ISSN: 0973-7510
E-ISSN: 2581-690X
This study explores the aldose reductase (AR) inhibitory potential of dinaphthodiospyrol H, a compound isolated from Diospyros kaki (Japanese persimmon). Aldose reductase plays a crucial role in the polyol pathway, a key factor in the progression of diabetic complications such as neuropathy and retinopathy. The isolated compound demonstrated the maximum AR inhibitory effect followed by the tested extract such as 87.34% and 49.09%, respectively. The AR inhibitory effect was supported by molecular docking studies highlighting its strong binding affinity to the AR active site. Complementary Density Functional Theory (DFT) analysis further elucidated the compound’s electronic properties, confirming its stability and effectiveness as an AR inhibitor. Docking studies carried out on the 3D crystallographic structure of Aldose Reductase; ALR2 (PDB ID = 2FZB) showed significant hydrophilic interactions with amino acid residues Ala299, Leu301, Ser302 and hydrophobic interactions with the Trp219. The findings suggest that dinaphthodiospyrol-H holds significant promise as a lead compound for developing novel therapeutic agents targeting diabetic complications through AR inhibition.
Diospyros Kaki, Dinaphthodiospyrol H, Aldose Reductase Inhibitory, Disease Molecular Docking, DFT
Diabetes research has long focused on understanding the mechanisms driving diabetic complications, with hyperglycemia recognized as a primary factor affecting various tissues, sensory organs, the nervous system, circulation, and renal function.1-3 A key pathway contributing to these complications is the polyol pathway, where the enzyme aldose reductase (AR) catalyzes the conversion of glucose into sorbitol, which is further metabolized into fructose. This pathway serves as an alternative route for nonphosphorylated glucose metabolism.4-6
The polyol pathway is directly linked to the development of chronic diabetic complications such as neuropathy, cataract formation, nephropathy, and retinopathy. Consequently, inhibiting aldose reductase has become a focal point in therapeutic strategies to mitigate these complications. ARIs have gained significant attention as potential therapeutic agents that could prevent or reduce long-term diabetic complications.7-9 Despite the emphasis on tight blood glucose control as the main approach for diabetes management, achieving stable glucose levels is challenging for many patients. This has heightened interest in AR inhibition as a complementary strategy for managing diabetic complications. Current research increasingly focuses on developing and evaluating ARIs as effective treatments for these conditions.10
In our study, we explored the inhibition of aldose reductase by Dinaphthodiospyrol-H, a bioactive compound isolated from Diospyros kaki, also known as Japanese or Oriental persimmon, belongs to the Ebenaceae family, which comprises around 500 species predominantly found in tropical and subtropical regions.11,12 The genus Diospyros which includes persimmons and ebony boasts over 249 species of considerable economic and medicinal value. Among them, D. kaki is particularly significant for its historical use in traditional medicine, especially in China, and is one of the high-yield fruit species,13,14 widely cultivated in Korea, China, Japan, and India.15,16 Persimmon leaves and fruits have garnered attention for their diverse biological and pharmacological properties, making them valuable in medicinal, nutritional, and cosmetic applications.17
Traditional Chinese medicine (TCM) has documented the medicinal properties of persimmon leaves for centuries. For instance, the Ming dynasty text Diannan Bencao by Lan Mao (1556) discusses using persimmon leaves for treating chronic lower leg ulcers, while Bencao Zaixin, a Qing dynasty materia medica (1841), highlights their efficacy in treating cough, hematemesis, and excessive thirst.18 More recently, TCM has incorporated persimmon leaves in formulations targeting heart disease, cerebral arteriosclerosis, hypertension, and internal bleeding.19 The Naoxinqing pill, a patented TCM included in the 2010 Chinese Pharmacopeia, is derived from persimmon leaf extract and used to treat cerebral arteriosclerosis.20 In Japan, persimmon leaf tea is popular for its high vitamin C content and is known for its anti-aging and health benefits.21,22 The flavonoids in persimmon leaves, metabolized by intestinal bacteria, are absorbed into the bloodstream, providing antioxidant effects.23,24
Our research focused on isolating and characterizing active compounds from D. kaki, with particular emphasis on Dinaphthodiospyrol H as an aldose reductase inhibitor. We investigated the structure-activity relationships of these compounds to understand their potential as ARIs. Additionally, we applied Density Functional Theory (DFT) analysis to evaluate the electronic properties and reactivity of these compounds, providing insights into their inhibitory mechanisms. The combination of traditional medicinal knowledge and modern scientific techniques offers a comprehensive approach to exploring the therapeutic potential of D. kaki and its constituents in combating diabetic complications.
This integration of ancient wisdom with contemporary scientific research highlights the multifaceted value of D. kaki in both traditional and modern medicine. Beyond their medicinal applications, persimmon leaves have cosmetic and nutritional benefits, such as brightening the complexion and serving as a rich source of vitamin C. This versatile plant continues to be explored for novel applications, solidifying its relevance in both traditional practices and cutting-edge therapeutic developments.
Plant collection
In December 2018, a D. kaki specimen was meticulously collected from the Toormang region of Lower Dir, located in Khyber Pakhtunkhwa, Pakistan. Dr. Muhammad Ilyas, a distinguished expert at the University of Swabi, KP, Pakistan, carried out the botanical identification and authentication of the plant. To ensure proper documentation and future reference, a voucher specimen, designated as UOS/Bot, 43, was carefully deposited in the botanical garden maintained by the University of Swabi, Pakistan, where it remains preserved for scientific study and verification.
Extraction and isolation of the compound
To acquire the plant material, 5 kg of D. kaki was carefully shade-dried and subsequently pulverized into a fine powder. This powdered material was introduced to a cold extraction process with chloroform for 14 days. The resulting reddish extract was concentrated using a rotary evaporator, yielding a crude extract with a mass of 56.12 grams.
To further purify the extract, the chloroform fraction underwent defatting using hexane in a Soxhlet extractor, which resulted in a refined chloroform extract weighing 48.87 grams.
For fractionation, 14 grams of the chloroform extract was employed in normal-phase column chromatography utilizing silica gel. The elution process was carried out with a gradient solvent system of n-hexane and ethyl acetate (ranging from 100:0 to 40:60), producing 16 distinct fractions labeled DK-1 through DK-17. Each fraction was analyzed via thin-layer chromatography (TLC) to assess its composition. Based on the TLC results, fraction DK-8 was selected for further purification. This fraction underwent repeated normal-phase chromatography with a solvent mixture of n-hexane and ethyl acetate (88:12), which resulted in the formation of red needle-like crystals. These crystals were further purified by washing with hexane and acetone, leading to the isolation of compound 1.
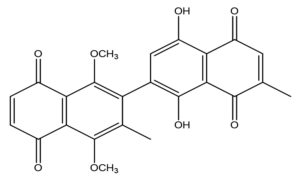
The isolated compound was identified as a naphthoquinone derivative, specifically 1′,4′-dihydroxy-1,4-dimethoxy-3,7′-dimethyl-[2,2′-binaphthalene]-5,5′,8,8′-tetraone. The chemical structure of this compound was confirmed by comparing its physical and spectroscopic properties with previously published data.25 The structure of the isolated compound is given in Figure 1.
Aldose reductase inhibitory
The assay was carried out following the methodology described by Imran et al.26 A reaction mixture totaling 100 μL was prepared, containing 20 μL of buffer solution (100 mM sodium phosphate, pH 6.2), 30 μL of bovine aldose reductase enzyme, 20 μL of the substrate DL-glyceraldehyde (10 mM), 20 μL of the cofactor NADPH (0.1 mM), and 10 μL of the test compounds at a concentration of up to 0.2 mM. Initially, the mixture was prepared without the cofactor and incubated at 32 °C for 10 minutes to allow pre-incubation. The enzymatic reaction was subsequently initiated by adding NADPH, and the reaction progress was monitored continuously for 5 minutes. D-Saccharic acid 1,4-lactone was employed as a reference inhibitor to validate the assay. The IC50 value, representing the concentration required to inhibit the enzyme by 50%, was determined using a similar procedure.
Computational methodology
DFT
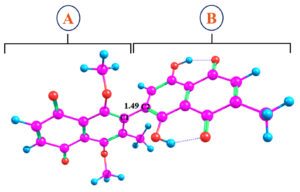
With the final optimized structure, analyses including Electrostatic Potential (ESP), Frontier Molecular Orbitals (FMO), and Density of States (DOS) were conducted. Figure 2 illustrates two distinct fragments of the optimized structure: Fragment A and Fragment B. These fragments are separated by a C-C bond with a bond length of 1.49 Å.
Molecular docking studies
The isolated compounds were docked utilizing the MOE into the binding pocket of 2FZB enzymes to investigate their binding mechanisms. The protein data bank obtained the crystal structure of human ALR2 with PDB codes 2FZB. The protein structure was created using the MOE preparation module, followed by 3-D protonation and energy minimization. For molecular docking, the MOE default parameters placement, the top-ranked conformations were chosen for protein-ligand interaction profile analysis based on docking score. Through the use of Discovery Studio, ligand interaction and visualization were performed.27
Aldose reductase inhibitory of Diospyros kaki
The inhibitory effects of the chloroform extract and dinaphthodiospyrol H, isolated from D. kaki, on aldose reductase are detailed in Table 1. The chloroform extract exhibited a moderate inhibition of 49.09% at a concentration of 0.2 µg, indicating its potential but relatively lower effect in comparison to dinaphthodiospyrol H which demonstrated a substantial aldose reductase inhibition (87.34%) at a concentration of 0.2 µM, with an IC50 value of 1.34 ± 0.38 µM, suggesting its strong inhibitory potential. For comparative purposes, D-Saccharic acid 1,4-lactone, a standard aldose reductase inhibitor, showed an even greater inhibition of 89.34% at the same concentration of 0.2 µM, with a lower IC50 value of 0.87 ± 0.31 µM. These results highlight the significant inhibitory potential of dinaphthodiospyrol H and D-Saccharic acid 1,4-lactone, while underscoring the chloroform extract’s moderate effectiveness.
Table (1):
Aldose Reductase Inhibitory Potential of Chloroform Extract and Dinaphthodiospyrol H Isolated from Diospyros kaki
Sample |
Concentration |
% Inhibition |
IC50 ± SEM (μM) |
|---|---|---|---|
Extract |
0.2 µg |
49.09 |
– |
Dinaphthodiospyrol H |
0.2 µM |
87.34 |
1.34 ± 0.38 |
D-Saccharic acid 1,4-lactone |
0.2 µM |
89.34 |
0.87 ± 0.31 |
Electrostatic potential (ESP)
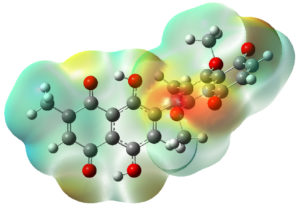
The ESP maps presented in Figure 3 were derived from an optimized molecular structure. Figure 3 illustrates that the red areas, representing negative electrostatic potential, are concentrated on the oxygen atoms in both Fragment A and Fragment B. This suggests that these oxygen atoms are susceptible to electrophilic or cationic attack. Conversely, the blue regions, indicating positive electrostatic potential, are primarily associated with the carbon and hydrogen atoms in both fragments. These areas are potential sites for nucleophilic attack, highlighting their role in molecular reactivity and interaction dynamics.
Frontier molecular orbital (FMO) analysis
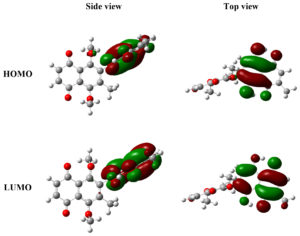
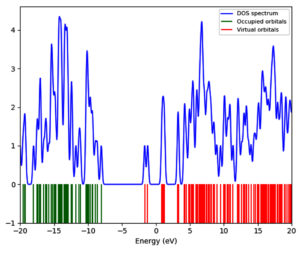
FMO analysis gives information into the optical and electronic properties of organic compounds. This analysis is particularly useful for understanding charge transfer processes within a molecule. The key components of FMO analysis are the HOMO and the LUMO. The HOMO, possessing the highest energy, can donate electrons to the LUMO, which has the lowest energy. The energy difference between the HOMO and LUMO is critical as it influences the stability and interaction characteristics of the compound under study.28 FMO analysis was carried out utilizing the same level of theory as the structure optimization (wB97XD/6-31G(d,p)). Figure 4 displays the HOMO and LUMO surfaces of the compound, while Table 2 lists their respective energy values. The results indicate that the isodensities are predominantly concentrated on Fragment 2 of the compound. The HOMO and LUMO energies are -8.05 eV and -1.66 eV, respectively, yielding an energy gap of 6.38 eV. This significant energy gap suggests substantial electronic interactions and stability within the molecule, reflecting its potential reactivity and electronic behavior.
Table (2):
HOMO-LUMO values (eV) and their energy gap
Complex |
HOMO (eV) |
LUMO (eV) |
EH-L (eV) |
|---|---|---|---|
dinaphthodiospyrol H |
-8.05 |
-1.66 |
6.38 |
Density of state (DOS) analysis
DOS analysis provides valuable insights into the distribution of molecular orbitals and the occupation of these orbitals by electrons across different energy levels. This analysis helps to understand the electronic transitions between the valence and conduction bands, with the Fermi level serving as a reference point for these transitions.29 By examining the DOS spectra, one can study the formation and arrangement of energy levels within the molecule. Figure 5 presents the DOS spectra of the compound. The spectra reveal that the HOMO is located at -8.05 eV, while the LUMO is situated at -1.66 eV. These findings are in agreement with the results obtained from the FMO analysis, further validating the consistency and reliability of the compound electronic structure. The alignment of these results underscores the accuracy of the electronic property assessments and provides a comprehensive understanding of the compound’s electronic behavior.
Docking studies
To evaluate the binding affinities and profile of inhibition of the compounds with the target including Aldose Reductase (ALR2). There are four ligands (L1-L4), and this work investigates how different ligand stoichiometry influences the formation of protein-ligand complexes, resulting in cooperative structural changes. Ligand L2 is arranged perpendicularly, creating hydrogen bonds with Leu301 and Ser302 and p–p stacking with L1’s naphthalene moiety. Ligands L3 and L4 occupy a tiny pocket at the interface of crystal packing, where L3 forms a salt bridge with Lys194 and L4 takes up an H-bond from Asn292.
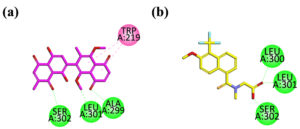
Binding mode of dinaphthodiospyrol H (a) and standard drug (b) in the active site of 2FZB
The visual inspection of the binding mode of compound (a) within the active site of ALR2 revealed significant hydrophilic interactions. Specifically, one of the methoxy moieties formed a hydrophilic interaction with Leu301. Additionally, a dione group attached to a cyclohexene ring, which possesses a dimethoxy substituent on the adjacent chain, established a hydrogen bond interaction with Ala299. Both the dione and the dimethoxy-substituted rings exhibited hydrophobic interactions with Trp219. Moreover, another dione group attached to a cyclohexene ring with a dihydroxy group on the adjacent chain formed a hydrogen bond with Ser302. Notably, the hydrophilic interactions with Leu301 and Ser302 mirrored those observed in the native ligand (L2).
In comparison, the co-crystallized selective inhibitor, the native ligand (b) of ALR2, demonstrated binding affinities with key residues. This included significant hydrophilic interactions with Leu300, Leu301, and Ser302 (Figure 6).
The search for safe, effective, and economical drug candidates for the treatment of management of DM is currently a big challenge to the medicinal chemist. In this regard, ample medicinal plants and their isolated constituents30 have been studied for the said health concerns.31,32 Among the medicinal plants, the genus pistasia is one leading plants as antidiabetic.33 The juice of D. kaki is used for the treatment of DM in alternative systems of medicines.34 Different triterpenoids isolated from D. kaki have been tested with a significant pharmacological effect.35 The current study tested a dihydroxylated compound isolated for D. kaki for anti-DM potential. The tested compound significantly inhibited AR. The AR inhibitors such as alrestatine have been discovered for the treatment of secondary complications of DM. These enzymes are present in almost all cells and create diabetic complications so the AR inhibitors are the best drug candidates that attenuate the chances of diabetes-related complications such as retinopathy. The chloroform extract of D. kaki exhibited moderate inhibition of aldose reductase (49.09%) compared to the strong inhibitory effect of dinaphthodiospyrol H (87.34%) at similar concentrations. Dinaphthodiospyrol H showed a high degree of inhibition, almost comparable to the standard drug D-saccharic acid 1,4-lactone (89.34%). ESP maps and FMO analyses further highlighted the electronic and reactivity features of dinaphthodiospyrol H. At the same time, docking studies revealed strong binding affinities with key residues in the aldose reductase enzyme’s active site. The aldose reductase inhibition results suggest that dinaphthodiospyrol H has significant potential as a therapeutic inhibitor, given its high inhibition percentage and IC50 value (1.34 ± 0.38 µM). The chloroform extract showed moderate efficacy, which may be due to a lower concentration of active constituents. The FMO analysis, which revealed an energy gap of 6.38 eV, suggests strong electronic stability and reactivity, correlating well with the observed biological activity. The ESP map, with negative potential localized around oxygen atoms, indicates potential sites for electrophilic attack, further explaining the molecular interactions observed during the docking studies. The hydrophilic and hydrophobic interactions of dinaphthodiospyrol H with residues such as Leu301 and Ser302, similar to the native ligand, support its strong binding affinity to aldose reductase. The results have significant implications for the development of aldose reductase inhibitors from natural sources. Dinaphthodiospyrol H, with its strong inhibitory potential and stable electronic properties, could serve as a lead compound for further drug development aimed at treating diabetic complications like cataracts and neuropathy, where aldose reductase plays a critical role. The insights gained from ESP and FMO analyses can aid in understanding how the compound’s electronic properties affect its biological activity, providing a framework for designing more effective inhibitors. The molecular docking results reinforce the therapeutic potential of dinaphthodiospyrol H, which, with further modifications, could achieve even higher binding affinities than the standard drug. The results of the study are limited to in vitro evaluation of the aldose reductase inhibition by dinapthodiospyrol and would need further validation through in vivo studies, for confirmation of the biological activity of dinaphthodiospyrol H. The molecular docking analysis, though insightful, is based on a static model and does not account for the dynamic nature of the enzyme or solvent effects. Additionally, while the FMO and DOS analyses provide valuable information on electronic structure, their relationship to biological activity is indirect and requires further validation through experimental studies.
Future studies should focus on conducting in vivo testing of dinaphthodiospyrol H to validate its aldose reductase inhibitory activity in biological systems. Structural modifications of dinaphthodiospyrol H could be explored to enhance its binding affinity and pharmacokinetic properties. Additionally, more comprehensive studies incorporating molecular dynamics simulations would provide a deeper understanding of the enzyme-ligand interactions in a dynamic environment. Expanding this research to other flavonoids or related compounds in Diospyros kaki may reveal other potential inhibitors.
This study demonstrates the significant aldose reductase inhibitory potential of dinaphthodiospyrol H isolated from D. kaki. The compound exhibited strong inhibitory activity (87.34%) with an IC50 value of 1.34 µM, comparable to the standard inhibitor, D-saccharic acid 1,4-lactone. In contrast, the chloroform extract showed moderate inhibition, suggesting that dinaphthodiospyrol H is likely the active component responsible for the observed effects. Molecular docking studies revealed strong binding interactions with key residues in the aldose reductase active site, reinforcing its potential as a therapeutic agent. Additionally, the ESP, FMO, and DOS analyses provided valuable insights into the compound’s electronic structure and reactivity, highlighting its stability and interaction potential. Despite the promising findings, further in vivo studies and molecular modifications are recommended to optimize its efficacy and explore its broader pharmacological applications. This work supports the use of natural compounds from D. kaki as potential therapeutic agents in managing diabetic complications.
ACKNOWLEDGMENTS
The authors extend their appreciation to the Taif University, Saudi Arabia, for supporting this work through project number TU-DSPP-2024-09.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AR conceptualized and supervised the study. WFA and ASA funding acquisition. SN and ZA applied methodology. UR, M, KA and DFT performed molecular docking. UR, M, KA, MR, WFA, ASA, MA, HS, NM and DFT performed formal analysis. SN performed data interpretation. SN wrote the original draft. ZA wrote the manuscript. ZA, MA, HS, NM and MR reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This research was funded by the Taif University, Saudi Arabia, Project number TU-DSPP-2024-09.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Ojo O. An overview of diabetes and its complications. Diabetes Res Open J. 2016;2(2):e4-e6.
Crossref - Amin N. An overview of diabetes mellitus; types, complications, and management. International Journal of Nursing Science Practice and Research. 2018;4(1):119-124.
- Vlacho B, Rossell-Rusinol J, Granado-Casas M, Mauricio D, Julve J. Overview on chronic complications of diabetes mellitus. Chronic Complications of Diabetes Mellitus. 2024:1-10.
Crossref - Yabe-Nishimura, C. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacological Reviews 1998;50(1):21-34.
Crossref - Thakur S, Gupta SK, Ali V, Singh P, Verma M. Aldose Reductase: A cause and a potential target for the treatment of diabetic complications. Arch Pharm Res. 2021;44(7):655-667.
Crossref - Balestri F, Moschini R, Mura U, Cappiello M, Del Corso A. In search of differential inhibitors of aldose reductase. Biomolecules. 2022;12(4):485.
Crossref - Kumar M, Choudhary S, Singh PK, Silakari O. Addressing selectivity issues of aldose reductase 2 inhibitors for the management of diabetic complications. Future Med Chem. 2020;12(14):1327-1358.
Crossref - Nadavapalli P, Nadavapalli P, Bojja KS, Gawli K. In silico analysis of potential inhibitors of aldose reductase. J Appl Pharm Sci. 2023;13(12):140-152.
Crossref - Tomlinson DR, Stevens EJ, Diemel LT. Aldose reductase inhibitors and their potential for the treatment of diabetic complications. Trends Pharmacol Sci. 1994;15(8):293-297.
Crossref - Jannapureddy S, Sharma M, Yepuri G, Schmidt AM, Ramasamy R. Aldose reductase: an emerging target for development of interventions for diabetic cardiovascular complications. Front Endocrinol. 2021;12:636267.
Crossref - Kurt A, Kaya E. Medical and cosmetic applications of persimmon (Diospyros kaki): toxicity assessment-A review. Int J Trad Complement Med Res. 2020;1(3):162-176.
- Sharma M, Goyal P, Mittal P. Therapeutic Potentials of Persimmon (Diospyros kaki): An Updated Review. Res J Pharm Technol. 2024;17(6):2757-2760.
Crossref - Puri AV. A Review on Phytochemistry and Pharmacology of Diospyros montana Roxb. Bioactives and Pharmacology of Medicinal Plants. 2022:295-310.
Crossref - Mallavadhani UV, Panda AK, Rao YR. Review article number 134 pharmacology and chemotaxonomy of diospyros. Phytochemistry. 1998;49(4):901-951.
Crossref - Funayama S, Hikino H. Hypotensive principles of Diospyros kaki leaves. Chem Pharm Bull. 1979;27(11):2865-2868.
Crossref - Rauf A, Uddin G, Patel S, Khan A, et al. Diospyros, an under-utilized, multi-purpose plant genus: A review. Biomed Pharmacother. 2017;91:714-730.
Crossref - Rauf A, AlOmar TS, Rashid U, et al. Dinaphthodiospyrol H: a natural α-glucosidase inhibibitor extracted from Diospyros kaki Lf. Nat Prod Res. 2024:1-5.
Crossref - Xie C, Xie Z, Xu X, Yang D. Persimmon (Diospyros kaki L.) leaves: a review on traditional uses, phytochemistry and pharmacological properties. J Ethnopharmacol. 2015;163:229-240.
Crossref - Han J, Kang S, Choue R, et al. Free radical scavenging effect of Diospyros kaki, Laminaria japonica and Undaria pinnatifida. Fitoterapia. 2002;73(7-8):710-712.
Crossref - Pei Y, Cai T, Zhang Y, Gao H. Methodological investigations and quality evaluations on the method of interference test of the bacterial endotoxin test in Chinese Pharmacopoeia. J Appl Virol. 2013;2(3):24-29.
Crossref - Tsurunaga Y, Takabayashi Y, Nishi M, Suzuki Y. Differences in the ascorbic acid, astragalin, and polyphenol contents, and the DPPH radical scavenging activity of 22 commercial persimmon leaf tea products. Journal of Home Economics of Japan. 2011;62:437-444.
- Martinez-Las HR, Quifer-Rada P, Andres AA, Lamuela-Raventos R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). J Funct Foods. 2016;23:370-377.
Crossref - Bei W, Zang L, Guo J, et al. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J Ethnopharmacol. 2009;126(1):134-142.
Crossref - Zhang S-h, Wang Y-z, Meng F-y, et al. Studies of the microbial metabolism of flavonoids extracted from the leaves of Diospyros kaki by intestinal bacteria. Arch Pharm Res. 2015;38:614-619.
Crossref - Rauf A, Aljohani AS, Alhumaydhi FA, Naz S. A novel compound from the bark of Diospyros lotus and their urease inhibitory activity. Chem Nat Compd. 2020;56:1005-1007.
Crossref - Imran A, Shehzad MT, Shah SJA, et al. Development, molecular docking, and in silico ADME evaluation of selective ALR2 inhibitors for the treatment of diabetic complications via suppression of the polyol pathway. ACS Omega. 2022;7(30):26425-26436.
Crossref - Qayum M, Kaleem WA, Rauf A, et al. In vitro Leishmanicidal evaluation and molecular docking simulations of bioactive compounds from the bark of Taxus wallichiana. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology. 2024;158(3):473-478.
Crossref - Thiruvangoth S. Exploring the spectral and nonlinear optical properties of phenyl isothiocyanate through density functional theory and molecular docking studies: A comprehensive analysis of a potent biologically active phytochemical. Hybrid Advances. 2024;6:100214.
Crossref - Adindu EA, Godfrey OC, Agwupuye EI, et al. Structural analysis, reactivity descriptors (HOMO-LUMO, ELF, NBO), effect of polar (DMSO, EtOH, H2O) solvation, and libido-enhancing potential of resveratrol by molecular docking. Chemical Physics Impact. 2023;7:100296.
Crossref - Nie T, Cooper GJ. Mechanisms underlying the antidiabetic activities of polyphenolic compounds: A review. Front Pharmacol. 2021;12:798329.
Crossref - Sivakumar T, Deepa B. A critical review on Antidiabetic Potential of Herbal plants and its their bioactive components. Journal of University of Shanghai for Science and Technology. 2023;25(01):303-314.
- Mueed A, Shibli S, Al-Quwaie DA, et al. Extraction, characterization of polyphenols from certain medicinal plants and evaluation of their antioxidant, antitumor, antidiabetic, antimicrobial properties, and potential use in human nutrition. Front Nutr. 2023;10:1125106.
Crossref - Rauf A, Al-Awthan YS, Muhammad N, et al. Pharmacological investigation of genus Pistacia. Nat Med Plants. 2022;2022:117.
Crossref - Ahmad M, Farooq U, Shafi A, Akhtar G, Hayat K. Preparation and quality evaluation of functional persimmon (Diospyros kaki) juice for diabetic patients. Agric Sci J. 2022;4(2):105-114.
Crossref - Van Nguyen H, Le NT, Le NTN, et al. T. Extraction, purification, and evaluation of bioactivities of total triterpenoids from Persimmon (Diospyros kaki L.f.) Leaves. Process Biochem. 2024;139:70-80.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.