ISSN: 0973-7510
E-ISSN: 2581-690X
Eco-friendly anti-algal agents are in demand for preventing the growth of unwanted algae. Green synthesized nanoparticles exhibit antimicrobial properties and have been used as a better alternative against chemical and physical processes. In the present study, treatment of silver nitrate with leaf extracts (5% w/v) of Tinospora cordifolia, a plant with proven antimicrobial effects, exhibited UV-visible absorption maxima between 440-460 nm after 1h indicating bioreduction of silver to nanoparticles. The green synthesised silver nanoparticles (5 mgl-1) exhibited inhibition zones against Chlamydomonas reinhardtii in in vitro agar assays. Treatment with green synthesised silver nanoparticles during exponential phase of algal growth resulted in significant reduction in algal population, carbohydrate, protein and chlorophyll contents confirming the anti-algal potential. This is the first report on the growth inhibitory potential of green synthesised silver nanoparticles against green algae.
Green synthesized silver nanoparticles, Tinospora cordifolia, Chlamydomonas reinhardtii, algicidal property
Nanotechnology is a rapidly growing domain with a wide range of applications in different sectors. Among the different methods of synthesis of nanoparticles, biological method is much preferred due to better yield, lesser expenditure and its environmentally-friendly nature. Green synthesis using plant extracts is a bottom-up approach involving reduction/oxidation reactions. Green synthesised nanoparticles are immediately coated with protein caps that helps to prevent aggregation and the natural capping can provide more shelf life, stability and altered properties than artificially synthesised nanoparticles. Green synthesised silver nanoparticles are reported to exhibit powerful antimicrobial activity, especially against bacteria and fungi.1,2
Algae form an important component of our ecosystem and often cause major problems in polyhouses, solar panels, fresh water bodies etc. as they reduce the amount of incident solar radiation. They are reported to cause diseases to plants and algal blooms in water bodies.3 Environmentally-friendly algicidal agents are of great demand for inhibiting the growth of unwanted algal growth. Anti-algal properties of green synthesised nanoparticles have not been much explored.
Chlamydomonas reinhardtii is a model alga that is commonly used for phytotoxicity study of various compounds due to its high responsiveness, easiness in culturing and comparatively lesser doubling time.4-9 In the present study, it was attempted to green synthesize silver nanoparticles using leaf extracts of the medicinal plant Tinospora cordifolia with proven antimicrobial properties and to evaluate their potential for the inhibition of growth of the model alga, Chlamydomonas reinhardtii by in vitro inhibitory assays and analysing the variation in algal growth rate and biochemical parameters.
Green synthesis of silver nanoparticles using Tinospora cordifolia
Green synthesis of silver nanoparticles was carried out using Tinospora cordifolia according to the protocol reported by Alex et al.10 Green and healthy leaves of Tinospora cordifolia were collected and five grams of the fresh leaves were thoroughly washed in Labolene (Thermo Fisher Scientific, India) and tap water, and twice in sterile deionised water. The leaves were cut into fine pieces and boiled in 100 ml sterile distilled water and filtered through Whatman No. 2 filter paper to get the leaf extract. Synthesis of silver nanoparticles was done by adding 10 ml of leaf extract of Tinospora cordifolia to 90 ml of 1mM silver nitrate solution and then heating for 40s in a microwave oven. The solution was then kept at room temperature for 1h for the formation of silver nanoparticles. It was centrifuged at 10,000 rpm for 15 minutes and the pellet was resuspended in sterile deionised water and again centrifuged at 10,000 rpm for 15 minutes. Finally, the pellet was resuspended in 1000 µl of sterile deionised water. Silver nitrate solution (90 mL) to which 10 ml of sterile double distilled water was added served as the control.
The formation of silver nanoparticles was monitored by scanning the bio-reduced silver nanoparticle solution at wavelengths from 300 to 600 nm in a UV-Vis spectrophotometer with sterile deionised water as reference. The reduction of silver ions was confirmed by qualitative testing of the supernatant obtained after centrifugation of the bio-reduced silver nitrate solution with 0.1 M sodium chloride.
In vitro assays for algal growth inhibition of green synthesized silver nanoparticles
Inhibition of growth of Chlamydomonas reinhardtii (Central Food Technology Research Institute, Mysore) on treatment with green synthesized silver nanoparticles was analyzed by performing in vitro inhibition assays namely agar well inhibition assay, filter paper disc assay and spot-on lawn assay. All the experiments were performed by spread plating the algae on Bold’s Basal Medium (BBM) agar plates and were replicated four times. The anti-algal property of the aqueous leaf extract (5% w/v) used for green synthesis was also checked. For agar well inhibition assay, wells (8 mm diameter) were cut out from four quadrants of the plate spread with the algal culture, with a sterile cork borer. The base of the wells was sealed with 100 µl molten BBM agar. After solidification of the base of the wells, 200 µl of test solutions were added, viz. green synthesised silver nanoparticles (5 mgl-1) and leaf extract (5% w/v). For filter paper disc assay, sterile filter paper discs each of 5 mm diameter were wetted with 10 µl of the test solutions. The discs were dried inside the laminar air flow chamber and then placed at the four quadrants of the plate seeded with the algal culture. For spot-on lawn assay 20 µl each of the test solutions were spotted on the discs after spread plating the algae and allowing it to dry. In all the assays, equal volumes of sterile deionised water served as the absolute control. All the plates were incubated at 25°C under fluorescent white light of 3000 lux and the diameter of inhibition zones around the treatments were measured after an incubation period of eight days.
Effect of green synthesized silver nanoparticles on growth rate and biochemical parameters of Chlamydomonas reinhardtii
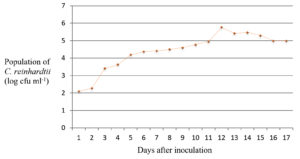
For the determination of the exponential growth phase, single colony of Chlamydomonas reinhardtii was inoculated into 50 ml BBM broth and incubated in a rotary shaker (100 rpm) at 25°C with an illumination of 3000 lux. The algal population was assessed by plating on BBM agar plates at an interval of 24 h for seventeen days. The log cfu per ml was plotted on a graph to obtain the growth curve of the algae (Population vs Time).
Effect on algal growth rate was assessed by the addition of 20 µl each of the test solutions (5 ppm solution of green synthesized silver nanoparticles and 5% w/v of leaf extract) to the growing culture of C. reinhardttii at the beginning of the exponential phase and then taking the viable plate count by serial dilution. Equal volume of deionised water was added in the untreated control. Four replications were maintained for each treatment.
Carbohydrate, protein and chlorophyll contents were analysed before and after treatment at three-day intervals. Total carbohydrate content was estimated by Anthrone method.11 Total protein content of the algal culture was estimated by Lowry’s method.12 Total chlorophyll content was estimated by warm extraction using methanol.13 Four replications were maintained for all the experiments.
Green synthesis of silver nanoparticles using Tinospora cordifolia
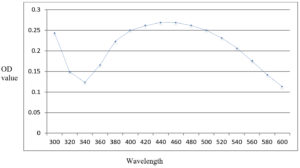
The silver nitrate solution after addition of leaf extract showed colour change to yellowish brown. The solution when tested for formation of silver nanoparticles after a period of one hour, exhibited a peak of UV-visible absorption spectrum between 440-460 nm (Fig. 1). The addition of sodium chloride did not produce any precipitate whereas in the control solution which was not treated with leaf extract, white precipitate was formed.
Algal growth inhibition by green synthesized nanoparticles
The green synthesised silver nanoparticles of T. cordifolia produced inhibition zones of 7.3 mm, 3.6 mm and 6 mm in agar well diffusion assay, filter paper disc assay and spot-on lawn assays respectively. The treatment with leaf extract of T. cordifolia alone and control in which deionised water was added did not exhibit any inhibition zone.
Effect of green synthesized silver nanoparticles on growth of Chlamydomonas reinhardtii
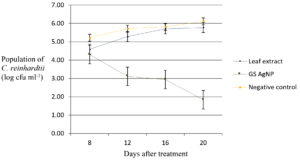
The exponential growth phase of C. reinhardtii was estimated to begin from eighth day onwards with the peak on the twelfth day (Fig. 2) and hence the treatments were applied on the eighth day and observations were recorded at three-day intervals. The population count of the alga showed a reduction to 1.01 log cfu ml-1 on the twelfth day after the application of green synthesised silver nanoparticles compared to the initial algal population of 2.09 log cfu ml-1. The population in the algal culture treated with Tinospora cordifolia leaf extract was 3.95 log cfu ml-1 on the twelfth day of application compared to initial population of 2.03 log cfu ml-1. On the same day, in the untreated control, the population count showed an increase to 5.94 log cfu ml-1 compared to the initial population of 2.08 log cfu ml-1 (Fig. 3).
Fig. 3. Effect of application of green synthesised silver nanoparticles on culture of C. reinhardtii.
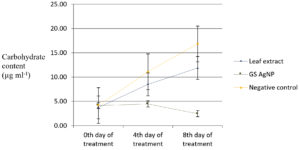
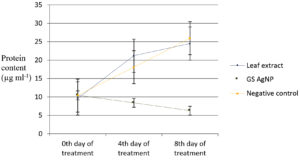
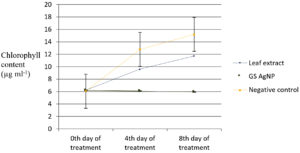
Variations in biochemical parameters viz., total chlorophyll, carbohydrate and protein content of Chlamydomonas culture after treating with green synthesized silver nanoparticles during exponential growth phase was observed. Carbohydrate content of the algal culture treated with green synthesised silver nanoparticles decreased to 2.46 µg/ml compared to the untreated control (16.84 µg/ml) on the twentieth day (Fig. 4). Protein content of the algal culture treated with green synthesised silver nanoparticles decreased from 10.43 µg/ml to 6.27 µg/ml compared to untreated control (25.93 µg/ml) on twentieth day (Fig. 5). The chlorophyll content did not exhibit an increase after the addition of green synthesised silver nanoparticles (6.19 µg/ml on twentieth day to 5.95 µg/ml on twelfth day) whereas in the untreated control approximately two and a half times increase was recorded (6.06 µg/ml on the twelfth day to 15.21 µg/ml on the twentieth day (Fig.6).
Fig. 4. Carbohydrate content of C. reinhardtii culture treated with green synthesised (T.cordifolia) silver nanoparticles.
Fig. 5. Protein content of C. reinhardtii culture treated with green synthesized silver nanoparticles.
According to Wei et al.,14 the peak of UV-visible absorption spectra gives an indication of the uniformity of size of green synthesised silver nanoparticles. In the present study, the UV-visible absorption peak of nanoparticles synthesised using T. cordifolia showed a narrow range of peak (440-460 nm) which indicates greater uniformity of the particles. Variation in concentration of the leaf extract and change in the reaction time can cause significant variation in the absorption maxima and its distribution on UV-visible spectrophotometric analysis. According to Morales-Lozoya et al.,15 the UV-visible absorption peak for green synthesised silver nanoparticles using aqueous extract of Morinda citrifolia was found to be 434, 436, and 438 nm. This variation in the range of absorption peak may be due to the difference in the temperature of incubation and the quantity of leaf sample used for green synthesis in both the studies.
According to Alex et al.,10 the complete conversion of AgNPs can be confirmed by the addition of 0.1 N NaCl to the supernatant obtained after centrifugation of the green synthesised AgNPs. Any free Ag+ will react with Cl– to form precipitate of silver chloride as a result of double displacement reaction between unreacted AgNO3 in the suspension and NaCl to yield sodium nitrate (NaNO3) and silver chloride (AgCl). In the present study, on addition of NaCl, no precipitate was formed, which is an indication that complete conversion has taken place within 1 h.
The green synthesised silver nanoparticles using leaf extract of T. cordifolia exhibited the inhibition zones in all the three inhibition assays, the maximum being in agar well diffusion technique. The inhibition was reconfirmed by the results obtained in population assays by treatment with silver nanoparticles at the exponential phase of the algal culture. In the present study, the leaf extract of T. cordifolia did not exhibit conspicuous inhibition zones in in vitro inhibition assays. Earlier reports indicate that T. cordifolia, exhibited anti-algal property when tested against three algal isolates namely, Anabaena circinalis, Scenedsmus quadricauda and Mougeotia scalaris.16 However, terpenes, alkaloids and phenols extracted from Tinospora cordifolia were used for the assay, whereas in the present study whole leaf aqueous extract was used (5% w/v ie., the same concentration that has been used for the green synthesis).
The carbohydrate content (2.46 µg ml-1) showed a significant reduction in the treated cultures whereas it was increased in the untreated control (16.84 µg ml-1) on the eighth day of inoculation. There was also a significant reduction in the protein content (6.27 µg ml-1) in the treated culture compared to untreated control (25.93 µg ml-1) on eighth day of inoculation. The chlorophyll content (5.96 µg ml-1) remained stable on the eighth day in the treated culture whereas in the untreated control, approximately 2.5 times increase was recorded (15.21 µg ml-1). Earlier reports17 have shown significant reduction in cell viability and chlorophyll content accompanied by increased Reactive Oxygen Species (ROS) in Chlamydomonas acidophila when exposed to silver nanoparticles at different pH levels (4-7).
The results indicate that green synthesised silver nanoparticles using T. cordifolia were effective in inhibiting the growth of C. reinhardtii. Higher surface area to volume ratio and unique physical and chemical properties may have contributed to the enhanced anti-algal activity of the green synthesised silver nanoparticles. The toxicity of silver nanoparticles is reported to be facilitated by their ability to form free radicals when in contact with the cell surface which eventually makes the cell wall porous, causing leakage of cell contents.18 Silver nanoparticles also interact with the phosphorous moiety of the DNA leading to interruptions in DNA replication which further leads to cessation of cell multiplication.19 Chemically synthesised silver nanoparticles of size 25 nm are reported to cause toxicity to the cells of C. reinhardtii and reduce their photosynthetic efficiency when the alga was exposed to varying concentrations of silver nanoparticles
(5-10 µmol l-1)20. The present study is the first report on green synthesised silver nanoparticles using leaf extracts of T. cordifolia exhibiting antialgal property against Chlamydomonas reinhardtii. The only earlier report on anti-algal property of green synthesised silver nanoparticles is on bamboo leaf extract that was found to be effective against green algae Dictyosphaerium pulchellum and Algoriphagus chordate.21 Further studies can aid in developing an ecofriendly approach for the control of invasive algae using green synthesized silver nanoparticles.
Results of the present study indicate that green synthesised silver nanoparticles using leaf extracts of T. cordifolia exhibit anti-algal property against Chlamydomonas. Transmission electron microscope analysis of the particles could reveal more details about the characteristics of the green synthesized silver nanoparticles, regarding their size and shape. Further studies can aid in developing an ecofriendly approach for the control of invasive algae using green synthesized silver nanoparticles.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the facilities provided by Kerala Agricultural University for the conduct of the work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This work was supported by funding received from ICAR-DARE for initiating algal research work in the project ‘Advanced Centre for Integrated Biotechnology for research and development’.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analysed during this study are included in the manuscript.
- Al-Otibi F, Perveen K, Al-Saif NA, et al. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J Biol Sci. 2021;28(4):2229-2235.
Crossref - Singh R, Hano C, Nath G, Sharma B. Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria. Biomolecules. 2021;11(2):299.
Crossref - Pozdnyakov DV, Pettersson LH and Korosov AA. Investigation of harmful/nuisance algal blooms in marine enviroments. In Exploring the Marine Ecology form Space. Springer. 2017;950-1040.
Crossref - Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M. Acclimatization of Chlamydomonas reinhardtii to different growth irradinaces. J Biol Chem. 2012;287(8):5833-5847.
Crossref - Blaby IK, Blaby-Haas CE. Gene expression analysis by arylsulfatase assays in the green alga Chlamydomonas reinhardtii. Reporter Gene Assays. 2018:149-161.
Crossref - Hong DD, Tam LT, Anh HTL, et al. The selection of appropriate cultural conditions for growth of recombiant green microalga Chlamydomonas reinhardtii in laboratory conition. Acad J Biol. 2018;40(2):203-213.
Crossref - Majewska M, Hajrshkova D, Gusciora M, Aksmann A. Phytotoxic activity of diclofenac:evaluation using a model green alga Chlamydomonas reinhardtii with atrazine as a reference substance. Chemosphere. 2018;209:989-997.
Crossref - Sasso S, Stibor H, Mittag M, Grossman AR. The natural history of model organisms: From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. Elife. 2018;7:392-433.
Crossref - Calatrava V, Hon E F, Llamas A, Fernandez E, Galvan A. Nitrogen scavening from amino acids and peptides in the model alga Chlamydomonas reinhardtii-the role of extracellular l-amino oxidase. Algal Res. 2019;38:101395.
Crossref - Alex S, Rani PMR, Soni KB, Nair DS, Reghunath BR. Biosynthesis of silver nanoparticles with antibacterial activity using leaf extract of Michelia Champaca. J Plant Sci Res. 2012;28(1):121-126.
- Hedge JE, Hofreiter BT. In: Whistler, R.L. Be Miller, J.N. (ed.): Methods in Carbohydrate Chemistry. Academic Press, New York. 1961; 190.
- Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265-275.
Crossref - Mckiney G. Absorption of light by chlorophyll soluutions. J Biol Chem. 1941;140(2):315-322.
Crossref - Wei S, Wang Y, Tang Z, et al. A novel green synthesis of silver nanoparticles by the residues of Chinese herbal medicine and their biological activities. RSC Advances. 2021;11(3):1411-1419.
Crossref - Morales-Lozoya V, Espinoza-Gomez H, Flores-Lopez LZ, et al. Study of the effect of the different parts of Morinda citrifolia L.(noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl Surf Sci. 2021;537:147855.
Crossref - Al-Haidari AMD, Al-Shahwany AW, Hassan GM. Evaluation of Tinospora cordifolia Willd. extracts against algal growth. Iraqi J Sci. 2016;57(2B):1103-1110.
- Oukarroum A, Samadani M, Dewez D. Influence of pH on the toxicity of silver nanoparticles in the green alga Chlamydomonas acidophila. Water, Air and Soil Pollution. 2014;225(8):1-8.
Crossref - Danilczuk M, Lund A, Sadlo J, Yamada H, Michalik J. Conduction electron spin resonance of small silver particles. Spectrochimica Acta Part A: Mol Biomol Spectroscc. 2006;63(1):189-191.
Crossref - Prabhu S, Poulose EK. Silver nanoparticles:mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2(1):32.
Crossref - Navarro E, Piccapietra F, Wagner B, et al. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol. 2008;42(23):8959-8964.
Crossref - Dhasarathan P, Devi NR, Sangeetha P, Navaraj SMG, Rajitsigh AJA, Padmalath C. Utilisation of green synthesised silver nanoparticles for water quality management. Adv Nanoparticles. 2018;7(4):77-84.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.