ISSN: 0973-7510
E-ISSN: 2581-690X
A study on potential biological control of 10 bacterial isolates isolated from healthy gouramy pond to inhibit the Saprolegnia sp. causal agent of saprolegniasis in gouramy egg was carried out. The ability of bacterial isolates to inhibit Saprolegnia sp. was evaluated in vitro by antagonistic assay on minimum salt medium agar with 2% colloidal chitin as C source, and conducted in vivo by examining all isolates to reduce saprolegniasis in gouramy egg. Hyphal abnormality as a result of antagonistic assay was examined. Adherence test of bacterial isolate cells to egg surface was performed. The result showed that all isolates, except that of PB08 and PB10 indicated to produce chitinase and other antifungal substances. All bacterial isolates showed to inhibit growth of the fungi except that of PB01 which was pathogen to gouramy eggs. Microscopic observation showed that hyphal necrosis was a common abnormality found, followed by hyphal tip necrosis, bent hyphae, and broken hyphae, respectively after antagonistic assay. All bacterial isolates were attached on the egg surface, except that of PB17 which was loosely attached on the egg surface. This study indicated that PB05, PB08, PB13, PB14, and PB15 could be used as potential biological control candidates against saprolegniasis.
Biological control, gouramy egg, Saprolegnia sp.
Fungal disease of fish by watermold is wide spread in fresh water, and is responsible for the most important and extensive commercial losses by reducing both quality and yield in fish production (Mousavi et al., 2009). Saprolegniasis caused by Saprolegnia spp. is one of major watermold disease in aquaculture (Bruno and Wood, 1999). This disease observes as superficial, cottony-like, white growth on the skin and gills of fish, and on fish eggs (Khoo, 2000; Osman et al., 2008). During eggs incubation, these fungi produce mycelia which grow and spread from the nonviable to the healthy eggs suffocating them and causing mortality (Mousavi et al., 2009). In Indonesia, Saprolegnia sp. causes high mortality and reduce survival rate of gouramy in hatchery.
Antifungal agent such as malachite green has been used for long period of time to control fungal growth in fish culture (Van West, 2006). Other chemicals such as hydrogen peroxide (Rach et al., 2004), formalin, and sodium chloride (Rasowo et al., 2007) have been proposed to alter malachite green. However, malachite green was an effective one (Van West, 2006), and has been used for controlling the disease until recently (Van West, 2006; Rasowo et al., 2007; Osman et al., 2008). The chemical practices to overcome fish disease problem have adverse environmental effects affecting non-target organisms and causing health hazards to humans (Khoo, 2000), besides demanding high costs (Mousavi et al., 2009).
Biological control of fish pathogenic fungi is an alternative to reduce chemical utilization to control the fungal growth. Biological control of pathogenic fungal using bacteria and fungi is based on the ability of microbes to produce chitinase and b-1,3-glucanase that lyse fungal cell wall (El-Katatny et al., 2000), since fungal cell wall is composed mainly by polysaccharide like chitin and glucan (Gohel et al. 2006; Anand & Reddy 2009;). Other antifungal metabolites have also shown to suppress fungal disease (Osman et al., 2010; Saravanakumar et al., 2010). Searching for potential microbes is one initial step in developing biological control of fish disease. Recently, the use of naturally occurring bacteria for biocontrol of Saprolegnia has been reported (Lategan et al., 2004; Osman et al., 2008; Saravanakumar et al., 2010). Watson et al. (2008) reviewed biological control of fish pathogenic fungi using bacterial isolates, in which Aeromonas media and Pseudomonas fluorescens were utilized. Unlike biological control of plant pathogen, biological control of fish disease still got less attention. In this study, we isolated antagonistic bacteria from healthy gouramy pond and evaluated the bacterial isolates to control Saprolegnia.
Isolation of Saprolegnia sp.
Infected gouramy eggs from hatchery pond were collected and placed in sterile glass bottle. Cotton-like hyphae of the suspected fungi was cultured on Subaround’s dextrose agar (SDA) with 25 mg/l chloramphenicol. Culture was incubated at 30°C for 2 days. Fungal identification was examined based on microscopic characteristics (Beakes et al., 1994; Rajan, 2000). Culture of Saprolegnia sp. was mantain on SDA and stored at 4°C.
Isolation and screening of bacterial isolates
Fifteen water samples were randomly colected from freshwater of healthy gouramy pond culture in Perbaungan, North Sumatra. Water sample was collected from 20-30 cm of the water surface using sterile glass bottle. All samples were placed in ice container and brought to the laboratory. Isolation of bacteria was conducted by inoculating 1 ml water sample in modified salt medium (MSMC) (0.7 g K2HPO4, 0.3 g KH2PO4, 0.5 g MgSO4.7H2O, 0.01 g FeSO4.7H2O, 0.001 g ZnSO4, and 0.001 g MnCl2 in 1000 ml) containing 2% (w/v) chitin colloidal (MSMC) agar. Bacterial colony with clear zone was transferred into different plates several times until a pure culture was obtained. Bacterial isolates were mantain on MSMC agar and stored at 4°C.
Preliminary screening test of chitinase and antifungal substances were done by growing the isolates in MSMC agar for 5 days and Candida albicans lawn in Muller Hinton Agar (MHA) for 1 day, respectively. All cultures were incubated at 30°C. Typical chitinolytic bacterial isolate was indicated by clearing zone around the colony. Antifungal producing bacteria showed to inhibit the C. albicans growth around their colonies. The semi-quantitative enzyme or antifungal activity was measured as diameter of clear or inhibition zone around the tested bacterial colony.
Simple morphological and biochemical characterization of bacterial isolates
Colony shape was observed directly. Cell shape and Gram staining were evaluated using a microscope. Motility was examined using semi-solid medium sulfide indole motility. Biochemical properties were characterized including gelatin test using gelatin nutrient medium, citrate test using Simons Citrate Agar, catalase test using 3% H2O2 solution, and starch metabolism using starch agar.
Assay of bacterial-fungal antagonism
Bacterial assays against Saprolegnia sp. were conducted to determine the antifungal activity of the bacterial isolates. An agar plug (Ø 5-mm) of Saprolegnia sp. from the margin of an actively growing culture was inoculated in the center of plate containing 20 ml of MSMC agar. Paper disc of 10-µl (»108 cells/ml) of each bacterial culture was place at the edge of plate opposite to the fungal inoculation at a distance of 3.5 cm from the center. Cultures were incubated at 30°C. Each treatment was repeated 3 times. Observation was taken after 5 days of incubation. Antagonistic activity was measured as radius of uninhibited mycellia substracted by radius of inhibited mycellia by bacterial activity.
Pathogenicity test of Saprolegnia sp. to gouramy egg
Test of pathogenicity of Saprolegnia sp. isolated from infected gouramy egg was conducted using its zoospore. Twenty gouramy healthy eggs were placed in 400 ml SDW (SDW) in flask. A 0.4 ml (»104 zoospores/ml) was put into the flask (Hanjavanit et al., 2008). Control was a treatment without zoospore inoculation. Hatching and infection rate were measured during 7 days of treatment.
Pathogenicity and adherence test of bacterial isolates to gouramy egg
It is important to know that our bacterial isolates as potential biological agent might not harm and infect the eggs. Twenty gouramy healthy eggs were placed in 400 ml sterilize distilled water (SDW) in flask. A 0.4 ml (»105 cells/ml) was put into the flask (Lategan et al., 2004). Control was a treatment without bacterial inoculation. Hatching and infection rate were measured during 7 days of treatment.
To know the ability of bacterial isolates to attach to the egg, eggs of 48-hours after bacterial treatment was taken and washed with SDW. Some eggs was grinded and then cultured in MSMC agar. Others were fixed with formaline buffer saline for histological observation to know the effect of bacterial treatment.
In vivo assay of potential biological control of bacterial isolates to Saprolegnia sp.
A series of glass container were prepared with 400-ml of SDW, in which 3-days old of 25 gouramy healthy eggs were put into. Oxygen was administered using aerator. Zoospore (»104 zoospores/ml) of Saprolegnia sp. (Hanjavanit et al., 2008) was inoculated into the glass container 1 day after the inoculation of bacterial cell (»105 cells/ml) (Lategan et al., 2004). (-) control was eggs without any microbe inoculation, while (+) control was egg treated with Saprolegnia sp. without bacterial inoculation. Each treatment was replicated three times. Hatching rate were measured after zoospore inoculation.
Microbial cell source and culture condition
Except mentioned, all fungal and bacterial cell sources were prepared as below. A 2-days old mycelia of Saprolegnia sp. was cut using cork borer # 2 with diameter of 5 mm. The mycelium was recultured in glucose yeast extract agar at 25°C for 1 day. Recultured mycelia was cut and washed three times with SDW, put into 20 ml SDW and incubated at 25°C for 1 day to produce zoospore. Bacterial isolate was grown in MSMC agar at 28-30°C for 1 day. All media were adjusted to pH 6.8.
Microscopic observation of hyphae and bacterial cell adherence
Inhibited hyphae of the fungi were cut by 1 cm2. The hyphae were examined under light microscope and compared with normal ones. Hyphal abnormality was calculated in percentage as number of abnormality type (lytic, twisted, and broken hyphae) divided by total number of abnormal hyphae. Bacterial cell adherence was observed by using scanning electron microscope (JEOL JSM 5310 LV) of Laboratory of Scanning Electron Microscope, Zoology Section, LIPI, Cibinong, Indonesia.
Hatching and infection rate measurement
Hatching and infection rate were measured as conducted by Hanjavanit et al., (2008). Hatching rate = number of hatching egg/total number of egg. Infection rate = number of infected egg/total number of egg.
Characterization of Saprolegnia sp. from gouramy egg
Infected gouramy egg was source of the pathogen Saprolegnia sp. Saprolegnia presented as superficial, cotton-like and white grew on fish eggs in water (Khoo, 2000). On SDA the fungi showed as brown colour mat all over the agar surface after 3 days of incubation (Fig. 1). Vegetative and asexual reproductive structure were observed on this study. A wet mount preparation of fish eggs showed the fungal morphology. The fungal hyphae were broad, branching, cenocytic and nonseptate (Figure 1). The tips of hyphae were capped by sporangia which appear darker and more granular (Khoo, 2000; Rajan, 2000).
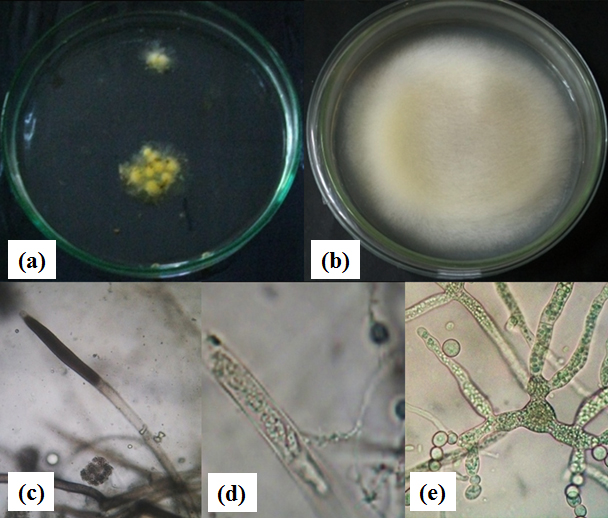
Figure 1. (a). Gouramy eggs infected by Saprolegnia sp., (b). Saprolegnia sp. colony on GYA, (c). Zoospongarium at hyphal tip, (d). Zoospongarium with mature zoospores, and (e). Asexual reproduction structure
Table (1):
Clearing zone showed by bacterial isolates in preliminary screening of chitinase and glucanase producing bacteria.
| Bacterial Isolates | Clearing zone (mm) | |
|---|---|---|
| Chitinase test | Glucanase test | |
| PB3A | 12.2 | 15.0 |
| PB01 | 11.6 | 6.0 |
| PB02 | 13.3 | 4.0 |
| PB05 | 12.9 | 4.5 |
| PB08 | 10.7 | 0 |
| PB10 | 16.8 | 0 |
| PB13 | 12.0 | 6.0 |
| PB14 | 14.5 | 5.5 |
| PB15 | 14.0 | 4.3 |
| PB17 | 18.0 | 6.8 |
Asexual reproduction structure of Saprolegnia sp. was typically unicelular and circular shape cell with flagella (zoospore) which is produced in zoospongarium (Beakes et al., 1994; Rajan, 2000). Gemmae and chlamydospore was found in the fungal isolated from gouramy eggs. Chlamydospore was asexual aplanospore formed through hyphal segmentation. Gemmae was irregular in shape and often occur in catenulate chain. They germinate to produce hyphae or hyphae bearing a terminal zoosporangium.
Preliminary screening and characterization of bacterial isolate producing chitinase and other potential antifungal compounds
Screening of bacterial isolates from water samples of healthy gouramy pond found 10 isolates to produce chitinase and other antifungal compounds. The test was aimed to assess the potential ability of the isolates to lyse fungal cell wall. All isolates, except that of PB08 and PB10 indicated to produce both chitinase and antifungal compounds (Table 1). To screen microbe producing chitinase and other antifungal compound/enzyme like glucanase may not be difficult since polysaccharides such as chitin and glucan are abundant in nature.
Chitinolytic bacteria were often characterized by their ability to produce a clear zone around their colony in chitin containing media. To degrade fungal cell wall composed more with glucan rather than chitin like in Saprolegnia sp., glucanase or other antifungal substance is needed. The tested was done using C. albicans (Table 1) of which cell wall was dominated by glucan (47-60%) (Chaffin et al., 1998). Hence, glucanase was most likely antifungal compound released by the bacterial isolates to inhibit C. albicans.
All bacterial isolates were gram-negative. In spite of different colony trait, PB01 and PB08 showed common biochemical traits. B3A, PB10, PB13, and PB15 shared the same characteristics. Similar morphological and biochemical traits indicated the same species (Table 2). Further identification test should be conducted to determine the species.
Table (2):
Simple morphological and biochemical characterization of the bacterial isolates.
| Bacterial isolates | Colony characterization | Cell shape | Gram | TSIA | Motility | Gelatin | Citrate | Catalase | Starch | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Sucrose | Lactose | Sediment | Splinter | |||||||||
| PB3A | Irregular, cream | rod | – | – | + | + | – | – | + | + | + | – | – |
| PB01 | Irregular, yellow | coccus | – | + | – | – | H2S | – | – | + | + | – | – |
| PB02 | Entire, cream | rod | – | + | – | – | – | – | + | + | + | – | + |
| PB05 | Irregular, cream | coccus | – | – | + | + | – | – | + | + | + | – | – |
| PB08 | Entire, cream | coccus | – | + | – | – | H2S | – | + | + | + | – | – |
| PB10 | Irregular, cream | coccus | – | – | + | + | – | – | + | + | + | – | – |
| PB13 | Irregular, cream | coccus | – | – | + | + | – | – | + | + | + | – | – |
| PB14 | Entire, cream | rod | – | – | + | + | – | – | + | + | + | – | + |
| PB15 | Irregular, cream | coccus | – | – | + | + | – | – | + | + | + | – | – |
Assay of bacterial-fungal antagonism
To see the potential ability of bacterial isolates in controlling Saprolegnia sp. growth, In vitro antagonistic assay has to be performed. Microbial antagonism implies direct interaction between two microorganisms sharing the same ecological niche (Alabouvette, 2006). Three main types of direct interactions (parasitism, antibiosis and competition for nutrients) may be involved. Antagonistic effects responsible for disease suppression results either from microbial interactions directed against the pathogen, mainly during its saprophytic phase, or from an indirect action through induced resistance of the host (Alabouvette, 2006).
The result showed that the bacterial isolates inhibited the growth of fungi with some extent. Inhibition zone was observed on 5-days of incubation (Table 3). Inhibition zone was an elevation clear zone on area between Saprolegnia and the bacterial isolates. The result showed that PB17 inhibited Saprolegnia sp. the most, while PB08 inhibited less. Many reported different ability of bacterial isolate to inhibit fungal growth. Pseudomonas fluorescens isolated from Rainbow Trout lession inhibited growth of Saprolegnia parasitica (Hatai and Willoughby, 1988). Non Pathogenic Aeromonas Strain (NPAS) had inhibition activity against Saprolegnia spp. (Osman et al., 2008). Most of the bacterial isolates showed potential inhibitory activity against the fungi on 4-5 days of observation. It seemed that inhibitory effect decreased after 5 days showed by fungal colonization to entire culture.
Table (3):
Inhibition zone of Saprolegnia sp. growth caused by antagonistic bacterial isolates .
Bacterial isolates |
Inhibition zone (mm) |
|---|---|
PB3A |
12.2 |
PB01 |
11.5 |
PB02 |
13.3 |
PB05 |
12.9 |
PB08 |
10.7 |
PB10 |
16.7 |
PB13 |
12.0 |
PB14 |
14.5 |
PB15 |
14.0 |
PB17 |
18.0 |
In vitro test bacterial isolate antagonism against Saprolegnia sp. after 7 days followed by microscopic observation of fungal hyphae showed hyphal abnormalities, such as hyphal necrotic, hyphal tip necrotic, broken hyphae, and bent hyphae (Fig. 2). This observation was conducted to determine the effect of bacteria on morphological structure of Saprolegnia sp. Abnormalities in fungal hyphae are morphological changes of impaired growth of fungi occur in the hyphae that should grow normally. Enzymatic activity of the bacterial isolates was believed to lyse the fungal cell wall. Fungal cell wall degrading enzymes produced by an antagonist were thought to be involved simultaneously in parasitism and antibiosis (Alabouvette et al., 2006).
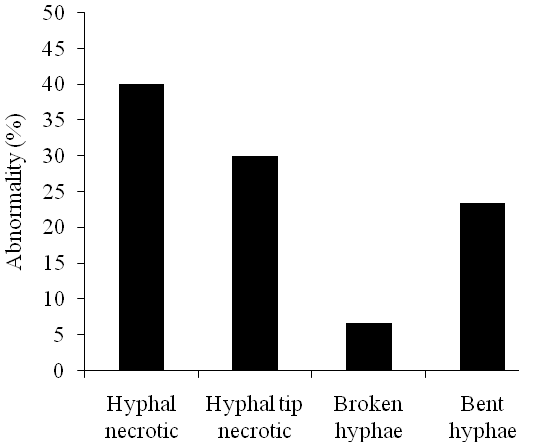 Figure 2. Effect of antagonistic bacterial isolates on hyphae of Saprolegnia sp.
Figure 2. Effect of antagonistic bacterial isolates on hyphae of Saprolegnia sp. Hyphal abnormalities such as necrotic, broken and bent hyphae were observed. Getha and Vikineswary (2002) noticed a lytic effect, hyphal distortion like swelling or bulbous growth caused by interaction between Streptomyces violaceusniger strain G10 and Fusarium oxysporum f.sp. cubense after 2 days of incubation. By the fourth day, distortion and lytic of the hyphae were more frequently noted. Other morphological abnormalities, such as abnormal branching of hyphae and the formation of hyphal protuberances, were also seen.
Pathogenicity of microbial isolates
Saprolegniasis is an important disease in fish hatchery. It increases mortality and decrease hatching rate of eggs. Saprolegniasis is a disease with symptoms that are commonly seen on the surface of the skin such as the formation of white cotton-like on the fish or eggs (Bruno and Wood, 1999). Clinical symptoms that appeared at each stage of the eggs formed different impairing larvae. In this study, we tested pathogenicity of Saprolegnia sp. isolated from infected gouramy eggs to the healthy ones. The eggs infected with Saprolegnia sp. had clinical symptom as color changed to more pale, and within 24 hours hyphae covered the eggs. The fungus seemed to affect the eggs by decreasing hatching eggs (Fig. 3). To know the causal agent of the disease, infected eggs were sampled and grown in GYA. It was shown that the same Saprolegnia was identified. This suggested Saprolegnia was causal agent of the disease.
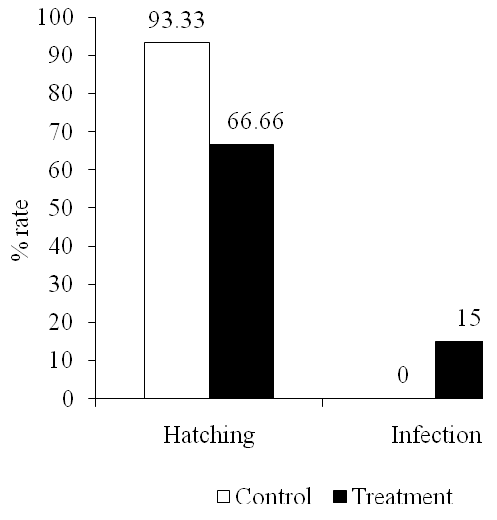 Figure 3. Effect of Saprolegnia sp. on hatching and infection rate of gouramy eggs
Figure 3. Effect of Saprolegnia sp. on hatching and infection rate of gouramy eggsIt was observed that clinical symptoms obviously appeared in dead eggs while in infecting egg the symptoms were not clear. Early clinical changes were not too visible on the eggs that did not have a tail and eyes (age of 4 days). Reddish abdomen was commonly found in 7-days egg. Larvae did not actively swim but floating on the container edge. The yolk still remained more than normal ones, and fins or skin were white. Bruno and Wood (1999) mentions that the clinical symptoms of early infection are skin lesions colored white or gray, which expand rapidly causing damage to the skin and muscle.
Our Saprolegnia sp. isolate showed relatively not to be infectious one, while Noga (1993) reported that Saprolegnia taken from fish lesions was more pathogenic rather than that of laboratory collection since the fish lesion isolate was more adapted to the host. On the other hand, Hanjavanit et al. (2008) reported that some Saprolegnia isolates of laboratory collection were pathogenic to catfish egg. Other reports suggested that infection in the artificial fish eggs requires some environmental stress. Environmental stresses such as high temperature and artificial wound on skin increased infection rate (Lategan et al., 2004).
Microbes used as biological control agents in aquaculture should have some requirements, one of which microbes may not cause the disease or pathogenic to the host (Verschuere et al., 2000). Pathogenicity test of bacterial isolates was performed to determine whether the bacteria were pathogenic to gouramy eggs by reducing egg hatching (Fig. 4.). Potential bacterial isolates were expected to have no impact to the organs. It was observed that PB3A infection caused significant abnormal spin in gouramy larvae. Other abnormalities were shown when the egg inoculated with PB01. The skin may pathologically or physiologically reflect fish body alteration.
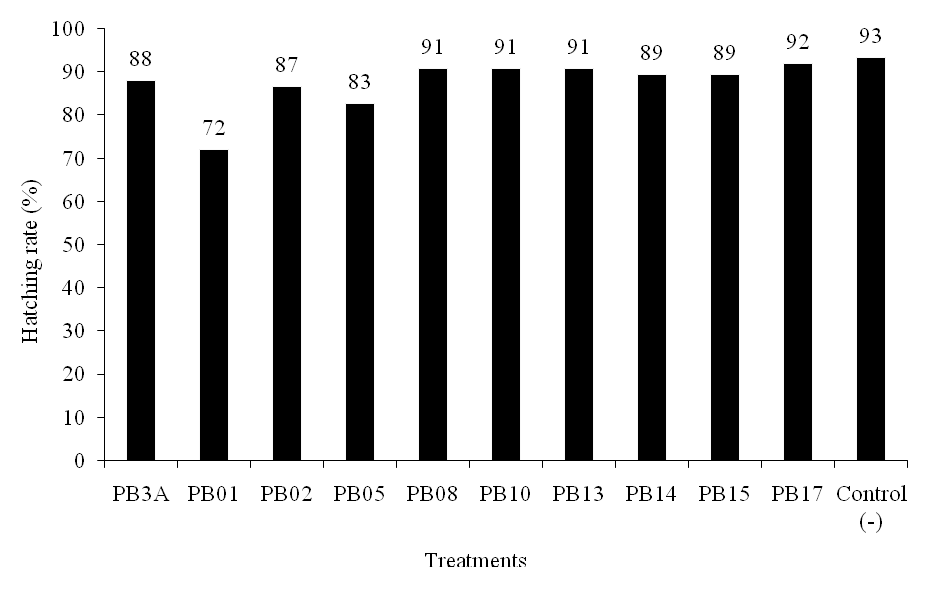 Figure 4.Effect of antagonistic bacterial isolates on hatching rate of gouramy eggs
Figure 4.Effect of antagonistic bacterial isolates on hatching rate of gouramy eggsReisolation of bacterial isolates from eggs using MSMC agar showed that reisolated bacterial isolates were chitinolytic. This suggested that the isolates were plug on the egg surface, but PB17, which showed loose attachment. Bacterial ability to attach on surface of intestinal mucosa is one pre-requirement of bacteria to be probiotics to compete with attached pathogens (Watson et al., 2008). The ability to grow and to attach to the intestinal mucosa and to the outer surface of fish body In vitro has been reported. Balcazar et al. (2007) reported that some strains of Lactic Acid Bacteria produced attachment and antagonist compound to inhibit the growth of the pathogen Flavobacterium psychrophillum.
SEM examination showed that the egg produced more mucous substances when inoculated with bacterial isolates, characterized by thick folding layer on the surface. Attached bacterial cell was not clearly observed since the surface was covered with excessive mucous as egg respond to bacterial infection (Fig. 5.). Martinez et al. (2004) noticed Flavobacterium psychrifillum caused excessive mucous excretion on fin to completely cover the bacteria. Cross section of mucous and microscopic high-magnification power revealed the buried bacterial cell. Decostere et al. (1999) reported that F. columnare cell on the surface of gill filaments and lamina were seemed as a mat using a low-magnification power, but appeared as a long-mat bacterial cell covered by fine mucous when using high-magnification power.
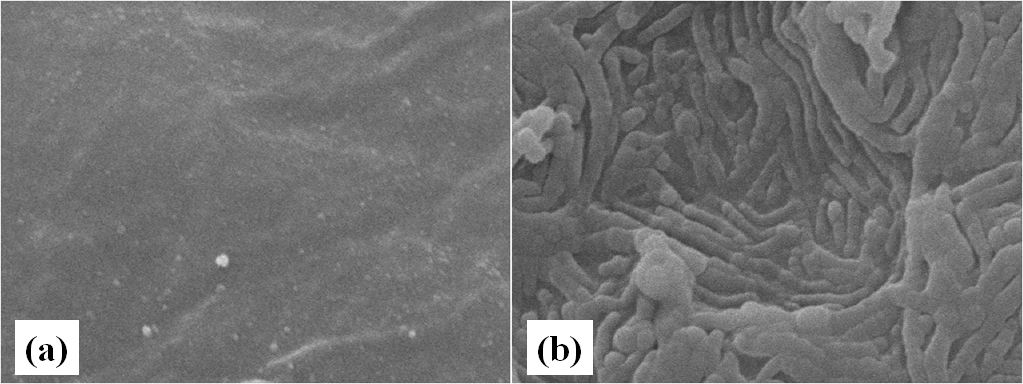
Figure 5. Bacteral cell adherance on gouramy egg. (a). without bacterial cell application, and (b). with bacterial application
Potential biological control of Saprolegnia sp.
Previous studies have reported the ability of bacteria as biological control of saprolegniasis on several species of fish in vivo (Lategan et al., 2004; Osman et al., 2008). Potential biological control of Saprolegnia sp. was examined by inoculating potential bacterial isolates to 3-days old healthy gouramy egg prior fungal inoculation. Potential inhibition of bacterial isolates against Saprolegnia sp. growth varied, indicating different potential genetic in antagonistic mechanism among the isolates. Three bacterial isolates PB08, PB15, and PB17 decreased Saprolegnia sp. infection to the egg caused more egg hatching (>90%) compared to (+) control (egg infected by Saprolegnia sp.), and did not differ from (-) control) control egg with no infection both with fungi and bacteria (Fig. 6.).
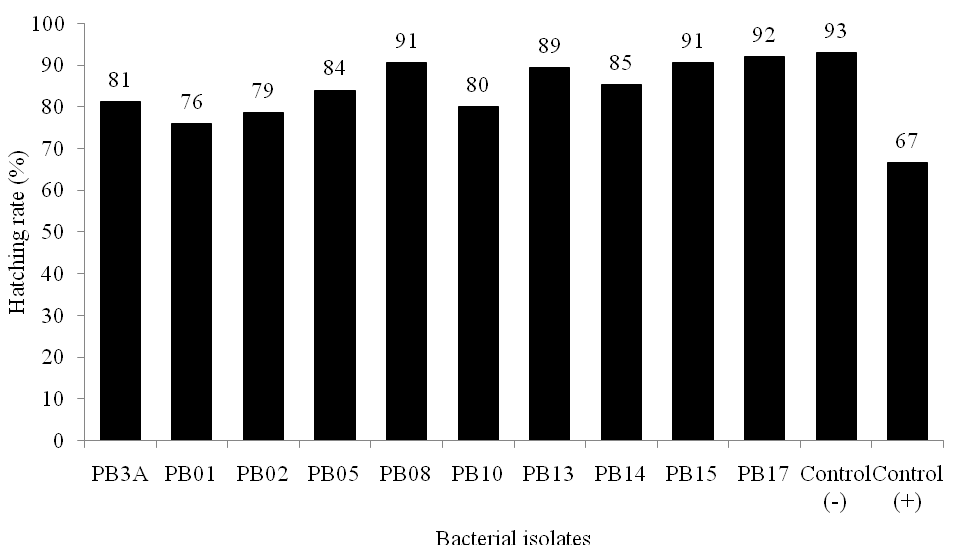
Figure 6. Hatching rate of gouramy eggs after antagonistic assay of bacterial isolates againts Saprolegnia sp. in vivo
Bacteria as biological control agents typically use their metabolites to suppress the growth of other microorganisms. Screening bacterial isolate in producing chitinase and other antifungal substance is an important step to determine potential of such a bacterial isolate to control pathogenic fungi. Though the fungal cell wall is made up of mainly of glucan and chitin (Gohel et al., 2006; Anand & Reddy 2009). the b-1,3-glucanase and chitinase are key enzymes responsible for fungal cell wall lytic and degradation (El-Katatny et al., 2000; Gohel et al., 2006). Glucanase secreted by potential bacterial isolates could degrade Saprolegnia sp. hyhae cell wall. Many bacteria were reported to produce both chitinase and glucanase. Serratia marcescens, Streptomyces viridodiasticus and Micromonospora carbonacea producing chitinase and glucanase (El-Tarabilya et al., 2000). Paenibacillus sp. 300 strain and Streptomyces sp. 385 produced chitinase and b-1,3-glukanase on the culture media (Singh et al., 1999).
The chitinase secreted by the bacterial isolates could be one of inhibition mechanism of the fungi but might not play the main role. Saprolegnia sp. is a member of Oomycetes in which cell wall is dominated by polysaccharides such as b-(1-3) and b-(1-6)-glucan, and cellulose. Chitin is minor component of the cell wall, composing less than 4% of total polysaccharide of the cell wall (Compos-Takaki et al., 1982). However, chitin plays an important role in in the fungi (Guerriero et al., 2010). Assay of chitinase showed that GlcNAc was slightly released in medium when inoculated with PB17, while PB08 and PB15 showed no GlcNAc released (unpublished data). GlcNAc was released when fungal cell wall degraded. It was speculated that chitinase might degrade chitin on tip of the fungal hyphae as seen in microscopic observation of hyphal tip necrotic.
Glucan became target for biological control mechanisme of Oomycetes because Saprolegnia sp. cell wall was dominated by glucan. Therefore, it was necessary to examine whether the isolates produced glucanase. However in this study we only tested the ability of the isolates to inhibit C. albicans as preliminary test to imply of glucanase producing bacteria. Morphological abnormality of fungal hyphae was dominated by hyphae lytic which indicated lytic mechanism caused by enzyme activity such as b-(1-3) glucanase. Diby et al., (2005) reported that this enzyme lysed hyphae cell wall and coagulated cytoplasm of Phytophtora capsici after treatment with Pseudomonas fluorescens.
The presence of other metabolites in addition to chitinase and glucanase was thought to be responsible for inhibiting fungal growth (Getha and Vikineswary, 2002). Potential bacterial isolates exhibited other mechanism against Saprolegnia sp. Aeromonas media A199 produced Indol (T1) an extracelular substance toxic to cytoplasm of the fungi S. parasitica (Lategan et al., 2006). Competition for certain metal ion could be the other mechanism of inhibition as shown by P. fluorescens (Hatai and Willoughby, 1988). Competition for iron in the tissues of fish by the bacteria inhibits the growth of pathogens (Veschuere et al., 2000). Antibiotics were also reported as substance that inhibit fungal growth. Antibiotics produced by P. fluorescens was actively against S. parasitica (Hatai and Willoughby 1988). In addition, competition for space might also be responsible. Lategan et al. (2004) reported extensive bacterial cell colonization in the epithelial cells of fish caused fungus could not compete to infect the cells. An increase in mucus on the surface of the egg indicate a colonization of bacteria on epithelial cells induced stimulation of mucus that would indirectly seek to eliminate the fungus that attaches to the mucosa of the egg.
Saprolegnia infection caused inflammation and vacuolar degeneration in the outer layer of the egg. Lesions to this egg layers were due to the fungus secrete enzymes that degrade components of the egg layer. The same lesions were reported by Giesker et al. (2006) in Saprolegnia infection in salmon Onchorhyncus mykiss. They observed hemorrhagic and mononuclear inflammation at the edge of the lesion, vacuolar degeneration in epithelial cells and degeneration of the connective tissue and muscle.
ACKNOWLEDGMENTS
We would like to thank Directorate General of Higher Education, Indonesian Ministry of Education and Culture for supporting this research.
- Alabouvette, C., Olivain, C., and Steinberg, C. Biological control of plant diseases: the European situation. Review. Eur. J. Plant Pathol. 2006; 114: 329-341.
- Anand, S. and Reddy, J. Biocontrol potential of Trichoderma Sp. against plant pathogens. Int J Agri Sci 2009; 1: 30-39.
- Balcazar, J.L., Vandrell, D., de-Blas, I., Ruiz-Zarzuela, I., Girones, O., and Muzquiz, J.L. In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Veterinary Microbiology 2007; 122: 373-380.
- Beakes, G.W., Wood, S.E., and Burr, A.W. Feature which characterize Saprolegnia isolate from Salmonids fish lesions a Review. Mueller, G.J (Ed.), Salmon saprolegniosis. Bonneville Power Administration, Portland: 1994; 33-66.
- Bruno, D.W. and Wood, B.P. Saprolegnia and other oomycetes. Woo, P.T.K. and Bruno, D.W. (Ed.), Fish diseases and disorders. Vol. 3: Viral, bacterial and fungal Infections. CABI Publishing, Wallingford, Owon, United Kingdom: 1999; 599-659.
- Chaffin,W.L., LÌpez-Ribot, J.L., Casanova, M., Gozalbo, M., Martinez, J.P. Cell wall and secreted proteins of candida albicans: Identification, function, and expression. Microbiology and Molecular Biology Reviews. 1998; 62: 130-180.
- Compos-Takaki, G.M., Dietrich, S.M.C., and Macarenhas, Y. Isolation and characterization of chitin from the cell walls of Achlya radiosa. Journal of General Microbiology 1982; 128 : 207 -209.
- Decostere, A, Haesebrouck, F., Turnbull J.F., and Charlier, G. Influence of water quality and temperature on adhesion of high and low virulence Flavobacterium columnare strains to isolated gill arches. Journal of Fish Disease 1999; 22:1-11.
- Diby, P., Saju, F.A., Jisha, P.J., Sarma, Y.R., Kumar,A., and Anandaraj, M. Mycolytic enzymes produced by Pseudomonas fluorescens and Trichoderma spp. against Phythopthora capsici, the foot rot pathogen of black pepper (Piper nigrum L). Annals of Microbiology 2005; 55: 129-133.
- El-Katatny, M.H., Somitsch, W., Robra, K-H., El-Katatny, M.S., and Gübitz, G.M. Production of chitinase and b-1,3-glucanase by Trichoderma harzianum for control of the phytopathogenic fungus Sclerotium rolfsii. Food Technol. Biotechnol., 2000; 38: 173-180.
- El-Tarabilya. K. A., Soliman, M.H., Nassar, A. H., Al-Hassani, H. A., Sivasithamparam, K., McKenna, F., and Hardy, G.E.St.J. Biological control of Sclerotinia minor using a chitinolytic bacterium and actinomycetes. Plant Pathology 2000; 49: 573-583.
- Getha, K. and Vikineswary, S. Antagonistic effects of Streptomyces violaceusniger strain G10 on Fusarium oxysporum f.sp. cubense race 4: Indirect evidence for the role of antibiosis in the antagonistic process. J. Ind. Microbiol. Biotechnol., 2002; 28: 303-310.
- Gieseker, C.M., Serfling, S.G., and Reimschuessel R. Formalin treatment to reduce mortality associated with Saprolegnia parasitica in rainbow trout, Oncorhynchus mykiss. Aquaculture 2006; 253: 120-129.
- Gohel, V., Singh, A., Vimal, M., Ashwini, P., and Chhatpar, H.S. Bioprospecting and antifungal potential of chitinolytic microorganisms. Review. Afr. J. Biotechnol. 2006; 5: 54-72.
- Guerriero, G., Avino, M., Zhou, Q., Fugelstad, J., Clergeot, P.H., and Bulone,V. Chitin synthases from Saprolegnia are involved in tip growth and represent a potential target for anti-oomycete drugs. Plos Pathogen 2010; 6: 1-12.
- Hanjavanit, C, Kitancharoen, N., and Rakmanee, C. Experimental infection of aquatic fungi on eggs of African catfish (Clarias gariepinus Burch). KKU Sci. J. 2008; 36(Supplement): 36-43.
- Hatai, K, and Willoughby, L.G. Saprolegnia parasitica from Rainbow Trout inhibited by the bacterium Pseudomonas fluorescens. Bull. Eur. Ass. Fish Pathol. 1988; 8: 27-29.
- Khoo, L. Fungal diseases in fish. W. B. Saunders Company. Seminar Avian and Exotic Pet Medicine 2000; 9: 102-111.
- Lategan, M.J, Torpy, F.R., and Gibson, L.F. Biocontrol of Saprolegniosis in silver perch Bidyanus bidyanus (Mitchell), by Aeromonas media strain A 199. Aquaculture 2004; 235: 77 – 88.
- Lategan, M.J., Booth, W., Shimmon, R., and Gibson, L.F. An inhibitory substance produced by Aeromonas media A199, an aquatic probiotic. Aquaculture 2006; 254: 115-124.
- Verschuere, L., Rombaut, G., Sorgeloos, P., and Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiology and Molecular Biology Reviews 2000; 64: 655-671.
- Martínez, J.L. Casado, A., and Enríquez, R. Experimental infection of Flavobacterium psychrophilum in fins of Atlantic salmon Salmo salar revealed by scanning electron microscopy. Diseases of Aquatic Organisms 2004; 59: 79-84.
- Mousavi, S.M., Mirzargar, S.S.Mousavi, E.Z., Baigi, R.O., Khasrovi, A., Bahonar, A., and Ahmadi, M.R. Evaluation of antifungal activity of new combined essential oils in comparison with malachite green on hatching rate in Rainbow Trout (Onchorhyncus mykiss) eggs. Journal of Fisheries and Aquatic Science 2009; 4: 103-110.
- Noga, E.J. Water mold infections of freshwater fish: Recent advances. Annual Rev. Fish Diseases 1993; 291-304.
- Osman, H.M., Noor El Deen, A.E., Waled, S.E.S., and Omima, A.A. A trial for induction of saprolegniosis in Mugel cephalus with special reference to biological control. Journal of American Science 2010; 6: 203-209.
- Osman, H.M., Solman, W.E., Noor El Deen, A.E., Mohamed, L.A. Induction of saprolegnasis in Oreochromis niloticus with special reference to its biological control. Global Veterinaria 2008; 2: 32-37.
- Rach, J.J., Valentine, J.J., Schreiera, T.M., Gaikowskia, M.P., and Crawford, T.G. Efficacy of hydrogen peroxide to control saprolegniasis on channel catfish (Ictalurus punctatus) eggs. Aquaculture 2004; 238: 135-142.
- Rajan, S.S. Practical manual of fungi. Anmol Publication PVT LTD. New Delhi: 2000; 52- 55.
- Rasowo, J., Okoth, O.E., and Ngugi, C.C. Effect of formaldehyde, sodium chloride, potassium permanganate and hydrogen peroxide on hatch rate of African catfish Clarias gariepinus eggs. Aquaculture 2007; 269: 271-277.
- Saravanakumar, R., Moomeen, H.S., Ronald, J., and Kannan, M. Control of fish bacterial pathogens, by antagonistic marine actinomycetes isolated from Gulf of Mannar Coast. World Journal of Fish and Marine Sciences 2010; 2: 275-279.
- Singh, P.P., Shin, Y.C.,Park, C.S., and Chung, Y.R. Biological control of Fusarium Wilt of cucumber by chitinolytic bacteria. Phytopathology 1999; 89: 92-99.
- Van West, P. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: New challenges for an old problem. Mycologist. 2006; 20: 99-104.
- Watson, A.K., Kaspar, H., Lategan, M.J., and Gibson, L. Probiotics in aquaculture : The need, principles and mechanisms of action and screening processes. Aquaculture 2008; 274: 1-14.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


