell. 2006;18(5):1292-1309.
Crossref - Guo M, Liu JH, Ma X, Luo DX, Gong ZH, Lu MH. The plant heat stress transcription factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front Plant Sci. 2016;7:114.
Crossref - Jin Y, Pan W, Zheng X, et al. OsERF101, an ERF family transcription factor, regulates drought stress response in reproductive tissues. Plant Mol Biol. 2018;98(1):51-65.
Crossref - Sukiran NL, Ma JC, Ma H, Su Z. ANAC019 is required for recovery of reproductive development under drought stress in Arabidopsis. Plant Mol Biol. 2019;99(1-2):161-174.
Crossref - Su Z, Ma X, Guo H, et al. Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and longterm acclimation in Arabidopsis. The Plant Cell. 2013;25(10):3785-3807.
Crossref - Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Biol. 1999;50:571-599.
Crossref - Penfield S. Temperature perception and signal transduction in plants. New Phytol. 2008;179(3):615-628.
Crossref - Deng X, Wang J, Li Y, Wu S, Yang S, Chao J, Tian W. Comparative transcriptome analysis reveals phytohormone signalings, heat shock module and ROS scavenger mediate the cold-tolerance of rubber tree. Sci Rep. 2018;8(1):4931.
Crossref - An D, Yang J, Zhang P. Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plants to cold stress. BMC Genom. 2012;13:64.
Crossref - Novillo F, Medina J, Salinas J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc Natl Acad Sci USA. 2007;104(52):21002-21007.
Crossref - Shi H, Ye T, Zhong B, Liu X, Jin R, Chan Z. At HAP 5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21. New Phytol. 2014;203(2):554-567.
Crossref - Hundertmark M, Hincha DK. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics. 2008;9:118.
Crossref - Hara M, Terashima S, Fukaya T, Kuboi T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta. 2003;217(2):290-298.
Crossref - Artus N, Uemura M, Steponkus PL, Gilmour SJ, Lin C, Thomashow MF. Constitutive expression of the coldregulated Arabidopsis thaliana COR15a gene affects both chloroplast and protoplast freezing tolerance. Proc Natl Acad Sci USA. 1996;93(23):13404-13409.
Crossref - Houde M, Dallaire S, N’Dong D, Sarhan F. Overexpression of the acidic dehydrin WCOR410 improves freezing tolerance in transgenic strawberry leaves. Plant Biotechnol. J. 2004;2(5):381-387.
Crossref - Bravo LA, Gallardo J, Navarrete A, et al. Cryoprotective activity of a cold induced dehydrin purified from barley. Physiol. Plant. 2003;118(2):262-269.
Crossref - Timperio AM, Egidi MG, Zolla L. Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J Proteomics. 2008;71(4):391-411.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.