ISSN: 0973-7510
E-ISSN: 2581-690X
Containment/dissemination of Leishmaniasis depends on the dominance of Th1/ Th2 immunity. IFN-g and IL-17A are well known for protection from leishmaniasis. Synergistic effects of these two cytokines are also known in various autoimmune diseases. However, the therapeutic, as well as adjunct therapeutic use of rIL-17A and rIFN-g in combination with sub-optimal dose of amphotericin-B (AmpB) is still not validated in visceral leishmaniasis. In the present study, we have evaluated the adjunct therapy in a mouse model of VL. After twenty-one days of post infection, in the therapeutic group, mice were intra-peritoneally injected with two doses of recombinant cytokines at one week interval. In adjunct therapeutic groups of mice, immune components were primed for three days with recombinant cytokine(s) followed by injection of sub-optimal dose of AmpB. Body weight, parasitic load in visceral organs and fold change in cytokines’ gene expression was evaluated. We observed significant gain in body weight, inhibition of parasitic load in visceral organs {(liver; 71.7% – 95%), (spleen; 70%-88.7%) (Bone marrow; 46.6 -87.1%)}; significant up regulation in fold change of pro-inflammatory cytokine(s) gene expression (TNF-g,iNOS, IL-2 and IL-12) as well as marginal increase of anti-inflammatory cytokine(s) gene expression (IL-4, IL-10&TGF-g} in adjunct therapeutic groups of mice. Our results suggest that though the therapeutic use of recombinant cytokine(s) is not the best option; however, use of recombinant cytokine(s) along with suboptimal dose of amphoterocin-B to reduce drug toxicity could have a way for better treatment options.
Balb/c mice, IL-17, therapeutic, adjunct therapy, Visceral Leishmaniasis
Visceral leishmaniasis (VL), also known as kala-azar (black fever) is a disease of reticulo-endothelial system caused by Leishmania-donovani and Leishmania infantum1,2. The clinical manifestations caused by these organisms are usually not distinguishable. However, the decisions for treatment usually do not require species identification. The infection spreads from skin (site of infected insect bite) to visceral organs, especially spleen, liver and bone marrow which additionally progress in spleenomegaly and hepatomegaly that ultimately leads to death, if not treated3. In experimental Leishmania donovani infection mice model susceptibility (BALB/c) and resistant (C57BL/6J) in mice acquire via a macrophage-activating, Th1 cell-dependent mechanism governed by different interdigitating cytokines4. Use of recombinant cytokine-based therapies, over the past few decades, boosts the clinical relevance, especially in the anticancer immune response5-7. Recombinant cytokines milieu virtually regulates the innate and adaptive immunity, including the imitation, execution and extinction of pathogen through the induced immune response.
Cytokines are known for their pleiotropic functions so that they function locally or at a distance to suppress or enhance the immunity8,9. Cytokine/cytokine-combinations that selectively induce the dominance of Th1 response might be useful for VL treatment option. Strong effective immunity against VL parasite is characterised by the emergence of strong parasite-specific Th1 response. Interferon (IFN)-g and its major inducer IL-12 dictate the blood circulating T cells and monocytes to assemble at the site of infection and engulf the infected macrophages10,11. Typically Th1 activating cytokines accompanied with tumor necrosis factor induces profound inflammation and activate inducible nitric oxide synthase (iNOS) that leads to parasite killing in infected macrophages12,13. However, the dominance of IL-4 and IL-10 alone or in additive are responsible for dissemination of parasite into visceral organs14,15. Murray et.al., first of all, paved the pathway for the use of cytokines as therapy in visceral leishmaniasis. They reported the killing of parasite in the liver of infected mice with the use of anti-IL-10 receptor antibody (anti-IL-10R mAb) through an inducible nitric oxide synthase-dependent mechanism16.
The available options for the treatment of visceral leishmaniasis are associated with severe problems like adverse effect, low efficacy, long treatment period and the development of resistance17,18. Hence there is an urgent need for an alternative treatment option for visceral leishmaniasis. In this context, we designed and executed this study in a mice model; in which we have studied three different aspects of the use of recombinant cytokine(s) for an alternative treatment options. i) Prophylactic use of recombinant cytokine(s) for protection from leishmaniasis ii) Therapeutic use of recombinant cytokine(s) once the disease establishes and iii) use of sub-optimal dose of amphotericin-Balong with recombinant cytokine(s) to reduce the drug induced toxicity.
We did not observe better response of recombinant cytokine(s) as a prophylactic or as a therapeutic agent. However, in adjunct therapy, we observed significant results (in terms of parasite clearance in visceral organs, increased pro-inflammatory cytokines response and decreased/marginal increase of anti-inflammatory cytokine response). These findings may help in redefining the treatment module in the treatment of visceral leishmaniasis and decreasing the drug induced toxicity (especially amphotericin-Binduced toxicity). Furthermore, this study can also be done in the drug unresponsive cases.
Animal and parasites
Mice from Animal House Facility of CDRI were used as experimental hosts. This study was compliance with Ethical standard. Clinical strains of Leishmania donovani parasite were taken from patients those who were admitted for diagnosis and treatment of kala-azar in Balaji Utthan Sansthan (BUS), Patna. The clinical isolate of parasite were further cultured aseptically in our laboratory under the standard in vitro conditions as described elsewhere19. Further, grown parasite was injected into mice to establish and maintained in mice model with specific infectivity. Serial passages were done to maintain the amastigote to amastigote.
Mice and Infection
A total of 60 mice were utilized in this study. A 0.1ml of inoculum containing 106 amastigotes/ml was injected intravenously through tail vein to 52 naive Balb/c mice (25-30 g in body weight) {instead of 45 infected mice required for this study} with a sterile 26 gauge needle while rest of the 8 animals were kept as an uninfected control {instead of 5 uninfected mice required for this study} (Table 1 & 2). The magnitude of infection in infected animals was assessed 21 days later by necropsy of two mice from infected group.
Table (1):
Experimental condition of Therapeutic groups of mice.
| Group of Mice | Conditions |
|---|---|
| Therapeutic groups | |
| M1 (Negative control) | Without infection: injected with only PBS (0.01ml) |
| M2 to M8 | Infected with amastigote parasite |
| M2 (Positive control) | Without any recombinant treatment |
| M6 | Injected with recombinant IL-17 Day 21:10µg/0.01ml Day 28: First booster dose (10µg/0.01ml) |
| M7 | Injected with recombinant IFN-γ Day 21:10µg/0.01ml Day 28: First booster dose (10µg/0.01ml) |
| M8 | Injected with recombinant IL-17+ IFN-γ Day 21:10µg/0.01ml Day 28: First booster dose (10µg/0.01ml) |
| Sacrifice at day 45 | |
Table (2):
Experimental condition of Therapeutic groups of mice.
| Group of Mice | Conditions |
|---|---|
| Adjunct therapeutic group | |
| M1 (Negative control) | Without infection: injected with only PBS (0.01ml) |
| M2 to M14 | Infected with amastigote parasite |
| M2 (Positive control) | Without any recombinant treatment |
| M10 | Injected Optimal dose of AmpB Day 21:5µg AmpB /mice Day 28: First booster dose (5 µg AmpB /mice) |
| M11 | Injected sub- Optimal dose of (AmpB) Day 21:1µg AmpB /mice Day 28: First booster dose (1µg AmpB /mice ) |
| M12 | Injected sub- Optimal dose of AmpB + rIL-17A Day 18: 1st priming with rIL-17A (10µg/0.01ml) Day 21:1µg AmpB /mice Day 25: 2nd priming with rIL-17A (10µg/0.01ml) Day 28: First booster dose (1µg AmpB /mice ) |
| M13 | Injected sub- Optimal dose of AmpB+ rIFN-γ Day 18: 1st priming with rIFN-γ (10µg/0.01ml) Day 21:1µg AmpB /mice Day 25: 2nd priming with rIFN-γ (10µg/0.01ml) Day 28: First booster dose (1µg AmpB /mice ) |
| M14 | Injected sub- Optimal dose of AmpB+ rIL-17A Day 18: 1st priming with rIFN-γ + rIL-17A (10µg/0.01ml each) Day 21:1µg AmpB /mice Day 25: 2nd priming with rIFN-γ + rIL-17A ( 10µg/0.01ml each) Day 28: First booster dose (1µg AmpB /mice ) |
| Sacrifice at day 45 | |
Treatment
Ten groups containing five mice each were used for the study. These were treated with different recombinant cytokines alone and in various combinations with suboptimal dose of AmpB. The mice of uninfected controls (M1) were given PBS only (negative Control) and the rest groups of mice were infected with 1×106 amastigote parasites through the tail vein.
Recombinant cytokine(s) as therapeutic treatment
On 21 days of post challenged (well established disease in mice) first dose of recombinant cytokine(s) (10mg/0.01ml/mice) (Group M6= rIL-17A; R & D System, Cat. No. 421-ML-025; Group M7= rIFN-g; R & D System, Cat. No. 485-MI-100 and Group M8= rIL-17A+ rIFN-g) was administered via intra peritoneal in respective group of mice. After seven days of the first dose, the second dose of the same amount of recombinant cytokines was again injected. After 10 days of second dose (i.e. day 45) mice were sacrificed and evaluated for study parameters (Table 1).
Adjunct therapeutic groups
From day 21 of post challenge, adjunct therapy was initiated in respective groups. In this, groups of mice where the only conventional drug, AmpB was used for therapy: two doses of optimal (5µg/mice) and sub-optimal (1µg/mice) AmpB (on day 21 and 28 post challenge respectively) was administered in M10 and M11 group of mice respectively. In groups of mice where sub-optimal dose of AmpB along with recombinant cytokine(s) used for therapy: Three days prior to sub-optimal dose of AmpB administration, mice were sensitized with recombinant cytokine(s) (GroupM12= rIL-17A; Group M13=rIFN-g and Group M14= rIL-17A+ rIFN-g). After 15 days of the second dose of drug (i.e. day 45) mice were sacrificed and evaluated for study parameters (Table 2).
Parasitic Burden Post Treatment
Necropsy of all the mice was done after 10 days post-treatment to assess the parasitic burden in spleen, liver and bone marrow. Therapeutic efficacy was assessed in terms of the parasitic load in each organ. Calculations for parasitic density were done as the number of amastigotes/1000 cell nuclei in each organ as compared to untreated controls (M2). Percentage inhibition was assessed in comparison to the infected control by following formula 20
[ No.of parasite counted from infected control-No. of parasite from study groups / No.of parasite counted from infected control ] X 100
Immunological assay
Cytokine gene expression by Real time-PCR
To assess the quantitative analysis of mRNA expression of various cytokines and inducible NO synthase (iNOS) in spleen cells of various experimental groups of mice, Real-time PCR was performed. Spleen tissues were taken for RNA isolation. RNA isolation was carried out using Tri-reagent (Sigma-Aldrich: Cat No. 93289-100ML) after day 10 post treatment and quantified by using Gene-quant (Bio-Rad). cDNA was synthesized from 1µg of total isolated RNA using a first-strand cDNA synthesis kit (Fermentas: Cat No. K1622). Real-time quantitative polymerase chain reaction (RT-PCR) was conducted as per the protocol described earlier20. Housekeeping gene GAPDH was used for normalization in all quantifications. To avoid contamination or non-specific reactions, a no-template cDNA was included. Cycle threshold (CT) values obtained indicated the number of PCR cycles which are required for the fluorescence signal to exceed the detection threshold value (background noise). Comparative CT method was used to calculate the differences in gene expression 21. The fold change in the expression of genes in recombinant cytokine treated groups as compared to that of the infected group was determined by the formula fold change in expression 2-DDCT 21.
Therapeutic use of recombinant cytokines (IL-17A and IFN-g ) after infection has no effect on change in body and organ weight of mice
Analysis for change/gain in body and organ weight during the course of treatment is an important indicator for evaluation of efficacy of any intervention22. In this study, we used booster doses of recombinant cytokines (IFN-g and IL-17) alone or in combination after Leishmania donovani infection to see the efficacy of these recombinant cytokines in mice model of VL. No significant change in body weight was observed among the groups (by comparing the body weight of mice at day zero and at day 45) (Figure 1A I). Similarly no significant change in liver weight was observed among treated groups (M6, M7 and M8) and untreated group (M2) when compared with non-infected control group (M1) (Figure 1A II) Although, in case of spleen, significant gain in spleen weight was observed in infection untreated group (M2) (mean ± SD;0.315 ± 0.034) compared with non-infected control (M1) (mean± SD;0.244 ± 0.025); however in other groups spleen weight was comparable with non-infected control (Figure 1A III).
Fig. 1A. Therapeutic use of recombinant cytokines (IL-17A and IFN-γ) after infection has no effect on change in body and organs weight of mice :(I) Change in body weight (II) Change in liver weight (III) Change in spleen weight.
Therapeutic use of recombinant cytokine(s) inhibit parasitic growth in visceral organs of Leishmania donovani infected mice
After infection, leishmania parasites from the local site (tissue) migrate through the lymph nodes to the internal organs (especially, liver, spleen and bone marrow). Hence, we compared the parasitic load in visceral organs in treated group of mice (M6, M7 and M8) and compared with non-treated group of mice (M2). In liver: significant decrease (49% to 56.6%) in parasitic load was observed in cytokine(s) treated groups; (Group M6: mean ± SD; 2950 ± 262.4: 49.0%), (M7: mean ± SD; 3404 ±273.5; 56.64%) and (Group M8: mean ± SD; 3347 ± 232.9; 55.69%) as compared with infected non-treated control group (M2: mean ± SD; 6009 ± 589.3; 0%) (Figure 1B I). In spleen: significant decrease (28% to 43%) in parasitic load in spleen was observed in cytokine(s) treated groups; (Group M6: mean ± SD; 2250 ± 207.5; 43.9%), (Group M7: mean ± SD; 2014 ± 292.1; 39.3%) and (M8: mean ± SD; 1477 ± 263.8; 28.84%) as compared with infected control group (Group M2: mean ± SD; 5120 ± 270.4; 0%) (Figure 1B II). In bone marrow: Significant decrease (52.8% to 60.1%) in parasitic load in bone marrow was observed in cytokine(s) treated groups; (Group M6: mean ± SD; 412 ± 40.9; 55.9%), (Group M7: mean ± SD; 443.3 ± 33.0; 60.19%) and (Group M8: mean ± SD; 389.1 ± 35.3; 52.85%) compared with infected control group (Group M2: mean ± SD; 736 ± 94.82; 0%) (Figure 1B III). No parasites were observed in M1 group of mice due to lack of infection.
Fig. 1B. Therapeutic use of recombinant cytokine(s) inhibit parasitic growth in visceral organs of Leishmania donovani infected mice: After sacrifice of mice,tissue specimens from visceral organs were used for estimation of parasitic load. Crushed cells were spread on the slide and after methanol fixation, stained with Giemsa stain and then observed under the microscope (10 X 100). (I) Parasitic load in liver (II) Parasitic load in spleen (III) Parasitic load in bone marrow
Change in pro-inflammatory cytokines gene expression in infected mice upon therapeutic use of recombinant cytokine(s)
Severe defect in the immune system of the host is the hallmark of visceral leishmaniasis23. Successful cure of VL depends on the immune status (especially shift of cytokines toward Th1). Thus in this study, we have evaluated RNA level of cytokines gene expression in the group of mice infected with L donovani parasite and then treated with recombinant cytokines (M6, M7 and M8) and compared it with gene expression of infected non-treated group of mice (M2). After sacrifice of mice, cDNA was prepared from extracted RNA of splenocytes and then gene expression was evaluated. Gene expression of TNF-g: In treated groups of mice, relative fold expression of TNF-a was up-regulated (2.4 to 3.4 times); {(Group M6; mean ± SD: 2.6 ± 1.4), (Group M7; mean ± SD: 2.9 ± 1.6) and (Group M8; mean ± SD: 3.4 ± 0.39)} compared with infected non-treated control group (GroupM2) (Figure 2A I). Gene expression of iNOS: In treated groups of mice relative fold expression of iNOS was up-regulated (2.7 to 4.5 times); {(Group M6; mean ± SD: 3.6 ± 1.4), (M7; mean ± SD: 4.5 ± 0.28) and (Group M8; mean ± SD: 2.7 ± 1.4)} compared infected non-treated control group (GroupM2) (Figure 2A II). Gene expression of IL-2: In treated groups of mice relative fold expression of IL-2 was up-regulated (1.8 to 2.6 times); {(Group M6; mean ± SD: 1.9 ± 0.81), (M7; mean ± SD: 1.8 ± 1.2) and (Group M8; mean ± SD: 2.6 ± 0.35)} compared with infected non-treated control group (Group M2) (Figure 2A III). Gene expression of IL-12: In treated groups of mice relative fold expression of IL-12 was up-regulated (1.3 to 2.6 times); {(Group M6; mean ± SD: 1.3 ± 1.0), (Group M7; mean ± SD: 1.8 ± 0.6) and (Group M8; mean ± SD: 1.6 ± 1.1)} compared with infected non-treated control group (GroupM2) (Figure 2A IV). The up-regulation of relative gene expression was not significant probably due to small sample size.
Fig. 2A. Change in pro-inflammatory cytokines gene expression in infected mice upon therapeutic use of recombinant cytokine(s): After sacrifice, splenocytes from mice were immediately stored in RNAlater and after that Trozole was added. mRNA was isolated and cDNA was prepared. RT-PCR based gene expression was estimated using GAPDH housekeeping for normalization of gene expression. Furthermore, mean gene expression of infected group of mice (M2) was used for comparisons (fold change in gene expression) in recombinant cytokines treated groups (M6, M7 and M8) of mice and uninfected group (M1) (I) Gene expression of TNF-a (II) Gene expression of iNOS(III) Gene expression of IL-2 (IV) Gene expression of IL-12.
Change in anti-inflammatory gene expression in infected mice upon therapeutic use of recombinant cytokine(s)
Similar to pro-inflammatory cytokine(s) gene expression, relative fold expression of anti-inflammatory cytokines gene expression was also evaluated in infected after cytokine treated groups. Gene expression of IL-4: In treated groups of mice relative fold expression of IL-4 was almost similar (0.9 to 1.3 times); {(Group M6; mean ± SD: 1.1 ± 0.03), (M7; mean ± SD: 0.96 ± 0.06) and (M8; mean ± SD: 1.3 ± 0.11) compared with infected non-treated control group (Group M2) (Figure 2B I). Gene expression of IL-10: In treated groups of mice relative fold expression of IL-10 was down regulated (0.17 to 0.59 times); {(Group M6; mean ± SD: 0.59 ± 0.25), (GroupM7; mean ± SD: 0.65 ± 0.23) and (Group M8; mean ± SD: 0.17 ± 0.24)} compared with infected non-treated control group (Group M2) (Figure 2B II). Gene expression of TGF-b: In treated groups of mice relative fold expression of TGF-b was down regulated (0.45 to 0.97 times); {(M6; mean ± SD: 0.89 ± 0.09), (Group M7; mean ± SD: 0.97 ± 0.10) and (GroupM8; mean ± SD: 0.45 ± 0.05)} compared with infected non-treated control group (GroupM2) (Figure 2B III).
Fig. 2B. Change in anti-inflammatory gene expression in infected mice upon therapeutic use of recombinant cytokine(s): Marginal change in relative fold expression of anti-inflammatory cytokines gene expression was observed. (I) Gene expression of IL-4 (II) Gene expression of IL-10 (III) Gene expression of TGF-β
Adjunct therapy of recombinant cytokines (IL-17A and IFN-g) along with sub-optimal dose of AmpB, influence significant improvement in body weight but not change in organ weight
Change/gain in body and organ weight was evaluated in groups of mice used for conventional drug therapy after infection: optimal (Group M10:AmpB; 5µg/mice) and sub-optimal (Group M11: 1µg/mice) dose of drug, alone, and sub-optimal dose of drug along with recombinant cytokines (rIL-17A and rIFN-g) alone or in combination (Adjunct therapy; M12, M13 & M14). After twenty- one (21) days of infection, therapy was started. Two doses of drugs and recombinant cytokines were given after a one-week interval. In order to prime the immune components to the fullest function of drug, recombinant cytokine(s) was given three (3) days prior to drug infusion in adjunct therapy groups of mice. After forty-five (45) days of infection body weight of mice was taken before sacrifice and organ weight was taken after sacrifice. Significant change in body weight was observed in all the group of mice (M10 to M14) when compared with body weight at zero-day (Figure 3A I). When we compared the organ weight (especially liver and spleen weight) with infected control (M2), no significant change was observed (Figure 3A II & 3A III).
Fig. 3A. Adjunct therapy of recombinant cytokines (IL-17A and IFN-γ) along with sub-optimal dose of AmpB, influence in significant improvement in body weight but not change in organ weight: (I) Change in body weight (II) Change in liver weight (III) Change in spleen weight.
Adjunct therapy of recombinant cytokines (IL-17A and IFN-g) along with sub-optimal dose of AmpB, inhibit the parasitic growth in visceral organs of Leishmaniadonovani infected mice
Further, we evaluated the parasitic load in visceral organs (especially, liver, spleen and bone marrow) of the study groups of mice (M1, M2, and M10 to M14) to see the effectiveness of drugs (optimal and suboptimal) alone or in combination with recombinant cytokine (suboptimal dose of drug). The parasitic load was compared with infected non-treated control group of mice (M2). In liver: significant decrease in parasitic load in optimal drug treated group (Group M10; mean± SD; 569 ±328.3; 90.53%) andin sub-optimal drug treated group (Group M11; mean± SD; 2675 ±124.3; 55.4%). Moreover, in sub-optimal drug along with recombinant cytokine(s) treated groups showed significant reduction in parasitic load: {IL-17A (Group M12:mean± SD; 1698 ±116.4; 71.7%)},{IFN-g (Group M13: mean± SD; 735.7 ±200.2; 87.7%)},{IL-17A + IFN-g (Group M14; mean± SD; 295 ±89.8; 95.0%)} as compared with infected non-treated control group (GroupM2: mean ± SD; 6009 ± 589.3; 0%) was observed (Figure 3B I). In spleen: significant decrease in parasitic load in optimal drug treated group (Group M10: mean± SD; 533 ±97.7; 89.5%); sub-optimal drug treated group (Group M11:mean± SD; 1721.0 ±372; 66.3%); sub-optimal drug along with recombinant cytokine(s) treated groups; {IL-17A (Group M12; mean± SD; 1536 ±310.8; 70.0%)},{IFN-g (Group M13; mean± SD; 779.5 ±38.4; 84.7%)}and {IL-17A + IFN-g (Group M14; mean± SD; 557 ±131.4; 88.7%)} as compared with infected non-treated control group (GroupM2: mean ± SD; 5120 ± 270.4; 0%) was observed (Figure 3B II). In bone marrow: significant decrease in parasitic load in optimal drug treated group (Group M10; mean± SD; 244.7 ±24.6; 66.7%); sub-optimal drug treated group (Group M11; mean± SD; 302.9 ±49.7; 58.8%); sub-optimal drug along with recombinant cytokine(s) treated groups; {IL-17A (Group M12; mean± SD; 393.1 ±27.7; 46.6%)}; {IFN-g (Group M13; mean± SD; 272.5 ±20.1; 63%)}and {IL-17A + IFN-g (Group M14; mean± SD; 94.8 ±2.9; 87.1%)} compared with infected non-treated control group (M2: mean ± SD; 736.4 ± 94.8; 0%) was observed (Figure 3B III).
Fig. 3B. Adjunct therapy of recombinant cytokines (IL-17A and IFN-γ) along with sub-optimal dose of AmpB, inhibit parasitic growth in visceral organs of Leishmaniadonovani infected mice: Giemsa stained slides of tissue specimen was used for parasite load estimation in visceral organs under the microscope (10 X 100). (I) Parasitic load in liver (II) Parasitic load in spleen(III) Parasitic load in bone marrow
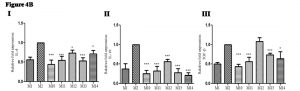
Change in pro-inflammatory cytokines gene expression in infected mice upon adjunct therapy of recombinant cytokines (IL-17A and IFN-g) along with sub-optimal dose of AmpB
Pro-inflammatory cytokine(s) gene expression was evaluated post-sacrifice in study groups of mice to see the effectiveness of optimal and sub-optimal dose of drug alone and suboptimal dose of the drug along with recombinant cytokine(s) . Gene expression of TNF-±: In treated groups of mice relative fold expression of TNF-a was up-regulated (2.9 to 7.8 times); {( Group M10: mean ± SD: 7.8 ± 0.33), {(Group M11: mean ± SD: 2.9 ± 0.44), {( Group M12: mean ± SD: 6.2 ± 0.37), {( Group M13; mean ± SD: 4.4 ± 0.13)and {( Group M14: mean ± SD: 6.9 ± 0.28) as compared with infected non-treated control group (M2) (Figure 4A I). Gene expression of iNOS: In treated groups of mice relative fold expression of iNOS was also up-regulated (3.2 to 7.5 times); {(Group M10: mean ± SD: 7.5 ± 0.33), {(Group M11: mean ± SD: 3.5 ± 0.74), {(Group M12: mean ± SD: 6.2 ± 0.32), {(Group M13: mean ± SD: 4.4 ± 0.13)and {( Group M14: mean ± SD: 6.9 ± 0.28) as compared with infected non-treated control group (M2) (Figure 4A II). Gene expression of IL-2: In treated groups of mice relative fold expression of IL-2 was up-regulated (1.2 to 5.3 times); {( Group M10: mean ± SD: 3.2 ± 0.14), {(Group M11: mean ± SD: 1.2 ± 0.14), {(Group M12: mean ± SD: 4.2 ± 0.15), {( Group M13; mean ± SD: 5.3 ± 0.24) and {(Group M14: mean ± SD: 3.4 ± 0.24) compared with infected non-treated control group (Group M2) (Figure 4A III). Gene expression of IL-12: In treated groups of mice relative fold expression of IL-12 was up-regulated (2.0 to 5.3 times); {(Group M10: mean ± SD: 4.3 ± 0.31), {(Group M11: mean ± SD: 2.0 ± 0.88), {(Group M12: mean ± SD: 4.2 ± 0.2), {(Group M13: mean ± SD: 3.2 ± 0.16)and {(Group M14: mean ± SD: 5.3 ± 0. 4) as compared with infected non-treated control group (M2) (figure 4A IV).
Fig. 4A. Change in pro-inflammatory cytokine(s) gene expression in infected mice upon adjunct therapy of recombinant cytokines (IL-17A and IFN-γ) along with sub-optimal dose of AmpB: (I) Gene expression of TNF-a(II) Gene expression of iNOS(III) Gene expression of IL-2(IV) Gene expression of IL-12
Change in anti-inflammatory gene expression in infected mice upon adjunct therapy of recombinant cytokines (IL-17A and IFN-g) along with sub-optimal dose of AmpB
Similar to pro-inflammatory cytokines gene expression, fold change in gene expression of anti-inflammatory cytokines gene was also evaluated. The expression of anti-inflammatory cytokines namely; Gene expression of IL-4: In treated groups of mice relative fold expression of IL-4 was down regulated (0.4 to 0.7 times); {(Group M10: mean ± SD: 0.44 ± 0.0.54), {(Group M11: mean ± SD:0.5 ± 0.1), {(Group M12: mean ± SD:0.73 ± 0.07), {(Group M13; mean ± SD: 0.53 ± 0.08) and {(Group M14: mean ± SD: 0.7 ± 0.08) as compared with infected non-treated control group (M2) (figure 4B I). Gene expression of IL-10: In treated groups of mice relative fold expression of IL-10 was down regulated (0.2 to 0.56 times); {(Group M10: mean ± SD: 0.25 ± 0.08), {(Group M11: mean ± SD: 0.32 ± 0.11), {(Group M12: mean ± SD: 0.56 ± 0.05), {(Group M13: mean ± SD: 0.27 ± 0.1)and {(Group M14: mean ± SD: 0.21 ± 0. 07) as compared with infected non-treated control group (M2) (Figure 4B II). Gene expression of TGF-²: In treated groups of mice relative fold expression of TGF-b was up-regulated (0.43 to 1.08 times); {( Group M10: mean ± SD: 0.43 ± 0.06), {(Group M11: mean ± SD: 0.56 ± 0.11), {(Group M12: mean ± SD: 1.08 ± 0.09), {(Group M13: mean ± SD: 0.73 ± 0.04)and {(Group M14: mean ± SD: 0.63 ± 0. 17) as compared with infected non-treated control group (M2) (figure 4B III).
The anti-leishmania drugs are the only treatment option for visceral Leishmaniasis till date24,25. This treatment option is always associated with adverse effect on the host like cost, toxicity, longer treatment duration, drug resistance etc24,25. Even though several research advances has been made to overcome the associated problems; drug resistance among VL patients is emerging as a major challenge. Researchers are continuously searching for alternative therapy and developing short duration combination therapy along with reducing drug dose in order to overcome the drug resistance24.
In the present study, author(s) interested in evaluating a new prophylactic and therapeutic strategy with recombinant cytokine(s) for better treatment of visceral leishmaniasis. Change in body weight during the course of treatment has been recognised as a relevant determinant for clinical outcome of the infection with leishmaniasis22,26. Galindo et al. in 2013, showed the gain in body weight of hamsters with progression of disease26. However, we observed no significant change in body weight in both prophylactic and therapeutic groups of mice when compared with control group with the course of treatment. This shows that the recombinant cytokine(s) have some role in amelioration of disease. In our findings, we did not observe any change in the weight of visceral organs among the study groups compared with controls. Additionally, significant gain in spleen weight was observed in untreated group of mice. This is an indicative of profound parasitemia in leishmaniasis in untreated group of mice. This further substantiates our finding of the role of recombinant cytokine(s) in disease management. Sudharshan et al. in 201127 has shown that a significant correlation between parasitic load and clinical outcome in patients undergone treatment with L-AmpB. Mary et al. in 2006 proposed the persistence of more than one parasite/ml of blood after treatment is associated with high risk of relapse16. Few more studies were also shown an increase of more than 10 parasite/ml of blood after treatment is a strong indicative of relapse16,27,28. In our study, we observed significant inhibition of parasitic load in visceral organs in both therapeutic and adjunct therapeutic group of mice with the use of recombinant cytokine(s). When we evaluated the sub-optimal dose of AmpB with cytokine(s) primed group of mice, we observed more than 95% of parasitic clearance.
Furthermore, balance and dynamic change of pro-inflammatory and anti-inflammatory cytokines may help in predicting the clinical outcome of disease14,29. On one hand increased pro-inflammatory response amplifies the inflammatory reactions that trigger the immune response to overcome the disease; on the other hand this heightened pro-inflammatory response may cause collateral tissue damage. Simultaneously the anti-inflammatory cytokines limit the pro-inflammatory cytokine response to prevent the collateral damage of tissue30. However, an excessive down regulation of pro-inflammatory cytokines may favour disease progression. Several reports show that the Th1 type of pro-inflammatory cytokine response (especially interferon-g, tumor necrosis factor-±, interleukin-2, interleukin-12 and iNOS) are the crucial factors in the initiation of protective immunity against visceral leishmaniasis. In our study, we did not find any clear cut cytokine dominance in therapeutic group of mice. Moreover, in adjunct therapeutic group of mice, we observed a clear shift toward Th1 type of cytokine. Furthermore, we also observed increased expression of anti-inflammatory cytokine genes (especially IL-4, IL-10 and TGF-b). Probably this over expression of anti-inflammatory cytokine(s) was the activation of compensatory mechanism to limit the over expressed pro-inflammatory response. This was further confirmed with significant parasitic inhibition in visceral organs of this group.
Based on the above findings we propose that use of recombinant IFN-g as well as IL-17A may curtain the parasitic load in visceral leishmaniasis. This is possibly due to Th1 induced macrophage activation of IFN-g/IL-12 axis, which is further facilitated by IL-17A. Therefore we suggest that both therapeutic, as well as adjunct therapeutic use of recombinant cytokine(s) is/are beneficial in redefining the disease treatment. However, the adjunct therapeutic use of recombinant cytokine(s) shows much better outcome as it reduces the parasitic load in visceral organs and skews the cytokine milieu dominantly towards Th1.
Conflict Of Interest
The authors declare that there is no conflict of interest.
- El Hajj R, El Hajj H, Khalifeh I. Fatal Visceral Leishmaniasis Caused by Leishmania infantum, Lebanon. Emerging infectious diseases, 2018; 24:906-907.
- Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clinical and diagnostic laboratory immunology, 2002; 9:951-958.
- Ready PD. Epidemiology of visceral leishmaniasis. Clinical epidemiology, 2014; 6:147-154.
- Alexander J, Brombacher F. T helper1/t helper2 cells and resistance/susceptibility to leishmania infection: is this paradigm still relevant? Frontiers in immunology, 2012; 3:80-80.
- Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers, 2011; 3:3856-3893.
- Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Annals of translational medicine, 2016; 4:261-261.
- Rider P, Carmi Y, Cohen I. Biologics for Targeting Inflammatory Cytokines, Clinical Uses, and Limitations. International journal of cell biology, 2016; 2016:9259646-9259646.
- Foster JR. The functions of cytokines and their uses in toxicology. International journal of experimental pathology, 2001; 82:171-192.
- Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Frontiers in immunology, 2014; 5:491-491.
- Ewunetu T DT, Gedle D, Kumera G, Diro E. Pro- and Anti-inflammatory Cytokines in Visceral Leishmaniasis. J. Cell Sci. Ther., 2015; 6:1-8.
- ALI MDAaN. Dynamicity of Immune Regulation during Visceral Leishmaniasis. Proc. Indian Natn. Sci. Acad., 2014; 80:247-267.
- Liu D, Uzonna JE. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Frontiers in cellular and infection microbiology, 2012; 2:83-83.
- Gupta G, Oghumu S, Satoskar AR. Mechanisms of immune evasion in leishmaniasis. Advances in applied microbiology, 2013; 82:155-184.
- Maspi N, Abdoli A, Ghaffarifar F. Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: a review. Pathogens and global health, 2016; 110:247-260.
- Gautam S, Kumar R, Maurya R, Nylén S, Ansari N, Rai M et al. IL-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. The Journal of infectious diseases, 2011; 204:1134-1137.
- Mary C, Faraut F Fau – Drogoul M-P, Drogoul Mp Fau – Xeridat B, Xeridat B Fau – Schleinitz N, Schleinitz N Fau – Cuisenier B, Cuisenier B Fau – Dumon H et al. Reference values for Leishmania infantum parasitemia in different clinical presentations: quantitative polymerase chain reaction for therapeutic monitoring and patient follow-up. Am J Trop Med Hyg., 2006; 75(5):858-863.
- Sundar S, Chakravarty J. An update on phrmacotherapy for leishmaniasis. Expert opinion on pharmacotherapy, 2015; 16:237-252.
- Singh OP, Singh B, Chakravarty J, Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infectious diseases of poverty, 2016; 5:19-19.
- Rai AK, Thakur Cp Fau – Kumar P, Kumar P Fau – Mitra DK, Mitra DK. Impaired expression of CD26 compromises T-cell recruitment in human visceral leishmaniasis. Eur. J. Immunol., 2012; 42(10):2782-2791.
- Kushawaha PK, Gupta R Fau – Tripathi CDP, Tripathi Cd Fau – Sundar S, Sundar S Fau – Dube A, Dube A. Evaluation of Leishmania donovani protein disulfide isomerase as a potential immunogenic protein/vaccine candidate against visceral Leishmaniasis. PLoS One., 2012; 7(4):e35670.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc., 2008; 3(6):1101-1108.
- Gómez-Galindo AM, Delgado-Murcia LG. Body weight as a determinant of clinical evolution in hamsters (Mesocricetus auratus) infected with Leishmania (Viannia) panamensis. Revista do Instituto de Medicina Tropical de Sao Paulo 2013;55:357-361.
- Kumar R, Nylén S. Immunobiology of visceral leishmaniasis. Frontiers in immunology, 2012; 3:251-251.
- Moore EM, Lockwood DN. Treatment of visceral leishmaniasis. Journal of global infectious diseases, 2010; 2:151-158.
- Ponte-Sucre A GF, Dujardin J-C, Barrett MP, López-Vélez R, García-Hernández R, et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis., 2017; 11(12):e0006052.
- Juliana Perrone Bezerra de Menezes CESG, Antônio Luis de Oliveira Almeida Petersen, Deborah Bittencourt Mothé Fraga, and Patrícia Sampaio Tavares Veras. Advances in Development of New Treatment for Leishmaniasis. BioMed Research International, 2015:11.
- Sudarshan M, Weirather Jl Fau – Wilson ME, Wilson Me Fau – Sundar S, Sundar S. Study of parasite kinetics with antileishmanial drugs using real-time quantitative PCR in Indian visceral leishmaniasis. J. Antimicrob. Chemother., 2011; 66(8):1751-1755.
- Bossolasco S, Gaiera G Fau – Olchini D, Olchini D Fau – Gulletta M, Gulletta M Fau – Martello L, Martello L Fau – Bestetti A, Bestetti A Fau – Bossi L et al. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J. Clin Microbiol., 2003; 41(11):5080-5084.
- Edhyana Sahiratmadja BA, Tjitske de Boer, Iskandar Adnan, Anugrah Maya, Halim Danusantoso, Ronald H. H. Nelwan, Sangkot Marzuki, Jos W. M. van der Meer, Reinout van Crevel, Esther van de Vosse, Tom H. M. Ottenhoff. Dynamic Changes in Pro- and Anti-Inflammatory Cytokine Profiles and Gamma Interferon Receptor Signaling Integrity Correlate with Tuberculosis Disease Activity and Response to Curative Treatment. Infection and Immunity 2007:820-829.
- Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiation research, 2012; 178:505-523.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.