Mosquitoes transmit life-threatening diseases to humans. The conventional mosquito control techniques that have been focused on population reduction by the application of insecticides or through source reduction by removing the larval habitat have become limited, and it has been evidenced by increased disease burden globally. This review focuses on advanced techniques that reduces and modify the mosquito population and limit their disease transmission by releasing the modified mosquitoes into the environment and that are presently under development and have the potential of controlling the mosquito-borne diseases.
Anopheles, Aedes, Culex, CRISPR/Cas9, RNAi, Mosquito Control, SIT, Wolbachia
Mosquito-borne diseases (MBDs) are the major global health concern.1 Culex, Anopheles, and Aedes are the major vector mosquitoes that transmit pathogens causing filariasis, malaria, dengue, and chikungunya, respectively. Among MBDs, the parasitic infection such as malaria is transmitted by the Anopheline mosquito, it affects around 249 million people and causes 608000 deaths every year globally. Dengue is the most widespread viral infection transmitted through Aedes mosquitoes and over 132 countries are under risk of contracting dengue. The estimated dengue cases are around 96 million and there are 40000 deaths every year.2 The other arbovirus diseases, such as chikungunya, Zika, and yellow fever, are of major concern.2 Growing resistance in vector mosquitoes against currently available insecticide and the toxicity of chemical insecticides on ecosystems has encouraged researchers to opt for the alternative vector control methods.3 As an alternative method, biotechnology offers several innovative techniques for mosquito control, focusing on reducing mosquito density or their capability to transmit diseases.4 The most prominent techniques used are sterile insect technique (SIT),5 release of insects having a dominant lethal (RIDL),6 Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas9),7 Wolbachia bacteria-based control,8 RNA interference (RNAi),9 and Paratransgenesis.10,11 This review provides the information on advanced techniques such as CRISPR/Cas9, RNAi, SIT, and Wolbachia used for vector control (Figure 1).
Clustered regularly interspaced short palindromic repeat and their associated protein (CRISPR/Cas9)
‘Genome editing’, a branch of genetic engineering, is a technique in which the target genome is modified by removing/inserting nucleotide sequences in the living system.12 CRISPR is one such genome editing tool found in prokaryotes, it is an adaptive immunity system that defends them against viruses or bacteriophages. CRISPR was first found during analysis of the gene alkaline phosphatase in Escherichia coli in 1987 by Japanese scientists.13 Later, the key role of CRISPR in the prokaryotic adaptive immune system against bacteriophages was experimentally proven during 2007.14 The CRISPR system comprises two essential proteins components, such as guide RNA (gRNA) and Cas9 proteins. The CRISPR/Cas9 method has three steps; they are detection, cleavage, and repair. Initially, the synthesized single guide RNA (sgRNA) identifies the sequence of interest in the target genome, and it forms a complementary base pair. In the second stage, Cas9 nuclease creates double-stranded breaks, and they are repaired by cellular mechanisms. CRISPR/Cas9 is the most promising genome editing method employed in different disciplines of science. It is widely employed in agriculture to increase the nutritional content of the food grains. CRISPR/Cas9 is being used to investigate cancers, HIV (human immunodeficiency virus), and gene therapy. CRISPR/Cas9 based gene drive mechanisms have been employed for mosquito vector control through population suppression or population replacement. Over the past decade, the CRISPR/Cas9 based technique has become a promising and potential method for effective control of vector mosquitoes.15-19
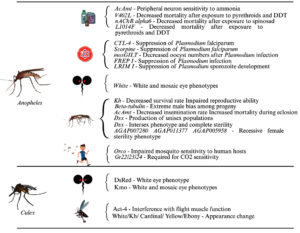
Traditional methods for controlling mosquito-borne diseases are becoming less effective due to vaccine development challenges and growing insecticide resistance. As a result, gene editing technologies like CRISPR-Cas9 are being explored as innovative alternatives. Researchers are targeting mosquito genes that influence pathogen reproduction, immune responses, and vector capacity (Figures 2 and 3). For malaria, genes like FREP1, LRIM1, and CTL4 in Anopheles mosquitoes have been edited to reduce Plasmodium infection and transmission while also affecting mosquito development and reproduction.20-22 Similar strategies have been applied to Aedes aegypti for arboviruses like dengue and Zika, with genes like Obp10, Obp22, AaRel1, and GCTL-3 being modified to suppress virus levels or transmission.23-26 Additionally, manipulating endogenous viral elements (EVEs) in mosquito genomes has shown potential in controlling viral replication through natural immune pathways.27 CRISPR-Cas9 and gene drives disrupt mosquito fertility and suppress the mosquito population. In male mosquitoes, genes like b2-tubulin,28,29 fruitless,30 and Nix31 have been edited to impair fertility, disrupt mating behavior, or increase the number of male progeny. In the female mosquito, genes such as tweedledee and tweedledum,32 CRVP379,33 β-Tubulin 85D,34 core clock CYC,35 kynurense hydroxylase kh,36 dsx37,38 were targeted to interfere with egg development and reduce fertility. Deletion of CYP9M10 gene in Ae. aegypti greatly reduces resistance to pyrethroids18 and mutation in the mJHBP gene increases mosquito susceptibility to bacterial infection, and it leads to sepsis.39 In Culex quinquefasciatus, the kmo gene, which is responsible for eye pigment, was the first target for CRISPR/Cas9-mediated gene knockouts in embryos, and it resulted in homozygous mutants with white-eye phenotypes in the following generation alone.40 Culex Hsu cell lines were used to express Cas9 and sgRNA using Culex-optimized plasmid. This system well edited the immune-related targets such as Dicer-2 and PIWI4, enabling the study of the antiviral gene function.41 Cas9 expressing Culex cell lines were established to validate the immune genes such as dicer 2, argonaute 2b, vago, piwi5, piwi6a, and cullin4a roll in antiviral responses.42 CRISPR-based split-gene drive system was developed to study the super-Mendelian inheritance rates in Cx. quinquefasciatus.43 These findings highlight the potential of gene editing in the control of mosquito-borne disease transmission.
Ribonucleic Acid interference (RNAi)
RNAi is a well known in vivo technique that reduces the mRNA transcripts through post-transcriptional modification with the help of a sequence that is complementary with double-stranded RNA.44 The two core proteins such as dicer and argonaut proteins, are involved in this pathway. The dicer protein is an endonuclease, which identifies the dsRNA and processes it into small RNAs. Later, the argonaut protein takes these small RNAs and searches for target complementary messenger ribonucleic acid (mRNA). In this process, the target mRNA will degrade, or its translation will halt. This process is called posttranslational gene silencing.45 The RNAi technique was first developed to manipulate the gene expression in the nematode, Caenorhabditis elegans,46 later this technique was successfully used to control the agricultural insect pests by using host-induced gene silencing (HIGS), spray-induced gene silencing (SIGS), and virus-induced gene silencing (VIGS) methods.47 As the RNAi technique is successfully employed in agriculture to control insect pests, researchers consider implementing this technique in mosquito control as a biopesticide. In mosquitoes, RNAi is one of the promising techniques to modify the mosquito gene expression (endogenous) and suppress the gene that encodes for pathogens in vivo. It disrupts the mosquito physiology by suppressing the gene required for blood feeding, reproduction, behavior pattern, longevity, and vector status, so thereby the burden of mosquito-borne diseases could be reduced.48
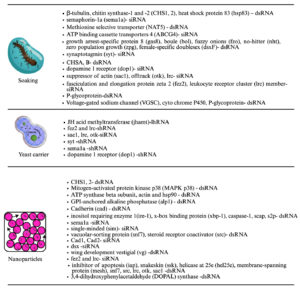
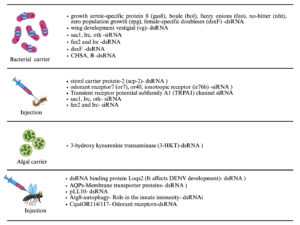
RNAi is a promising, species-specific method for mosquito control. By knocking down essential genes or those related to sex determination or fertility, RNAi can kill mosquitoes or reduce their reproduction without disturbing other species.47 Interfering RNAs (iRNAs), like small interfering RNAs (siRNAs), short hairpin RNAs (shRNAs), and double-stranded RNAs (dsRNAs), are delivered through soaking, feeding, injection, or using nanoparticles. Oral delivery is preferred for large-scale use. RNAi can also support genetic control programs by improving sexing strategies for male-only mosquito releases.49 Insects, including mosquitoes, possess the RNAi machinery, and RNAi is widely used to study the function of the gene and mosquito-pathogen interactions, mainly through adult-stage injections.50 Genome sequencing of major mosquito species enabled broader RNAi applications. More recently, RNAi has been successfully applied to mosquito larvae, with gene silencing confirmed by molecular and phenotypic changes like mortality. Delivery method is crucial for RNAi success. Two main delivery categories exist: non-vector mediated (e.g., soaking, injection, nanoparticles)44 and vector mediated (e.g., using bacteria, yeast, algae, and viruses)51 (Figures 4 and 5).49
Figure 4. Different delivery techniques such as soaking, yeast carrier and Nanoparticles used to introduce RNAi into the host mosquito
Figure 5. Different delivery techniques such as injection, algal and bacterial carriers used to introduce RNAi into the host mosquito
Injection is an effective method for delivering dsRNA into mosquitoes, especially in larvae and adult stages, as it ensures direct entry into the hemocoel and bypasses barriers like the cuticle and gut.52 The cold-anesthetizing mosquitoes were injected with a small quantity (nanoliter) of dsRNA into the thorax using fine glass needles to knock down the target genes in mosquitoes. Most of the studies were conducted using adult mosquitoes to block the pathogen replication.53 Yeast has proven to be an effective RNAi delivery system and attractant for mosquito larvae and females.54-57 For adult mosquito control, sugar-based baits (Attractive Targeted Sugar Baits (ATSBs)) show promise for oral delivery of RNAi,58,59 while other strategies like viral vectors and symbiotic microbes are under development. RNAi could also be used to block disease transmission or alter mosquito behavior.51 RNAi can cause both local and systemic effects and may influence both larvae and adults, making it a promising tool for mosquito control strategies.
Sterile insect technique (SIT)
SIT is a safe and species-specific insect population control method that works on the systematic release of a large number of sterilized male insects into the ecosystem.60 Sterile males mate with wild females, resulting in no offspring, thereby reducing the reproductive potential of the target insect. The release of sterile males in adequate quantities over a prolonged duration can result in considerable suppression or even local elimination of the targeted species.61 SIT has been effectively implemented in a larger scale. During 1989, SIT was successfully employed in the United States, Mexico, Central America, and Libya to eradicate the screwworm fly Cochliomyia hominivorax.62 Some other insect populations that were reduced using SIT technology were the Ceratitis capitata,63 Pectinophora gossypiella,64 and Cydia pomonella65 from America, Africa, Europe, and Asia. The magnitude of these operations can be considerable; for instance, the El Pino facility in Guatemala generates approximately two billion sterile male C. capitata weekly, which is roughly equivalent to 20 tons, primarily for deployment in California and Central America.60 SIT has demonstrated itself to be a cost-effective method to suppress or eradicate the insect populations.
The success of SIT encouraged researchers to implement the technique in vector control programs to reduce or to replace the mosquito populations. The preliminary study using SIT against mosquitoes was initiated during 1959 on the genera Culex, Anopheles, and Aedes. The trails were impeded due to lack of information on vector biology and ecology.66 Sterility in mosquitoes was induced by chemicals, due to difficulty in adjusting the chemical concentration in the sterility process, later it was replaced with irradiation.67 Repeated SIT attempts improved the technique and marked it as a standard method for vector control.68 SIT, when applied in urban settings against pupae and adult mosquitoes of Aedes albopictus, has achieved significant outcomes, inducing 70%-80% sterility in field populations.69 A large number of irradiated males need to be released into the surrounding area to facilitate a 10:1 or 5:1 ratio of sterile:wild mosquitoes to compete with wild males in mating with wild females.70 A behavioral and survival study on sterile Ae. aegypti mosquitoes revealed reduced flying distance and decreased survival time from 54 days to 27 days when the mosquitoes were sterile with 90 gray.71,72
SIT is highly species-specific, it lowers the population of the target mosquito species alone, which reduces the population or eradicates it overtime. With the success of SIT in vector control, the World Health Organization/Special Programme for Research and Training in Tropical Diseases (WHO/TDR) and the Food and Agriculture Organization of the United Nations and the International Atomic Energy Agency (FAO/IAEA) released the guidelines for pilot testing of SIT. Pilot studies against Ae. aegypti and Ae. albopictus are in progress in Brazil, Cuba, Malaysia, Mexico, the USA, Thailand, Singapore, France, Germany, Greece, Italy, Mauritius, and Spain, respectively.73,74
Wolbachia
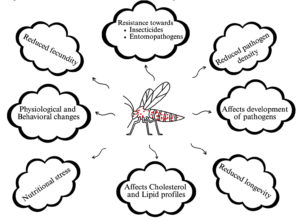
Wolbachia is often isolated from mosquito gut; it is an obligate, Gram-negative gut parasitic bacteria. It was first isolated from gut of Cx. pipiens during 1924 by Hertig, later the bacteria was named Wolbachia pipientis.75 Over the past two decades, several Wolbachia strains have been isolated from mosquitoes. Wolbachia forms endosymbiotic relationships with mosquitoes, ranging from parasitism to mutualism. As a parasite, Wolbachia interferes in mosquito physiology, immunity, and mosquito development, and it also reduces the reproductive ability and longevity of the mosquito, but its mutualism increases mosquito resistance towards viral infection.76,77 Wolbachia transmits vertically through eggs into the next generation, and it transmits horizontally by infection. Horizontal transmission was frequently recorded in Aedes and Culex mosquitoes but not reported from Anopheles and Ae. aegypti.78 Physiological and behavioral changes have been reported from Wolbachia-infected Culex, it alters the host temperature preference, for instance, Culex infected with supergroup A Wolbachia prefers cold temperature, while supergroup B-infected mosquitoes prefer warm.79 Wolbachia increases the insecticidal resistance in mosquitoes, field-collected mosquitoes infected with high-density Wolbachia showed higher resistance towards insecticides compared to infection with lower density Wolbachia. Similarly, wPipSJ-infected Cx. quinquefasciatus showed resistance towards infection of entomopathogenic bacteria (Figure 6).80,81
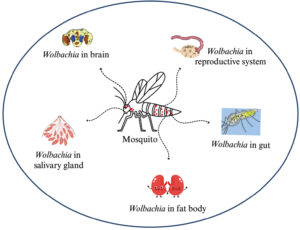
The Wolbachia technique has become a promising, alternative method to control mosquito-borne diseases, particularly in Aedes. It was found that infected female mosquitoes can mate with uninfected males and give rise to Wolbachia-infected offspring.82 In contrast, infected male mosquitoes mating with uninfected females give rise to the production of non-viable eggs.83 Wolbachia colonizes in the midgut, fat body, brain, and salivary gland of mosquito, but more prevalence was found in the reproductive tissues of the mosquito (Figure 7).84 Mosquito host-seeking behavior and oviposition would affect when Wolbachia infects the mosquitoes brain.85 Caragata et al. reported that Wolbachia brings nutritional stress to the mosquitoes, and it alters the cholesterol and lipid profiles, which increases the nutritional competition between Wolbachia and other pathogens as a result, it shortens the mosquito longevity (Figure 6).86
In Anopheles, it was first isolated from reproductive tissue of field-collected Anopheles gambiae, later from An. funestus, followed by An. stephensi.87-89 It was demonstrated that Wolbachia colonizes throughout the body parts of the mosquitoes, and it affects the development of the Plasmodium parasite inside the mosquito. Wolbachia showed varied effects on malaria parasites depending on their species, P. berghei oocyst density was increased in the midgut of An. gambiae infected with the Wolbachia strain wAlbB, while oocyst development was suppressed when mosquitoes were infected with the wMelPop strain.90 wAlbB reduced fecundity and male mating ability in An. stephensi, and it decreased parasite density.91 Reduced P. falciparum sporozoite was found in field-collected wAnga-infected An. coluzzii, and it was evidently reported that Wolbachia infection decreases the mosquito longevity and provides resistance to Anopheles mosquitoes against pathogen infection.92
The conventional, insecticide-mediated vector control strategies are unable to control vector-borne disease due to their inefficiency and increased resistance in the vector mosquitoes. A number of advanced techniques have proven potential, and they may become an effective intervention in reducing the disease transmission. These advanced techniques either modify the mosquito population or suppress them. Wolbachia, SIT, RIDL, and IIT are the techniques that have been tested at the field level, and most of these techniques do not require reapplication; therefore, they reduce the application cost, and they are also environmentally safe. These techniques have significant potential in controlling the mosquito population and limiting their vector capability, making them potential alternatives to conventional methods. The current research on these techniques has become vital in identifying new techniques that would be used effectively in controlling the disease transmission. However, the implementation of these techniques requires a thorough understanding of interactions between mosquitoes, pathogens, and the environment, and the evaluation of the associated risks and benefits should also be monitored.
ACKNOWLEDGMENTS
The author thanks the Dean, MMCH & RI, Kanchipuram, for providing the necessary facilities and support at the institute. The author gratefully acknowledges the staff of the CRL, MMCH & RI, Kanchipuram, for their constant support throughout the work.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- World Health Organization (WHO). 2017. Global vector control response 2017-2030. Special Programme for TDR SCI. Pg.No. 51. ISBN: 9789241512978. Copyright: CC BY-NC-SA 3.0 IGO
- WHO. 2024. Vector-borne diseases. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases
- Liu N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu Rev Entomol. 2015;60:537-559.
Crossref - Catherine G, Pascal B, Jean-Christophe P. Benefits and limitations of emerging techniques for mosquito vector control. Comptes Rendus Biologies. 2019;342(7-8):270-272.
Crossref - Oliva CF, Benedict MQ, Collins CM, et al. Sterile Insect Technique (SIT) against Aedes Species Mosquitoes: A Roadmap and Good Practice Framework for Designing, Implementing and Evaluating Pilot Field Trials. Insects. 2021;12(3):191.
Crossref - Aldridge S. Genetically modified mosquitoes. Nat Biotechnol. 2008;26(7):725.
Crossref - Macias VM, McKeand S, Chaverra-Rodriguez D, et al. Cas9-Mediated Gene-Editing in the Malaria Mosquito Anopheles stephensi by ReMOT Control. G3 (Bethesda). 2020;10(4):1353-1360.
Crossref - Wang GH, Gamez S, Raban RR, et al. Combating mosquito-borne diseases using genetic control technologies. Nat Commun. 2021;12(1):4388.
Crossref - Lopez SBG, Guimaraes-Ribeiro V, Rodriguez JVG et al. RNAi-based bioinsecticide for Aedes mosquito control. Sci Rep. 2019;9:4038.
Crossref - Ratcliffe NA, Pacheco JPF, Dyson P, et al. Overview of paratransgenesis as a strategy to control pathogen transmission by insect vectors. Parasit Vectors. 2022;15(1):112.
Crossref - de Melo KR, Cintra AM, Burini BC, Marinotti O, Souza-Neto JA, Rocha EM. Biotechnological Potential of Microorganisms for Mosquito Population Control and Reduction in Vector Competence. Insects. 2023;14(9):718.
Crossref - Asmamaw M, Zawdie B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics. 2021;15:353-361.
Crossref - Ishino Y, Krupovic M, Forterre P. History of CRISPR-Cas from encounter with a mysterious repeated sequence to genome editing technology. J Bacteriol. 2018;200(7):580-617.
Crossref - Ibrahim AU, Ozsoz M, Saeed Z, Tirah G, Gideon O. Genome engineering using the CRISPR Cas9 system. Biomed Pharm Sci. 2019;2(2):1-7
- Kistler KE, Vosshall LB, Matthews BJ. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 2015;11(1):51-60.
Crossref - Gantz VM, Jasinskiene N, Tatarenkova O, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A. 2015;112(49):E6736-E6743.
Crossref - Hammond A, Galizi R, Kyrou K, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34(1):78-83.
Crossref - Itokawa K, Komagata O, Kasai S, Ogawa K, Tomita T. Testing the causality between CYP9M10 and pyrethroid resistance using the TALEN and CRISPR/Cas9 technologies. Sci Rep. 2016;6:24652.
Crossref - D’Amato R, Taxiarchi C, Galardini M, et al. Anti-CRISPR Anopheles mosquitoes inhibit gene drive spread under challenging behavioural conditions in large cages. Nat Commun. 2024;15(1):952.
Crossref - Dong Y, Das S, Cirimotich C, Souza-Neto JA, McLean KJ, Dimopoulos G. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog. 2011;7(12):e1002458.
Crossref - Dong Y, Simoes ML, Marois E, Dimopoulos G. CRISPR/ Cas9 -mediated gene knockout of Anopheles gambiae FREP1 suppresses malaria parasite infection. PLoS Pathog. 2018;14(3):e1006898.
Crossref - Inbar E, Eappen AG, Alford RT, et al. Knockout of Anopheles stephensi immune gene LRIM1 by CRISPR-Cas9 reveals its unexpected role in reproduction and vector competence. PLoS Pathog. 2021;17(11):e1009770.
Crossref - Wu P, Sun P, Nie K, et al. A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe. 2019;25(1):101-112.e5.
Crossref - Li HH, Cai Y, Li JC, et al. C-type lectins link immunological and reproductive processes in Aedes aegypti. iScience. 2020;23(9):101486
Crossref - Dong S, Ye Z, Tikhe CV, Tu ZJ, Zwiebel LJ, Dimopoulos G. Pleiotropic odorant-binding proteins promote Aedes aegypti reproduction and flavivirus transmission. mBio. 2021;12(5):e0253121.
Crossref - Bui M, Benetta ED, Dong Y, et al. CRISPR mediated transactivation in the human disease vector Aedes aegypti. PLoS Pathog. 2023;19(1):e1010842.
Crossref - Suzuki Y, Baidaliuk A, Miesen P, et al. Non-retroviral endogenous viral element limits cognate virus replication in Aedes aegypti ovaries. Curr Biol. 2020;30(18):3495-3506.e6.
Crossref - Galizi R, Hammond A, Kyrou K, et al. A CRISPR-Cas9 sex-ratio distortion system for genetic control. Sci Rep. 2016;6:31139.
Crossref - Chen J, Luo J, Wang Y, et al. Suppression of female fertility in Aedes aegypti with a CRISPRtargeted male-sterile mutation. Proc Natl Acad Sci U S A. 2021;118(22):e2105075118.
Crossref - Basrur NS, De Obaldia ME, Morita T, et al. Fruitless mutant male mosquitoes gain attraction to human odor. Elife. 2020;9:e63982.
Crossref - Hall AB, Basu S, Jiang X, et al. A male-determining factor in the mosquito Aedes aegypti. Science. 2015;348(6240):1268-1270.
Crossref - Venkataraman K, Shai N, Lakhiani P, et al. Two novel, tightly linked, and rapidly evolving genes underlie Aedes aegypti mosquito reproductive resilience during drought. Elife. 2023;12:e80489.
Crossref - Tikhe CV, Cardoso-Jaime V, Dong S, Rutkowski N, Dimopoulos G. Trypsin-like inhibitor domain (TIL)-harboring protein is essential for Aedes aegypti reproduction. Int J Mol Sci. 2022;23(14):7736.
Crossref - Li X, Xu Y, Zhang H, et al. ReMOT Control Delivery of CRISPR-Cas9 Ribonucleoprotein Complex to Induce Germline Mutagenesis in the Disease Vector Mosquitoes Culex pipiens pallens (Diptera: Culicidae). J Med Entomol. 2021;58(3):1202-1209.
Crossref - Shetty V, Meyers JI, Zhang Y, Merlin C, Slotman MA. Impact of disabled circadian clock on yellow fever mosquito Aedes aegypti fitness and behaviors. Sci Rep. 2022;12(1):6899.
Crossref - Adolfi A, Gantz VM, Jasinskiene N, et al. Efficient population modification gene-drive rescue system in the malaria mosquito Anopheles stephensi. Nat Commun. 2020;11(1): 5553.
Crossref - Simoni A, Hammond AM, Beaghton AK, et al. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat Biotechnol. 2020;38(9):1054-1060.
Crossref - Kyrou K, Hammond AM, Galizi R, et al. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol. 2018;36(11):1062-1066.
Crossref - Kim IH, Castillo JC, Aryan A, et al. A mosquito juvenile hormone binding protein (mJHBP) regulates the activation of innate immune defenses and hemocyte development. PLoS Pathog. 2020;16(1):e1008288.
Crossref - Anderson ME, Mavica J, Shackleford L, et al. CRISPR/Cas9 gene editing in the West Nile Virus vector, Culex quinquefasciatus Say. PLoS One. 2019;14(11):e0224857.
Crossref - Torres TZB, Prince BC, Robison A, Ruckert C. Optimized In Vitro CRISPR/Cas9 Gene Editing Tool in the West Nile Virus Mosquito Vector, Culex quinquefasciatus. Insects. 2022;13(9):856.
Crossref - Walsh E, Torres TZB, Prince BC, Ruckert C. Generation of Cas9 Knock-In Culex quinquefasciatus Mosquito Cells. DNA. 2025;5(1):1.
Crossref - Feng X, López Del Amo V, Mameli E. et al. Optimized CRISPR tools and site-directed transgenesis towards gene drive development in Culex quinquefasciatus mosquitoes. Nat Commun. 2021;12: 2960.
Crossref - Sen GL, Blau HM. A brief history of RNAi: the silence of the genes. FASEB J. 2006; 20(9):1293-1299.
Crossref - Escobar MA, Dandekar AM. Post-Transcriptional Gene Silencing in Plants. In: Madame Curie Bioscience Database. Austin (TX): Landes Bioscience; 2000-2013.
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Patent and specific genetic interference by double stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):206-211.
Crossref - Christiaens O, Whyard S, Velez AM, Smagghe G. Double-Stranded RNA Technology to Control Insect Pests: Current Status and Challenges. Front Plant Sci. 2020;11:451.
Crossref - Yadav M, Dahiya N, Sehrawat N. Mosquito gene targeted RNAi studies for vector control. Funct Integr Genomics. 2023;23(2):180.
Crossref - Munawar K, Alahmed AM, Khalil SMS. Delivery Methods for RNAi in Mosquito Larvae. J Insect Sci. 2020;20(4):1-8.
Crossref - Kataki MS, Singh J, Rajkumari A, Kakoti BB. Regulatory issues and toxicological concerns related to nanocarriers, Nanocarriers Based Colon Targeting. 2025:759-794.
Crossref - Airs PM, Bartholomay LC. RNA Interference for Mosquito and Mosquito-Borne Disease Control. Insects. 2017;8(1):4.
Crossref - McFarlane M, Laureti M, Levee T, Terry S, Kohl A, Pondeville E. Improved transient silencing of gene expression in the mosquito female Aedes aegypti. Insect Mol Biol. 2021;30(3):355-365.
Crossref - Clayton AM, Cirimotich CM, Dong Y, Dimopoulos G. Caudal is a negative regulator of the Anopheles IMD pathway that controls resistance to Plasmodium falciparum infection. Dev Comp Immunol. 2013;39(4):323-332.
Crossref - Mysore K, Flannery EM, Tomchaney M, Severson DW, Duman-Scheel M. Disruption of Aedes aegypti olfactory system development through chitosan/siRNA nanoparticle targeting of semaphorin-1a. PLoS Negl Trop Dis. 2013;7(5):e2215.
Crossref - Duman-Scheel M. Saccharomyces cerevisiae (baker’s yeast) as an interfering RNA expression and delivery system. Curr Drug Targets. 2019;20(9):942-952.
Crossref - Mysore K, Li P, Wang CW, et al. Characterization of a yeast interfering RNA larvicide with a target site conserved in the synaptotagmin gene of multiple disease vector mosquitoes. PLoS Negl Trop Dis. 2019a;13(5):e0007422.
Crossref - Mysore K, Li P, Wang CW, et al. Characterization of a broad-based mosquito yeast interfering RNA larvicide with a conserved target site in mosquito semaphorin–1a genes. Parasit Vectors. 2019b;12(1):256.
Crossref - Coy MR, Sanscrainte ND, Chalaire KC, et al. Gene silencing in adult Aedes aegypti mosquitoes through oral delivery of double-stranded RNA. J Appl Entomol. 2012;136(10):741-748.
Crossref - Fiorenzano JM, Koehler PG, Xue RD: Attractive toxic sugar bait (ATSB) for control of mosquitoes and its impact on non-target organisms: a review. Int J Environ Res Public Health. 2017;14(4):398.
Crossref - Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 2010;10(3):295-311.
Crossref - Dyck VA, Hendrichs J, Robinson AS. Sterile insect technique: principles and practice in area-wide integrated pest management Springer, Dordrecht, The Netherlands. 2005:3-36.
Crossref - Alphey L. SIT 2.0: 21(st) Century genetic technology for the screwworm sterile-insect program. BMC Biol. 2016;14:80.
Crossref - Duarte F, Caro A, Delgado S, et al. Sterile insect technique (sit) effectiveness to control Ceratitis capitata (Diptera: Tephritidae) and medfly catches in two mass trapping layouts. Int J Pest Manag. 2022;68(4):402-413.
Crossref - Morrison NI, Simmons GS, Fu G, et al. Engineered repressible lethality for controlling the pink bollworm, a lepidopteran pest of cotton. PLoS One. 2012;7(12):e50922.
Crossref - Thistlewood HMA, Judd GJR. Twenty-five Years of Research Experience with the Sterile Insect Technique and Area-Wide Management of Codling Moth, Cydia pomonella (L.), in Canada. Insects. 2019;10(9):292.
Crossref - Moran-Aceves BM, Marina CF, Dor A, Liedo P, Toledo J, Sex separation of Aedes spp. mosquitoes for sterile insect technique application: a review. Entomol Exp Appl. 2021;169(10):918-927.
Crossref - Salim KU, Chan-Golston AM, Naughton CC, Ha S, Bradman A, Joyce A. Sterile insect technique and incompatible insect technique, emerging alternatives to insecticides for adult mosquito control, J Integr Pest Manag. 2025; 16(1):10.
Crossref - Klassen W, Curtis CF, Hendrichs J. History of the Sterile Insect Technique. Sterile Insect Technique: Principles and Practices in Area-wide Integrated Pest Management, 2nd edn (eds. by VA Dyck, J Hendrichs & AS Robinson). CRC Press, BocaRaton, FL, USA. 2021:1-44.
Crossref - Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. Pilot Field Trials With Aedes albopictus Irradiated Sterile Males in Italian Urban Areas. J Med Entomol. 2013;50(2):317-325.
Crossref - Guo J, Zheng X, Zhang D, Wu Y. Current status of mosquito handling, transporting and releasing in frame of the sterile insect technique. Insects. 2022;13(6):532.
Crossref - Carvalho DO, Morreale R, Stenhouse S, et al. A sterile insect technique pilot trial on Captiva Island: defining mosquito population parameters for sterile male releases using mark-release-recapture. Parasit Vectors. 2022;15(1):1-4.
Crossref - Bond JG, Osorio AR, Avila N, et al. Optimization of irradiation dose to Aedes aegypti and Ae. albopictus in a sterile insect technique program. PLoS One. 2019;14(2):e0212520.
Crossref - International Atomic Energy Agency (IAEA). Guidance Framework for Testing the Sterile Insect Technique as a Vector Control Tool against Aedes-Borne Diseases. https://iris.who.int/bitstream/handle/10665/331679/9789240002371-eng.pdf
- Bouyer J. Current status of the sterile insect technique for the suppression of mosquito populations on a global scale. Infect Dis Poverty. 2024;13(1):68.
Crossref - Minwuyelet A, Petronio GP, Yewhalaw D, et al. Symbiotic Wolbachia in mosquitoes and its role in reducing the transmission of mosquito borne diseases: updates and prospects. Front Microbiol. 2023;14:1267832.
Crossref - Allman MJ, Fraser JE, Ritchie SA, Joubert DA, Simmons CP, Flores HA. Wolbachia’s deleterious impact on Aedes aegypti egg development: the potential role of nutritional parasitism. Insects. 2020;11(11):735.
Crossref - Kaur R, Shropshire J, Cross KL, et al. Living in the endosymbiotic world of Wolbachia: a centennial review. Cell Host Microbe. 2021;29(6):879-893.
Crossref - Inacio Da Silva L, Dezordi FZ, Paiva MHS, Wallau GL. Systematic review of Wolbachia symbiont detection in mosquitoes: an entangled topic about methodological power and true symbiosis. Pathogens. 2021;10(1):39.
Crossref - Hague MTJ, Caldwell CN, Cooper BS. Pervasive effects of Wolbachia on host temperature preference. MBio. 2020;11(5):e01768-e01720.
Crossref - Echaubard P, Duron O, Agnew P, et al. Rapid evolution of Wolbachia density in insecticide resistant Culex pipiens. Heredity. 2010;104(1):15-19.
Crossref - Diaz-Nieto LM, Gil MF, Lazarte JN, Perotti MA, Beron CM. Culex quinquefasciatus carrying Wolbachia is less susceptible to entomopathogenic bacteria. Sci Rep. 2021;11(1):1094.
Crossref - O’Neill SL. The Use of Wolbachia by the World Mosquito Program to Interrupt Transmission of Aedes aegypti Transmitted Viruses. Adv Exp Med Biol. 2018;1062:355-360.
Crossref - Beebe N, Pagendam D, Trewin BJ, et al. Releasing incompatible males drives strong suppression across populations of wild and Wolbachia-carrying Aedes aegypti in Australia. Proc Natl Acad. Sci U S A. 2021;118(41):e2106828118.
Crossref - Mejia AJ, Dutra HLC, Jones MJ, Perera R, Mcgraw EA. Cross-tissue and generation predictability of relative Wolbachia densities in the mosquito Aedes aegypti. Parasit Vectors. 2022;15(1):128.
Crossref - Turley AP, Smallegange RC, Takken W, Zalucki MP, O’neill SL, Mcgraw EA. Wolbachia infection does not alter attraction of the mosquito Aedes (Stegomyia) aegypti to human odours. Med Vet Entomol. 2014;28(4):457-460.
Crossref - Geoghegan V, Stainton K, Rainey SM, et al. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat Commun. 2017;8(1):526.
Crossref - Baldini F, Segata N, Pompon J, et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat Commun. 2014;5:3985.
Crossref - El-Hadji AN, Bassene H, Makoundou P, Fenollar F, Weill M, Mediannikov O. First report of natural Wolbachia infection in wild Anopheles funestus population in Senegal. Malar J. 2018;17:408.
Crossref - Waymire E, Duddu S, Yared S, et al. Wolbachia 16S rRNA haplotypes detected in wild Anopheles stephensi in eastern Ethiopia. Parasit Vectors. 2022;15(1):1-11.
Crossref - Hughes G, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043.
Crossref - Baldini F, Rouge J, Kreppel K, et al. First report of natural Wolbachia infection in the malaria mosquito Anopheles arabiensis in Tanzania. Parasit Vectors. 2018;11(1):635.
Crossref - Wong ML, Liew JWK, Wong WK, et al. Natural Wolbachia infection in field-collected Anopheles and other mosquito species from Malaysia. Parasit Vectors. 2020;13(1):1-15.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.