ISSN: 0973-7510
E-ISSN: 2581-690X
Lactobacilli are important organisms which are recognized for their fermentative ability as well as their health and nutritional benefits. Antimicrobial properties of Lactobacilli are of special interest in food industry. Lactobacillus sp., was isolated from plant sources. The isolate was biochemically identified and confirmed by 16SrRNA sequencing. The partial sequence was submitted in GenBank and was assigned with the accession number KX098332. The isolate was screened for antibacterial activity against several pathogenic organisms viz E.coli, Staphylococcus and Bacillus. The antibacterial activity was measured statistically and the results were significant. Further antioxidant activity and their bacteriocin activity was checked under normal mesophilic conditions. The isolate showed less inhibitory effect in the presence of 1% bile salt Sodium Deoxy Cholate in a 4 hour delay of growth. The crude extract containing the bacteriocin was extracted and subjected to FTIR. The functional groups were compared with standard Lactobacilli extract and was confirmed with the presence of relevant peaks that matched with the standard. The present study describes the possible ability of the Lactobacillus and is evident that it can be used as a probiotic that could withstand bile acids, a potent antioxidant and in controlling pathogenic forms of bacteria.
16SrRNA sequencing, Lactobacillus, Bacteriocin, Antioxidant activity.
Lactobacilli belong to the group of Lactic Acid Bacteria (LAB), that have several distinguished abilities such as production of lactic acid, enzymes such as â-Galactosidase organic acids, free fatty acids, ammonia, reuterin, diacetyl, hydrogen peroxide and bacteriocin, which have the capacity to inhibit the growth of food spoilage and pathogenic organisms. Lactic acid bacteria are traditionally used as starters for food fermentations. Since they have the ability to inhibit spoilage and pathogenic bacteria, they are important in food preservation and intestinal prophylaxis. Lactic acid bacteria are the most important groups for industrials purposes, since their fermentative activity involves a notable preservative capacity as a result of the drop in the pH and the antimicrobial activity of their metabolites.
The first bacteriocin isolation study was achieved by using a Gram-negative bacterium Escherichia coli and the isolated molecules were named as Colisins in 1925. Many lactic acid bacteria, including members of great genera Lactococcus, Lactobacillus, Carnobacterium Enterococcus and Pediococcus, are known to secrete bacteriocin. Bacteriocins are in general cationic, amphipathic molecules as they contain an excess of lysyl and arginyl residues. Bacteriocins are among the most promising preservatives in the food industry, and are a family of microbial defense system, which meant they may prohibit the invasion of other strains or the change of the environment, both biotic and abiotic (Riley and Wertz, 2002)
These microorganisms are ubiquitous in nature found in milk, meat, fermented products, fermented vegetables and beverages sometimes as dominating microflora. Lactic acid bacteria isolate was first discovered in milk, soil, water, manure, sewage and other environments. In the recent years, different kinds of bacteriocins have been found from different bacteria; however, only one bacteriocin named nisin has been used for food preservation. Earlier in 1969, WHO announced that nisin was a kind of food preservative with high efficiency and safety, and later in 1983, FDA declared that nisin was generally recognized as a safe food preservative (Han et al., 1991) Since LAB and their metabolites have been consumed in high quantities by generations of people as cultured foods with no adverse effects, the LAB continue as the preferred source for food-use bacteriocins, either in the form of purified compounds or growth extracts. Bacteriocins differ from most therapeutic antibiotics in being proteinaceous and generally possessing a narrow specificity of action against strains of the same or closely related species. The low molecular weight bacteriocins of Gram-positive bacteria demonstrate bactericidal activity which is directed principally against certain other Gram-positive bacteria (Tagg et al., 1976).
Isolation and purification of lactic acid bacteria from food samples
Samples of fresh fruit wastes were collected from the field and market of Yelahanka, Bangalore. The samples were washed with sterile distilled water, chopped with sterile cutter in small pieces weighing 10g (Trias et al., 2008). For isolation of LAB, serial dilution agar technique was used. Ten grams of each sample taken was dissolved into 90 ml of MRS broth. After dissolving into MRS broth they were shaken homogeneously and were incubated at 37°C for 24 hours in an aerobic condition. In the serial dilution agar plate technique, 10ml of a stock solution was suspended and agitated in 90 ml water blanks to form a microbial suspension. Serial dilution of 10-2, 10-3, 10-4, 10-5 and 10-6 were made by pipetting 10ml into 90ml water blanks. 10 ml of each dilution was inoculated to MRS agar plates (prepared by pouring 15ml of sterile and cooled molten media in plates) and incubated at 37°C for 24 hours for bacterial growth. The plates were observed for appearance of colonies and number of colonies produced on each plate of different dilution was recovered. Bacteria were purified by streak plate method on MRS agar and incubated at 37°C for 24hrs and transferred to MRS agar slants and then maintained in refrigerator at 40°C till further analysis.
Standardization of test microorganism
The tested microorganism was standardized by using 0.5 Mc Farland standard. Mc Farland Standard was used as reference to adjust the turbidity of microbial suspensions so that their number will be within a given range. 0.5 Mc Farland gives approximate cell density of 1.5 x 108 CFU/ml, having absorbance of 0.132 at wavelength of 600 nm. The microbial suspensions were prepared in their respective sterile nutrient broth and are compared either visually or by measuring the absorbance with that of the standard (Eliane et al., 2016).
Biochemical and Molecular identification
The isolate was microscopically examined for gram stain reaction, cell morphology and cellular arrangement. The bacterial strains isolated from all the samples were identified up to generic level by employing the standard morphological and biochemical characteristics described in Bergey’s manual of systematic bacteriology (Holt et al., 1994). Amplification, cloning and sequencing of 16S rRNA gene analysis: Genomic DNA was extracted according to Ausubel et al., (1988). 16S rRNA genes of the bacterial isolates were amplified with genomic DNA isolates as template and 8F and 1490R primers (34) in the following composition and amplification cycle. Each reaction mixture contained 2 µl of template DNA (100 ng), 0.5 µM of two primers, and 25 µl of Enzyme Master Mix (Bioron). The PCR program consisted of an initial denaturation step at 94°C for 5 min, followed by 30 cycles of DNA denaturation at 92°C for 30 sec, primer annealing at 50°C for 1 min, and primer extension at 72°C for 2 min was carried out in Thermal Cycler (ThermoHybaid). After the last cycle, a final extension at 72°C for 20 min was added. The PCR products were purified by QIAquick PCR purification kit as described by the manufacturer and cloned using QIAGEN PCR cloning plus kit as described by the manufacturer. Clones were selected and isolated plasmids with insert were sequenced with M13 Sequencing Primers using ABI Biosystems automated sequencer. The sequences obtained were searched in GenBank of National Center for Biotechnology Information (NCBI) using Basic local alignment search tool (BLAST) (Altschul et al.,1997) to obtain the best homologous sequences.
Screening of isolated bacterial cultures for antimicrobial activity
MRS broth was used for antimicrobial metabolite production from lactic acid bacteria, 500mL flasks each containing 200 ml MRS broth autoclaved at 121°C for 15 minutes and inoculated with colony of a LAB isolate grown on MRS agar. The inoculated flasks were incubated at 37°C for 2-3 days under stationary condition. Then it was centrifuged at 10000 rpm for 10 minutes. Antimicrobial activity of culture supernatant (100µl/well) and broth (100µl/well) was tested by agar well diffusion method. An overnight culture of pathogens grown in their respective medium at 37°C was diluted to a turbidity equivalent to that of a 0.5 McFarland standard.
Study on bile tolerance
According to Hyronimus et al., (2000) the difference time between the control (growth in MRS broth without bile) and the test culture (growth in MRS broth with bile -1% w/v) was considered to be the delay in growth caused by the inhibitory action of bile. After 24 h incubation, viable cell count was determined by plating serial dilutions (in 0.05 M phosphate buffer pH 7.0) on MRS agar. These plates were then incubated at 37°C in anaerobic atmosphere for 48 hours. The percentage of growth was calculated as follows:
Production of bacteriocin
The strain was grown in MRS broth at 37°C for 48 h. After incubation, the broth was centrifuged at 5000 rpm for 10 minutes and the cells were separated. The cell free supernatant was used as crude bacteriocin. The extract obtained was subjected to Fourier transform infrared spectroscopy (FTIR) spectrum for knowing the physiological changes in the above samples and characterization was done by employing model NDXUS-672. The spectrum was taken in a mixed IR 400 -4000 cm-2 with 16 scan speed and was recorded using attended total reflectometer (ATR)
Anti oxidant assay
The bacterial strains isolated from vegetables and fruit wastes were subjected to FRAP (Ferric Reducing Antioxidant Power) assay (Antolovich et al., 2002) to evaluate the Antioxidant activity. The Scavenging activity of the extracts of samples can be calculated by using the formula:-
Scavenging Activity = (Control O.D-Test O.D÷Control O.D) ×100
The bacteriocin producing strain was isolated from the source of vegetables and fruit wastes based on its physiological and biochemical characteristics (Table 1). The isolate was gram positive, rod shaped and negative for oxidase and catalase test having smooth round colonies on the MRS agar media. The strain was capable of producing gas in glucose. The discrete band was observed in 1% agarose gel on UV illumination after loading the genomic DNA sample isolated. The sequence obtained was searched in nucleotide database using BLAST software in NCBI server. BLAST results showed that the sequences were having similarity with listed microorganisms and the sequences were deposited in Genbank using Bankit software and assigned accession number (Table-2).
Table (1):
Biochemical Characteristics of the Isolate
S. No |
Tests Performed |
Observation |
|---|---|---|
1 |
Morphology |
Rods |
2 |
Gram staining |
Positive |
3 |
Motility |
Negative |
4 |
Indole |
Negative |
5 |
Methyl red |
Negative |
6 |
Voges Proskauer |
positive |
7 |
Citrate |
Negative |
8 |
Catalase |
Negative |
9 |
Oxidase |
Negative |
10 |
Chloroform |
Sensitive |
11 |
Gas Production |
Positive |
Table (2):
Genomic Identification of Identified Bacterial species
S.No |
BLAST results |
Gen bank accession number |
|---|---|---|
1 |
Lactobacillus sp., |
KX098332 |
Bacteriocin production was strongly dependent on pH, nutrients source and temperature various physicochemical factors seemed to affect bacteriocin production as well as its activity. The bacteriocin suspension of Lactobacillus spp. grown in MRS broth had the best inhibitory effect against wide spectrum of bacteria. The present study demonstrated the production of the bacteriocin by Lactobacillus isolate under different defined culture conditions. The isolate is a mesophile and shows maximum growth rate at an optimum temperature of 37oC. Its antimicrobial potency, pH stability, activity retention in low and high temperatures suggested its wide applicability in acidic pH conditions and in pre-processed food products. Further research though, should be performed to develop extraction techniques for lactic acid and bacteriocins and test further their production on the nutrient media. The isolated strain was grown in MRS broth at 37 for 48 h. Supernatant containing crude bacteriocin was used to determine the antimicrobial activity. An agar well diffusion method was used to access the production of antimicrobial compounds by the selected isolate against three pathogens. The susceptibilities of various Gram-positive and Gram – negative bacteria to growth inhibition by the crude bacteriocin showed inhibitory activity against E.coli, Bacillus and Staphylococcus (Table -4). The extract shows significant inhibition on growth of some pathogenic forms. The Strain pronounced the survival rate of more than 90% in 1.% of bile salt (sodium deoxy cholate) and was used for further studies. Bile tolerance is one of the most crucial properties required for lactobacilli to survive in the small intestine, enabling them play a role in the physiological function of this organ. Bile salt can destabilize membrane integrity of bacterial cells. High concentration of bile in human was reported up to approximately 8% in the gall bladder and 2% in the intestine (Gunn, 2000). Therefore, it is necessary to search for the strains with sufficient ability to resist higher bile concentration. Good probiotic bacterial strain should survive in the presence of bile salts and be able to colonize the intestine surface. The concentration of bile salts used in this experiment was extremely higher compared to many other reports and it was used as a strong tool for selection of potential LAB (Park et al., 2006). The wide variation of bile sensitivity was observed among various species of Lactobacillus (Du Toit et al., 1998). The presence of bile salt caused less inhibitory effect to Lactobacillus isolated, the value of tolerance was 95.6% in a 4 hour delay of growth in the presence of 1% bile salt.(Table -3)
Table (3):
Bile tolerance test
S. No |
Bilesalt Concentration (%) |
Delay of growth (Hours) |
Percentage of growth (%) |
|---|---|---|---|
1 |
1 |
4 |
95.6 ±0.3 |
2 |
2 |
4 |
85.9 ± 0.1 |
3 |
3 |
4 |
63.9 ± 0.2 |
Values are mean ± standard deviations, n=3.
Table (4):
Antimicrobial activity
Pathogens |
Well diffusion*Zone of Inhibition(mm) |
Disc diffusion**Zone of Inhibition(cm) |
|---|---|---|
E. coli |
5.2 ± 0.9 |
10.3 ± 1.0 |
Staphylococcus |
2.3 ± 0.1 |
7.5 ± 1.0 |
Bacillus |
4.7 ± 1.3 |
10.0± 0.8 |
*- The diameter of inhibition was calculated as the difference between the total of inhibition zone and the diameter of growth spot of selected strains (mean ± standard deviation, n=4).
**- The diameter of inhibition zone given in mm included the size of the cork borer (3 mm) with the examined strain (mean ± standard deviation, n=4).
FTIR pattern obtained for isolate the showed homology with the standard graph of Lactobacillus (Figure 1 & 2) indicating the presence of OH stretch (3319 cm-1), C=C conjugated diene (1636 cm-1), CH- deformation for CH 3 group (1465 and 1361 cm-1), C=O stretch for COC group (1222 cm-1) and chloride peak (66 cm-1) were noticed. A peak at 3 312 cm-1 indicates OH stretch. Peaks at 2668 and 2430 cm-1. indicate the CH aliphatic stretch and a peak at 1638 cm-1 indicates the C=C conjugated diene. Peaks at 3938, 3408 cm-1 indicate the presence of C=C aromatic nuclei (carboxylic acid) while C=C stretch for C-O-C group has been noticed at 1117 and 1398 cm-1. The FTIR pattern of the crude extract matched the pattern of the Lactobacillus standard.
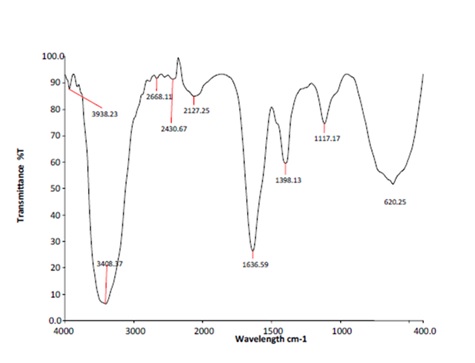
Fig. 1. FTIR Analysis Standard pattern for Lactobacillus sp.
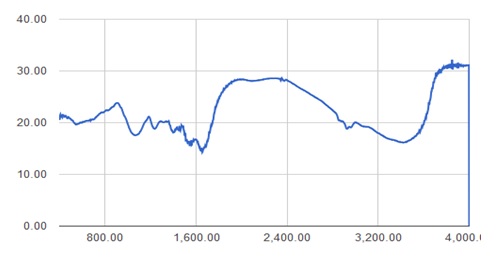
Fig. 2. FTIR pattern for Isolate
This study elucidates the antibacterial effects and some mechanism of action of Lactobacillus isolated from plant wastes. Similar results were reported by Von Mollendorff et al (2007). The antioxidant activity of Lactobaillus strain was determined by FRAP method with respect to reducing power and free radical scavenging activity. Interestingly, the isolate showed the scavenging activity of 33.3%. This high free radical scavenging activity of Lactobacillus suggest a high efficacy of these strains as promising probiotics with potential antioxidant activity for health promotion of the host (Klaenhammer, 1988). The safety and ability to adhere the host intestinal epithelium, the properties of high tolerance to an extremely stressed condition in gastrointestinal (GI) tract are of the most important issue. The present study clearly states that the isolate can tolerate the stressed condition and can overcome the growth of other pathogenic forms by producing certain inhibitory substances under normal conditions. Moreover, Makras et al., in 2006 proved that the good probiotics should have other abilities such as antagonistic activity against pathogenic bacteria and antioxidant activity in order to enhance health promotion of the host.
As per the results discussed in the present study, other bacteriocins of mesophilic Lactobacilli have been reported to be effective against closely related species and therefore considered as potential natural food preservatives (Daeschel 1993). However, studies relating to the antibacterial properties (Reddy et al., 1984) of these organisms have been limited and not fully exploited for use. Further in vivo studies will elucidate the potential of the bacteriocin extracted from the Lactobacillus.
- Altschul Stephen, F., Thomas, L., Madden, A., Alejandro Schaffer, Jinghui Zhang, Zheng Zhang, Webb Miller David, Lipman, J. Gapped BLAST and PSI-BLAST A new generation of protein database search programs. Nucleic Acids Res., 1997; 25: 3389-3402
- Antolovich, M., Prenzler, P.D., Patsalides, E., McDonald,S., Robards, K. Methods for testing antioxidant activity Analyst ,2002; 127: 183–198.
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidelman,Struhl, J .G. Current protocols in molecular biology. John Wiley and Sons, New York, 1988.
- Comparison of pH and bile resistance of Lactobacillus acidophilus strains isolated from rat, pig, chicken, and human sources. World J. Microbiol. Biotech ., 22: 35–37.
- Daeschel, M.A., Steenson Hoover, D.G. Applications and interactions of bacteriocins from lactic acid bacteria in foods and beverages. In Bacteriocins of Lactic acid Bacteria, eds, London: Academic Press., 1993; 63-91.
- Du Toit, M., Franz, C.M.A.P., Dicks, L.M.T., Schillinger, U., Haberer, P., Warlies, B., Ahrens, F., Holzapfel, W. H. Characterisation and selection of probiotic lactobacilli for a preliminary minipig feeding trial and their effect on serum cholesterol levels, faeces pH and faeces moisture content. Int. J. Food Microbiol.,1998; 40: 93-104.
- Eliane Maurício Furtado Martins, Afonso Mota Ramos, Maurilio Lopes Martins , Bruno Ricardo de Castro Leite Junior. Fruit salad as a new vehicle for probiotic bacteria. Food Sci. Technol (Campinas)., 2016; 36(3).
- Gunn, J.S. Mechanisms of bacterial resistance and response to bile. Microb. Infect., 2002; 2: 907•913.
- Han. Daba, Pandian S., Gosselin, J. F., R. E., Simard, J., Huang, C., Lacroix. “Detection and Activity of Bacteriocin Produced by Leuconostoc mesenteriodes. Applied and Environmental Microbiology., 1991; 57(12): 3450-3455.
- Holt, J.G., N.R. Kreig, P.H.A., Sneath, J.T., Staley,S.T., Williams. Bergey’s manual of determinative bacteriology Williams and Wilkins, Maryland, USA.,1994; 9th Edn.
- Hyronimus, B., Le Marrec, C., Sassi, A.H., Deschamps, A. Acid and bile tolerance of spore-forming lactic acid bacteria. Int. J.Food Microbiol.,2000; 61: 193–197.
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria. Biochime.,1988; 70: 337-349
- Makras, L., Triantafyllou, V., Fayol-Messaoudi, D., Adriany, T.,Zoumpopoulou, G., Tsakalidou, E., Servin, A., De Vuyst, L. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar typhimurium reveals a role for lactic acid and other inhibitory compounds. Res. Microbiol., 2006 ; 157: 241-247.
- Park, S.C.,Hwang, M.H., Kim, Y.H., Kim, J.C.,Song, J.C., LeeK.W., Jeong, K.S., Rhee, M.H.,Kim, K.S., Kim, T.W. Comparison of pH and bile resistance of Lactobacillus acidophilus strains isolated from rat, pig, chicken, and human sources. World J.Microbiol. Biotech.., 2006;.22:35–37.
- Reddy G.C., Shahani K.M., Friend B.A., Chandan R.C. Natural antibiotic activity of Lactobacillus acidophilus and bulgaricus, production and partial purification of L.bulgaricus cultured. J. Dairy Products., 1984; 8: 15-19.
- Riley, MA.. Wertz, J.E. Bacterioicins: Evolution, Ecology and Application. Annual Review of Microbiology., 2002; 56: 117-137.
- Tagg J.R., Dajani A.S., Wannamaker L.W. Bacteriocins of gram positive bacteria. Bacteriol. Rev.,1976; 40: 722-756.
- Trias, R., Baneras, L., Badosa, E. and Montesinos, E. Bioprotection of Golden delicious apples and Iceberg lettuce against foodborne bacterial pathogens by lactic acid bacteria. Int J Food Microbiol., 2008; 123:50-60.
- Von Mollendorf, J.W., Todorov, S.D., Dicks, L.M.T. Factor Affecting the adsorption of bacteriocins to Lactobacillus sakei and Enterococcus sp. Appl. Biochem. Biotechnol.,2007; 142: 209-220.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


