ISSN: 0973-7510
E-ISSN: 2581-690X
Pseudomonas syringae pv. syringae (Pss) is a gram negative plant pathogenic bacteria that cause severe disease on more than 150 various plant species. Pss is the most frequently bacterial pathogens on crops that make high economical damages in Iran. Studies showed that this bacterium cause significant yield losses in main crops in Iran. During infection of host plants by this organism, damage to plant cells will occur by production of virulence factors.This pathovar produce two cyclic lipodepsipeptide phytotoxin families including syringomycins (SR) and syringopeptins (SP). The strains of Pss were isolated from apricot, cherry, peach and wheat in Southwest Iran. To find out whether production of syringomycin and syringupptin in different isolates is affected by plant material, inhibitory effect of these toxins on Geotrichum candidum and Bacillus megaterium were studied. When no plant extracts were added to the SRM medium, the most intense inhibitory effect on the G. candidum was observed in isolate C1. The highest production of syringomycin was achieved by this strain in the presence of Takdane cultivar leaf extract. The production of syringomycin and syringopeptin by all isolates was significantly higher in the presence of plant extracts on SRM and PDA agar medium.
Iran; Plant extracts; Pss; Toxin.
Due to its geographic location and climate, Iran is one of the major producers of stone fruits in the world. One of the ways to increase garden products is to fight against pests and diseases of these plants. One of the most dangerous diseases of stone fruits is bacterial canker caused by Pseudomonas syringae pv. syringae (Pss). This disease reduces the quality and quantity of the product and reduces the life of the garden, especially in cherries, and can also damage young and old gardens sometimes up to 85 percent (Bultreys & Kaluzna, 2010). Currently, bacterial canker of stone fruits in most parts of the world damage apricots, plums, peaches and cherries. In Iran, bacterial canker disease was reported for the first time from apricot trees in Isfahan and P. syringae was reported as causal agent (Bahar et al., 1982). It seems that the concept of pathovar for this bacteria is not applicable, because it infect more than 150 different plant species (Kennelly et al., 2007). Epidemiologic studies indicate that the disease has two epiphytic and endophytic phases. Therefore, the bacteria can be safely multiply in the leaves of the host plants throughout the growth season. During contamination, Pss uses various secretion systems to transfer proteins into plant cells (Feil et al., 2005). This bacterium produces many pathogenicity factors that can infect wide range of plants. Many strains of Pseudomonas syringae pv. syringae are known to produce cyclic lipodepsipeptides (CLPs) as secondary metabolites such as syringomycin and syringopeptin. The CLPs are considered to be plant virulence factors and antifungal agents (Quigley & Gross, 1994). They affect plant membrane activities and induce necroses at relatively high concentrations (Guenzi et al., 1998), but the relationship of these effects to plant diseases has not been clearly established. Both toxins have strong antibacterial and antifungal activity. It has been shown that these secondary metabolites help colonizing of bacteria in the host and promote bacterial growth in intercellular space (Lu et al., 2002). Apparently, syringomycin production was unique to Pss strains and was not reported by other pseudomonas spp. The aim of this study was to investigate the effect of leaf extracts of three different cherry cultivars (cultivated in Iran) on the production of syringomycin and syringupoptin by Pseudomonas syringae pv. syringae.
Bacterial strains
The bacterial isolates used in the study are listed in Table 1. The strains of Pss were isolated from apricot, cherry, peach and wheat in Fars and Kohgiluyeh and Boyer-Ahmad provinces. The isolates were examined for gram reaction, catalase, oxidase, ultraviolet fluorescence, argenin dihydrolase, levan production, gelatin liquefaction, aesculin hydrolysis, hypersensitive reaction on tobacco leaves and pectolytic capability (Schaad et al., 2001). All isolates were stored in water suspensions (106 cells/ml) at 4°C and subcultured on king medium B (Little et al., 1998).
Table (1):
Characteristics of bacterial strains used in this study.
Strain |
Isolate |
Host of isolate |
Location collected |
|---|---|---|---|
Pseudomonas syringae pv. syringae |
A1 |
Apricot |
Yaouj (Iran) |
Pseudomonas syringae pv. syringae |
P1 |
Peach |
Shiraz (Iran) |
Pseudomonas syringae pv. syringae |
P2 |
Peach |
Yasouj (Iran) |
Pseudomonas syringae pv. syringae |
W1 |
Wheat |
Yasouj (Iran) |
Pseudomonas syringae pv.syringae |
C1 |
Sweet cherry |
Yasouj (Iran) |
Pseudomonas syringae pv. syringae |
C3 |
Sweet cherry |
Yasouj (Iran) |
Pseudomonas fluorescens |
CHA0 |
Soil |
Switzerland |
Virulence testing on sweet cherry fruitlets
Freshly collected immature sweet cherry fruits cv. ‘Takdane’ were disinfected by dipping in 50% ethanol for 3 min and then rinsed three times in sterile distilled water. Afterwards, fruitlets were wrapped with paper towel to remove the excess of water. Each fruitlet was inoculated by pricking in two places to the depth of 2 mm with sterile needle previously immersed in suspension of each strain. After inoculation each fruitlet was immediately placed on moist filter paper in sterile Petri dish and incubated at 24°C for four days. The reference strains and sterile distilled water were included as positive and negative control, respectively. Ten fruitlets were used for testing of each strain.
Bioassays for syringomycin production
Different strains of Pss were used to determine the amount of syringomycin and syringopeptin production in the absence of leaf extract and in the presence of leaf extract of Cherry cultivars Takdane, Surati and Ghaheri. These strains were evaluated for syringomycin production on SRM (Syringomycin minimal) media by using standard bioassays as previously described by Scholz-Schroeder and associates (2001). 15 ml of this culture medium was transferred in each Petri dish. 10 µl of 107 CFU/ml suspension from each bacterium was placed in the center of each SRM medium and kept at 23°C for five days. Petri dishes were sprayed with the suspension of the fungus Geotrichum candidum and after 24 to 48 hours the diameter of the inhibition zones was measured (Schaad et al., 2001). For the detection of syringopeptins Bacillus megaterium should be used as toxin indicator strain, since G. candidum is insensitive to syringopeptins. In order to investigate the effect of leaf extract on the production of toxin, 10 g of leaf tissue in 50 ml sterilized distilled water was completely dissolved. The extract was then sterilized through 0.45 µL pore size filter. For each 15 ml of SRM medium, 5 ml of herbal extract was used.
Bioassays for syringopeptin production
The PDA (Potato Dextrose Agar) medium was used to study the production of syringopeptin. P. fluorescense was also used as a negative control. For each 15ml culture medium, 5ml of leaf extract of different cultivars of cherry was added. In each Petri dishes, about 15-20ml of medium was used. 10 µl of bacterial suspension was placed in the center of each Petri dishes, and stored for six days at 23°C. After six day, the suspension of Basillus megaterium, sprayed onto Petri dishes. After 24-48 hours, the inhibition zone diameter was measured.
Statistical analysis
The experiment was carried out in a completely randomized design and data were subjected to ANOVA and means were separated according to the Duncan’s multiple range test. Data were analyzed by SAS software.
The strains of Pss were isolated from apricot, cherry, peach and wheat in Fars and Kohgiluyeh and Boyer-Ahmad provinces. All P. syringae pv. syringae strains used in this study were negative for oxidase, potato rot, and arginine dihydrolase, but, positive for levan production and the hypersensitive response on tobacco. All strains caused symptoms showing disorders around wounds on fruitlets. After 24h from fruit inoculations with strains of Pss deep black brown necroses were observed, while strain of P. fluorescens did not induce any symptoms. To find out whether production of syringomycin and syringupptin in different isolates is affected by plant material, inhibitory effect of these toxins on G. candidum and B. megaterium were studied. When no plant extracts were added to the SRM medium, the most inhibitory effect on the G. candidum was observed in isolate C1 (Fig. 1). The highest production of syringomycin was achieved by this strain in the presence of Takdane cultivar leaf extract. Most of the isolates produced more syringomycin in the presence of Takdane leaf extract. Syringomycin inhibition was not significantly different between three strains A1, W1 and P1 on the media containing leaf extract of Ghaheri cultivar (Fig. 1). The inhibitory difference of all isolates in the presence of leaf extract of all three cherry cultivars except P2 was significant in comparison to absence of leaf extract. leaf extract of Surati variety had a lower effect on the increasing of syringomycin and syringopeptin production (Fig. 1 and 2). After Takdane cultivar, Ghaheri and Surati cultivars increased the production of toxin. As a result, Takdane, Qahiri and Surati cultivars were more susceptible to bacterial canker of stone fruits respectively. Significant increase in the production of syringomycin and syringopeptin in the presence of leaf extract in comparison to the conditions without the presence of leaf extract indicates the high effect of plant signal molecules on the expression of pathogenicity genes of Pss. The production of syringomycin and syringopptin is significantly related to the amount of plant signal molecules, especially phenolic and sugar compounds (Wang et al., 2006). Among the isolates tested, inhibition zones were observed for all strains of P. syringae pv. syringae (Fig. 1 and 2). The largest inhibition zones were obtained with strains A1 and C1 in presence of plant extracts. The zones of inhibition were generally larger on medium supplemented with extracts of cherry cultivar Takdaneh, but the sizes of the inhibition zones varied by strain. The strains A1 and C1 produced substantially more syringomycin and syringopeptin on SRM and PDA agar media in presence of all plant extracts. In SRM medium without any extracts, the strain A1 formed 7-mm zones of inhibition of G. candidum, compared to 14-mm zones on medium supplemented with Takdaneh extracts and 11mm zones on medium with Ghaheri extracts (Fig. 1). Similar results were observed on PDA agar developed specifically for the production of syringopeptin under defined culture conditions (Fig. 2). Syringomycin and syringopeptin production by the strains was relatively sensitive to plant extracts. The production of syringomycin and syringopeptin by all isolates was significantly higher in the presence of plant extracts on SRM and PDA agar medium. On SRM agar medium, strains A1 and C1 produced zones of antifungal activity whose diameters were almost two times those produced in the absence of the plant extracts. Also on PDA agar medium, strains W1, A1 and C1 produced zones of antibacterial activity whose diameters were almost two times those produced in the absence of the plant extracts. There is growing evidence that virulence genes in bacteria respond to environmental stimuli (Mo et al., 1995), and Pss is no exception. Because virulence determinants are not constitutively expressed in most bacteria, activation of virulence genes upon perception of a specific chemical or physical stimulus imparts order and balance to pathogenesis that will optimize the bacterium’s chances for long term survival. The induction of toxigenesis in P. syringae pv. syringae by specific plant signal molecules reflects an ability of the bacterium to adapt to a dynamic plant environment. It was reported that syringomycin production is activated by specific plant signal molecules in diverse strains of P. syringae pv. syringae (Quigley & Gross, 1994). It recently was established that phytotoxin production by Pss is modulated by the perception of specific plant metabolites (Mo & Gross, 1991). Certain phenolic glucosides, such as arbutin, serve as signals that induce production of syringomycin, a cyclic lipodepsinonapeptide toxin that causes necrotic symptoms in host plants. In addition, the syrB gene, which is conserved in toxigenic strains of Pss and is predicted to encode a synthetase (Quigley & Gross, 1994), is activated by phenolic signal compounds. Cherry tissues appear to contain plant signal molecules that are perceived by Pss based on evidence that the syrB-ZucZ fusion is transcriptionally activated in an environment containing plant constituents (Mo & Gross, 1991). Inoculations of immature cherry fruits demonstrated that there is a rapid and strong expression of syrB in situ. When SRM medium was not amended with the cherry leaf extracts, strains failed to increase syringomycin production. This demonstrated that the cherry leaf extract contained a constituent or signal that was sensed by the bacterium, eventually leading to activation of genes responsible for toxin induction. Studies by Krzesinska et al., (1993) indicated that susceptibility of cherry genotypes to bacterial canker is correlated with signal activity. Extracts from the stems of 12 cherry genotypes were tested for syrB-inducing activity, and the genotypes most susceptible to bacterial canker contained higher signal activities than resistant genotypes. Consequently, both the quantity and quality of plant metabolites with signal activity may have an acute effect on disease development. Because high amounts of flavonoid glycoside signals occur in cherry leaves, one can speculate that their sudden release would be quickly sensed by the bacterium and lead to activation of genes, such as syrB, necessary for phytotoxin production. In addition, it appears that a broad spectrum of Pss strains would be capable of recognizing the flavonoid glycoside signals from cherry. This is based on evidence that most strains of Pss attack a wide range of plant hosts and that they recognize phenolic signal molecules that are found in the leaves, bark, and flowers of many plant species (Mo & Gross, 1991; Quigley & Gross, 1994). Host resistance as observed, for example, in cherry cultivars (Krzesinska et al., 1993) may reflect qualitative and quantitative differences in signal molecules or the balance of plant substances that antagonize induction by plant signals.
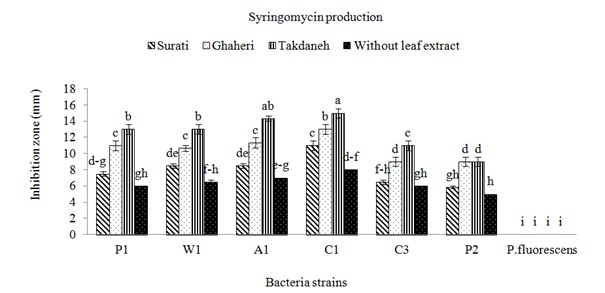 Fig. 1: Effects of plant extracts on syringomycin production by different strains of Pseudomonas syringae pv. syringae. The amounts of syringomycin produced were measured as the diameters of inhibition zones of the fungus G. candidumon SRM agar medium
Fig. 1: Effects of plant extracts on syringomycin production by different strains of Pseudomonas syringae pv. syringae. The amounts of syringomycin produced were measured as the diameters of inhibition zones of the fungus G. candidumon SRM agar medium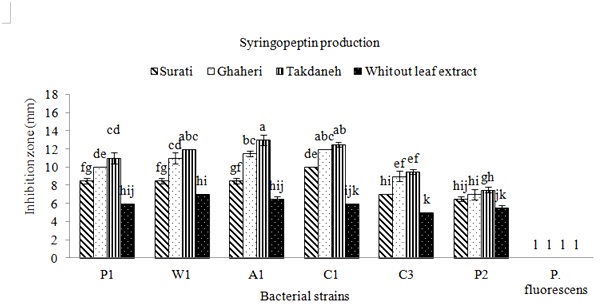 Fig. 2: Effects of plant extracts on syringopeptin production by different strains of Pseudomonas syringae pv. syringae. The amounts of syringopeptin produced were measured as the diameters of inhibition zones of the bacterium B. megaterium on PDA agar medium
Fig. 2: Effects of plant extracts on syringopeptin production by different strains of Pseudomonas syringae pv. syringae. The amounts of syringopeptin produced were measured as the diameters of inhibition zones of the bacterium B. megaterium on PDA agar mediumIn conclusion, it was found that the cultivars of Takdaneh, Ghaheri and Surati respectively induce more toxin production by Pss strains. These cherry cultivars in southwest Iran have the highest cultivation area. In susceptible cultivars, the pathogenicity factors of Pss is produced at a higher level compared to resistant cultivars.
ACKNOWLEDGMENTS
The authors would like to thank the Yasouj University, Iran, for the providing support for this study.
- Bahar, M., Mojtahedi, H., Akhiani, A. Bacterial Canker of Apricot in Isfahan. Iran. J. Plant Pathol., 1982; 18: 58-68.
- Bultreys, A., Kaluzna, M. Bacterial cankers caused by Pseudomonas syringae on stone fruit species with special emphasis on the pathovars syringae and morsprunorum race 1 and race 2. J. Plant Pathol., 2010; 92: 21-33.
- Feil, H., Feil, W.S., Chain, P., Larimer, F., DiBartolo, G., Copeland, A., Lykidis, A., Trong, S., Nolan, M., Goltsman, E. Comparison of the complete genome sequences of Pseudomonas syringae pv. syringae B728a and pv. tomato DC3000. Proceedings of the National Academy of Sciences, 2005; USA 102: 11064-11069.
- Guenzi, E., Galli, G., Grgurina, I., Gross, D.C., Grandi, G. Characterization of the syringomycin synthetase gene cluster: a link between prokaryotic and eukaryotic peptide synthetases. J. Biol. Chem., 1998; 273: 32857-32863.
- Hösel, W. Glycosylation and glycosidases, 1981; p. 725-753. In: E.E. Conn (ed.). The biochemistry of plants. vol. 7. Secondary plant products. Academic, New York.
- Kennelly, M.M., Cazorla, F.M., De Vicente, A., Ramos, C., Sundin, G.W. Pseudomonas syringae diseases of fruit trees: progress toward understanding and control. Plant Dis., 2007; 91: 4-17.
- Krzesinska, E.Z., Azarenko, A.N., Gross, D.C. Inducing the syrB gene in Pseudomonas syringae pv. syringae in twig extracts from cherry genotypes. Hortscience., 1993; 28: 335-337.
- Little, E.L., Bostock, R.M., Kirkpatrick, B.C. Genetic characterisation of Pseudomonas syringae pv. syringae strains from stone fruits in California. App. Environ. Microbiol., 1998; 64: 3818-3823.
- Lu, S.E., Scholz-Schroeder, B.K., Gross, D.C. Characterization of the salA, syrF, and syrG regulatory genes located at the right border of the syringomycin gene cluster of Pseudomonas syringae pv. syringae. Mol. Plant-Microbe Interact., 2002; 15:43-53.
- Mo, Y.Y., Gross, D.C. Plant signal molecules activate the syrB gene, which is required for syringomycin production by Pseudomonas syringae pv. syringae. J. Bacteriol., 1991; 173: 5784-5792.
- Mo, Y.Y., Geibel, M., Bonsall, R.F., Gross, D.C. Analysis of sweet cherry (Prunus avium L) leaves for plant signal molecules that activate the syrB gene required for synthesis of the phytotoxin, syringomycin, by Pseudomonas syringae pv. syringae. Plant Physiol., 1995; 107: 603- 612.
- Quigley, N.B., Gross, D.C. Syringomycin production among strains of Pseudomonas syringae pv. syringae: conservation of the syrB and syrD genes and activation of phytotoxin production by plant signal molecules. Mol. Plant-Microbe Interact., 1994; 7: 78-90.
- Schaad, N.W., Jones, J.B., Chun, W. Laboratory Guide for Identification of Plant Pathogenic Bacteria. 3nd Edition, 2001; American Phytopathological Society Press, St. Paul., Minnesota, USA, 373 PP.
- Scholz-Schroeder, B.K., Hutchison, M.L., Grgurina, I., Gross, D.C. The contribution of syringopeptin and syringomycin to virulence of Pseudomonas syringae pv. syringae strain B301D on the basis of sypA and syrB1 biosynthesis mutant analysis. Mol. Plant-Microbe Interact., 2001; 14: 336-348.
- Wang, N., Lu, S.E., Yang, Q., Sze, S.H., Gross, D.C. Identification of the syr–syp Box in the promoter regions of genes dedicated to syringomycin and syringopeptin production by Pseudomonas syringae pv. syringae B301D. J. Bacteriol., 2006; 188: 160-168.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


