Herpes simplex virus 1 (HSV1) is a neuro-invasive virus causing lifelong latent infection in humans. It increases the risk of dementia by entering inside the brain. Herpesviruses have been implicated in Alzheimer’s disease (AD) development. More than 50 million people worldwide are affected by Alzheimer’s disease. Alzheimer’s disease is becoming more prevalent with the increase age-related neurodegenerative diseases, dementia, etc. Therefore, there is an urgent need for better understanding of the pathogenesis of AD as well as its early detection. HSV-1 is a risk factor of for the occurrence of neurodegenerative diseases, sporadic Alzheimer’s disease, prior diagnosis of disease cycle of Herpes Simplex Virus Type 1 in brain tissue will help us to prevent AD in elderly patients. Serological assays were the first choice of detection including whole-antigen-based (non-gG-specific) methods and western blotting (WB) assays, but quantitative PCR (qPCR) & genomic sequencing has shown better efficiency. Recently RNAscope, a novel in situ RNA hybridization assay is developed to visualize and detect the multiple phases of HSV-1. In this review, we discussed about early detection of tau and β-amyloid protein which are biomarkers of AD and track the deposition of amyloid plaques reminiscent in brain. We also discussed the current work on HSV detection through RNAscope hybridization technique & summarized the role of dietary habits as a precautionary measure of the infection as well as anthropological diversification of dementia in India and factors influencing it. We also pointed out some knowledge gaps that are further required to be taken after detection of the infection.
Herpes Simplex Virus, Alzheimer’s Disease, RNAscope Hybridization, Neurodegenerative Diseases, Plant-Derived Antiviral Peptides
Herpes Simplex Virus (HSV) is very prominent worldwide and known since ancient times. It’s a neuroinvasive virus which infects humans causing mild to very complicated lethal mucocutaneous infection.1,2 HSV is a hypoxia-inducible pathogen frequently causing lifelong latent infection in humans. It has a linear, enveloped double-stranded DNA genome which is surrounded by an icosapentahedral capsid.3 HSV-I manifest latency after initial infection in sensory neurons terminating virion production along with lytic cycle gene expression of virus. However, only Latency Associated Transcript (LAT), 5 well-defined microRNAs (miRNAs), and two small non-coding RNAs (sncRNA1 and sncRNA2) are abundantly expressed.4 Physical or emotional stress, cerebral insults, immunosuppression are the various factors which are responsible for HSV-1 infection in the patient.5,6
Alzheimer’s disease (AD) is one of the most common causes of dementia in elderly population affecting millions of people worldwide. Two different types of proteins are present ie., amyloid-β (Aβ) and tau. Amyloid-β peptides are proteolytic fragments which are synthesized from amyloid precursor protein (APP) which accumulate into oligomers and fibrils to form Aβ-containing plaques measuring 200-650 μm2 in size.7,8 Tau proteins are mostly microtubule-associated proteins found in neurons and are associated with microtubules stabilization. However, in case of AD tau protein gets accumulated resulting in formation of neurofibrillary tangles (NFTs) due to hyperphosphorylation. Their size generally ranges from 10 to 500 μm2 .9
Association of HSV-1 in AD
There is many evidence suggesting that the HSV-1 is linked with AD. Aβ and Tau proteins are two biological hallmarks of AD which gets accumulated in brain leading to inflammation and dementia.10,11 Thus, it has been observed that HSV-1 virus once enters the lytic phase due to immunosuppression, physical and emotional stress, it causes viral reinfection leading to brain impairment and inflammation which is alike early AD clinical findings. A clinical investigation was carried out in a group of AD patients which showed greater abundance of HSV-1 latency-associated transcripts (LATs) in older patients.12 HSV-1 virus can further lead to different mechanisms like neuroinflammation, oxidative stress, mitochondrial damage, synaptic dysfunction, and neuronal apoptosis which can be linked to AD pathologies. APOE is a gene that generally codes for apolipoprotein E protein (apoE) which is a risk factor for developing AD. There are 3 versions of APOE gene: ε2, ε3, and ε4 allele. Carriers with ε4 allele has the strongest risk factor of developing AD in elderly stage of their life or late onset alzheimer disease (LOAD) symptoms of which generally starts to appear after the age of 65. Apolipoprotein E (APOE), phosphatidylinositol binding clathrin assembly protein (PICALM), complement receptor 1 (CR1) and clusterin (CLU) are different genes susceptible to AD are also involved in HSV-1 life cycle. The polymorphisms may affect brain susceptibility to HSV-1 infection.13,14
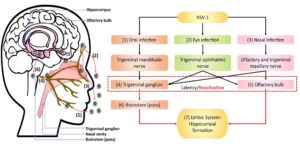
Once the HSV-1 virus starts to replicate in the oral, corneal, olfactory epithelial cells, the virus will start to utilize neuronal retrograde transport machinery and will outstretch to Trigeminal ganglion trigeminal ganglion and olfactory bulb where HSV-1 virus will enter latency and further leads to stress-induced reactivation. HSV-1 will be reactivated due to anterograde axonal transport to penetrate the brain. After that from trigeminal ganglion HSV-1 will travel to brainstem pons, which links our brain to our spinal cord and finally moves to limbic system where it inhibits the hippocampal memory system. HSV-1 alternately can infect hippocampal-entorhinal circuit directly via olfactory bulb a part of the limbic system15 (Figure 1 ).
Diagnosis
Serological Assays
Immunofluorescence assays, Western blot assays, and enzyme-linked immunosorbent assays (ELISAs) are the various types of serological tests are used for the detection and diagnosis of anti-HSV-1 and anti-HSV-2 antibodies. Irrespective of HSV-1 or HSV-2 infection, antibodies react with almost equal efficiency to both subtypes and leads to the cross reactivity. Limited sequence homologies and elicit type specific virus response are usually present in gGs of both HSV-1 & HSV-2.16,17
Molecular Assays
The real time PCR has setup a milestone in detecting a wide variety of nucleic acids through its mechanism. The concept of synthetically made oligonucleotides or primer itself has showed impeccable results in detecting load of pathogenic nucleic acids in varities of samples. It’s a chain reaction process where primer binds to the open chain of DNA and with the help of enzyme mostly (Taq or Thermus Aquaticus) starts the process of adding dNTPs and making new complementary strand of template DNA. The complete process is divided into three steps:
- Denaturation: The template double stranded DNA starts denaturing and separates out at 90°C to 95°C. which helps the DNA strand free for the primer to get binds on open strand.
- Annealing: Primer anneals to the denatured single stranded DNA at 55°c to 60°c and binds specifically to the binding region.
- Extension: Addition of dNTPs with the help of enzyme takes place at 72°c to 75°c just after the annealing of primer.
Recently, shotgun sequencing and modified Viromescan bioinformatics analysis are also applied to detect initial and low levels of HSV-1 gene expression between its reactivation and latent stage.12
RNAscope Technology
RNAscope hybridization is like traditional RNA ISH which is based on the concept that RNA probes can be organized in a way so that it can hybridized specifically to RNA of interest. For the detection of target RNA, a pair of ‘Z’ probes are used other than traditional ISH where a single RNA sequence is allowed to conjugate with labelled fluorophore or a digoxigenin.18,19 The ‘Z’ probe contains three major elements- bottommost region that hybridizes to RNA molecule. The bottommost region with ‘Z’ probe tail is joined by linker sequence. And the tail which binds to the pre-amplifier region (Figure 2A). Through series of sequential actions signal amplification is achieved when the lower portion (RNA specific sequence) of double ‘Z’ probe binds to their target sequence. Conjugation occurs when chromogenic or fluorescent labelled probes binds specifically on the binding site of amplifier molecules. The complementary base pairing multiple amplifier sequences binds subsequently to the preamplifier at the top of each double ‘Z’ pair (Figure 2B). 20
RNAscope probes are designed in such a way that it binds specifically to each transcript. In contrast to serological or RT-PCR assays, this method plays an important role in detection of HSV-1 reactivation status in the brain. One of the most important features of RNA scope is that it acts on multiple RNA targets and can detect neuronal HSV-1 latent LAT and lytic UL37 transcripts in mouse brain tissues. This combination detection tool can also be used to study different diseases in human brain samples such as HSV-1 induced neurodegeneration.21 Molecular and serological based HSV-1 detection assays are extensive techniques for its detection in clinical diagnosis. Low level of HSV-1 transcripts detection is a very challenging task. Therefore, to detect the low expressed multiple HSV-1 latent or lytic transcripts in infected brains with high specificity and sensitivity, RNAscope technology has proved to open a new technology in the field of neurodegenerative disorders.22
Viral infections such as HSV is not completely curable, but it can be prevented through a mix of life-style changes and dietary supplements. Since the beginning of mankind, naturally grown fruits and vegetables are widely used for different nutritional features.23-25 It has been observed that there are different Plant-Derived Antiviral Peptides (AVP) which plays an important role against DNA Viruses such as HSV-1 & HSV-2. From Sorghum bicolor seeds (Poaceae family), mostly found in Africa, a cationic, amphipathic peptide which is a 2 kD peptide is isolated which plays an important role by exerting HSV-1 virucidal activity by targeting viral replication cycles. This peptide plays an important role in covering the viral envelope protein and thereby making them inactive. From the leaves of Melia azedarach L., also called “Chinaberry tree, a Plant-Derived Antiviral Peptides (AVP), Meliacine was isolated. Meliacine contains polypeptides which can stop the replication cycle of HSV-1 polypeptides and viral DNA in the infected cell. The treatment of 50 µg/mL of meliacine in the infected patient cell was found to stop the spread of viral particles significantly. 26
Diets for Alzheimer’s Disease
The occurrence of Alzheimer’s disease is also greatly depended on the nutrition and healthy diet. So, saturated fatty acids such as meat is strictly prohibited in the patients with AD.36,37 This diet also slower the progression of AD by reducing the loss of brain volume.38,39 A specified type of diet named DASH (Dietary Approaches to Stop Hypertension) which involves high consumption of fruits, vegetables, whole grains and low-fat dairy products.40 So, a combination of DASH along with Mediterranean diet also known as MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) is designed which improves cognitive performance thereby reducing the risk of developing AD. The following diet combinations have significant correlation with less chance of cognitive disorder [Table].41
Table:
Antiviral properties of edible fruit extracts and fruit juices against HSV-1.27
| Plant used | Plant Parts used | Viruses examined | Observation |
|---|---|---|---|
| Almond [Prunus dulcis (Mill.) D.A. Webb] | Fruit skin extract | HSV-1 | By addition of extracts 1 h post-infection, entry of viral particles was found to be blocked.28,29 |
| Apple pomace (Malus domestica Borkh.) | Methanolic extract | HSV-1, 2 | At 1000 and 1200 µg/mL concentration, inhibition of viral replication is found to show cytopathic effect. By the use 56 µg/ml in oral epithelial cells, HSV-1 replication was found to be inactivated by 90%.30-32 |
| Blackberry (Rubus sp.) | Fruit juice | HSV-1 | |
| Black currant Ribes niagrum L. | Fruit extract | HSV-1 | Blocking the viral adsorption.33 |
| Pistachios (Pistacio vera L.) | Kernels | HSV-1 | Viral DNA synthesis was decreased.34 |
| Pomegranate (Punica granatum L.) | Fruit rind | HSV-1 | Viral replication was found to be stopped.35 |
Prevalance of Alzheimer disease in India
As per the recent study, 3.74 million people, out of which 2.93 million people from India are affected by Alzheimer disease.42,43 According to Clinical Dementia Rating (CDR), about 0.8% in individual’s ≥55 years of age and 1.3% in individuals ≥65 years of age was found to be affected by dementia.44 In the study conducted in 52,377 participants from Kolkata, showed dementia is the major disorders found in 1.0% in individuals aged ≥60 years.45-47 According to survey report, 5.3 million Indians aged >60 years is suffering from dementia till 2022 which is expected to exceed 14 million by 2050.48-50
Over the past two decades, the development in the field of neuroimmunology has provided us various new insights regarding different inflammatory disease that ultimately contribute to the development of Alzheimer’s Disease. AD generally had an increasing challenge to both public health as well as health care system so early detection of AD is very much important to prevent it. Various studies have shown the pervasiveness of HSV in most of the AD patients. The association of HSV with host have resulted in neurodegenerative diseases that leads to AD. Serological assays were the first line of choice for the detection, but it comes with the drawback of detecting slow and less quantitative virions. Moving over the period molecular techniques became the best option for the detection of viral load but limitation of detecting at latent stage infection and low level of viral copies is the major problem. RNAscope technique has shown way better method of detecting HSV in brain tissue through its methodology and number of viral transcripts as well. Measurement and growth of Amyloid beta and Tau protein should be monitored soon after the detection of HSV to avoid any further causalities. Some dietary supplements should also get included in diet. Soon after HSV-1 virus gets detected in the body, we should keep tracking the amyloid plaque formation in the brain of the patient via PET scanning or a spinal tap. Gadolinium-staining MRI is also used in the detection of amyloid plaques in-vivo. So, specific blood-based tests are also developed like the C2N test, called Precivity AD which uses mass spectrometry technique, that can measure low level of beta amyloid plaques in blood, and it is relatively cheaper. In future, cost effective and fast diagnosis technology should also be there for the detection of other opportunistic pathogens like CMV, EBV, VZV, PAN neurotrophic as, transcripts of these viruses have been reported earlier. The health organization should track the statistical analysis and association of HSV-1 and Alzheimer’s disease every year to monitor the complexity and its linkage in the dementia as recent data is not sufficient to monitor its relation. Nevertheless, specific healthcare system should be developed to increase awareness of dementia and its preventive measures.
ACKNOWLEDGMENTS
The authors are grateful to the Department of Botany, University of Science & Technology Meghalaya and Unipath Speciality Laboratory, Ahmedabad, for providing the laboratory facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357(9267):1513-1518.
Crossref - Murphy MJ, Fani L, Ikram MK, Ghanbari M, Ikram MA. Herpes simplex virus 1 and the risk of dementia: a population-based study. Sci Rep. 2021;11(1):8691.
Crossref - Zhang S, Zeng J, Zhou Y, et al. Simultaneous Detection of Herpes Simplex Virus Type 1 Latent and Lytic Transcripts in Brain Tissue. ASN Neuro. 2022;14:17590914211053505.
Crossref - Harrison KS, Jones C. Regulation of herpes simplex virus type 1 latency-reactivation cycle and ocular disease by cellular signaling pathways. Experimental Eye Research. 2022;218:109017.
Crossref - Doll JR, Thompson RL, Sawtell NM.Infectious Herpes Simplex Virus in the Brain Stem Is Correlated with Reactivation in the Trigeminal Ganglia. J Virol. 2019;93(8):e02209-18
Crossref - Marcocci ME, Napoletani G, Protto V, et al. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020;28(10):808-820.
Crossref - McManus RM.The Role of Immunity in Alzheimer’s Disease. Adv Biol. 2022;6(5):e2101166.
Crossref - Serrano-Pozo A, Mielke ML, Muzitansky A, et al. Stable size distribution of amyloid plaques over the course of alzheimer disease. J Neuropathol Exp Neurol. 2012;71(8):694-701.
Crossref - Maziuk BF, Apicco DJ, Cruz AL, et al. RNA binding proteins co-localize with small tau inclusions in tauopathy. Acta Neuropathol Commun. 2018;6(1):71.
Crossref - Roberts RO, Christianson TJH, Kremers WK et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and alzheimer disease dementia. JAMA Neurol. 2016;73(1):93-101.
Crossref - Harris SA, Harris EA. Molecular mechanisms for herpes simplex virus type 1 pathogenesis in Alzheimer’s disease. Front Aging Neurosci. 2018;10:48.
Crossref - Readhead B, Haure-Mirande JV, Funk CC, et al. Multiscale analysis of independent alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99(1):64-82.e7.
Crossref - Husain MA, Laurent B, Plourde M. APOE and Alzheimer’s Disease: from lipid transport to physiopathology and therapeutics. Front Neurosci. 2021;15:85.
Crossref - Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15(9):501-518.
Crossref - Yong SJ, Yong MH, Teoh SL, et al. The hippocampal vulnerability to herpes simplex virus type i infection: relevance to alzheimer’sdisease and memory impairment. Front Cell Neurosci. 2021;15:312.
Crossref - Burbelo PD, Hoshino Y, Leahy H, et al. Serological diagnosis of human herpes simplex virus type 1 and 2 infections by luciferase immunoprecipitation system assay. Clin Vaccine Immunol. 2009;16(3):366-371.
Crossref - Wald A, Ashley-Morrow R. Serological Testing for Herpes Simplex Virus (HSV)–1 and HSV-2 Infection. Clin Infect Dis. 2002;35(Suppl. 2): S173–S182.
Crossref - Atout S, Shurrab S, Loveridge C. Evaluation of the Suitability of RNAscope as a technique to measure gene expression in clinical diagnostics: A Systematic Review. Mol Diagn Ther. 2022;26(1):19-37.
Crossref - Chan S, de L’Etang A. F, Rangell L, et al. A method for manual and automated multiplex RNAscope in situ hybridization and immunocytochemistry on cytospin samples. Plos One. 2018;13(11):e0207619.
Crossref - Wang F, Flanagan J, Su N, et al. RNAscope: A Novel in Situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14(1):22-29.
Crossref - Jolly S, Lang V, Koelzer VH, et al. Single-Cell Quantification of mRNA Expression in The Human Brain. Sci Rep. 2019; 9(1):1-9.
Crossref - Roussarie JP, Yao V, Rodriguez-Rodriguez P, et al. Selective neuronal vulnerability in Alzheimer’s Disease: A network-based analysis. Neuron. 2020;107(5):821-835.e12.
Crossref - Sudomova M, Berchova-Bimova K, Mazurakova A, Samec D, Kubatka P, Hassan STS. Flavonoids target human herpes viruses that infect the nervous system: mechanisms of action and therapeutic insights. Viruses. 2022 13;14 (3):592.
Crossref - Jantan I, Ahmad W, Bukhari SNA. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front Plant Sci. 2015;6:655.
Crossref - Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr Rev Food Sci Food Saf. 2018;17(3):512-531.
Crossref - Alche LE, Barquero AA, Sanjuan NA, Coto CE. An antiviral principle present in a purified fraction from Melia azedarach L. leaf aqueous extract restrains herpes simplex virus type 1 propagation. Phytother Res. 2002;16(4):348-352.
Crossref - Santhi VP, Sriramavaratharajan V, Murugan R, et al. Edible fruit extracts and fruit juices as potential source of antiviral agents: a review. Food Measure. 2021;15(6): 5181-5190.
Crossref - Arena A, Bisignano C, Stassi G, et al. Immunomodulatory and antiviral activity of almond skins. Immunol Lett. 2010;132(1-2):18-23.
Crossref - Bisignano C, Mandalari G, Smeriglio A, et al. Almond Skin Extracts Abrogate HSV-1 replication by blocking virus binding to the cell. Viruses. 2017;9(7):178.
Crossref - Suarez B, Alvarez AL, Garcia, YD, et al. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010b;120(1):339-342.
Crossref - Alvarez AL, Melon S, Dalton KP, et al. Apple Pomace, a By-Product from the Asturian Cider Industry, Inhibits Herpes Simplex Virus Types 1 and 2 In Vitro Replication: Study of Its Mechanisms of Action. J Med Food. 2012;15(6):581-587.
Crossref - Danaher RJ, Wang C, Dai J, Mumper RJ, Miller CS. Antiviral effects of blackberry extract against herpes simplex virus type 1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(3):e31-e35.
Crossref - Ikuta K, Hashimoto K, Kaneko H. Anti-viral and anti-bacterial activities of an extract of blackcurrants (Ribes nigrum L.). Microbiol Immunol. 2012;56(12): 805-809.
Crossref - Musarra-pizzo M, Pennisi R, Ben-amor I, Smeriglio A, Mandalari G, Sciortino MT. In Vitro Anti-HSV-1 Activity of Polyphenol-Rich extracts and pure polyphenol compounds derived from pistachios Kernels (Pistacia vera L.). Plants. 2020;9(2):267.
Crossref - Houston DMJ, Bugert JJ, Denyer SP, Heawrd CM. Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLos One. 2017;12(6):e0179291.
Crossref - Petersson SD Philippou E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
Crossref - Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006-1015.
Crossref - Aridi YS, Walker JL, Wright ORL. The Association between the Mediterranean Dietary Pattern and Cognitive Health: A Systematic Review. Nutrients. 2017; 9(7): 674.
Crossref - Samadi M, Moradi S, Moradinazar M, Mostafai R, Pasdar Y. Dietary pattern in relation to the risk of Alzheimer’s disease: a systematic review. Neurol Sci. 2019;40(10):2031-2043.
Crossref - Solfrizzi V, Custodero C, Lozupone M, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer’s Disease and late-life cognitive disorders: A Systematic Review. J Alzheimers Dis. 2017;59(3):815-849.
Crossref - Sliwinska S, Jeziorek M. The role of nutrition in Alzheimer’s disease. Rocz Panstw Zakl Hig. 2021;72(1):29-39.
Crossref - Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016;15(9):913-924.
Crossref - Mathew R, Mathuranath P. Issues in evaluation of cognition in the elderly in developing countries. Ann Indian Acad Neurol. 2008;11(2):82-88.
Crossref - Rodriguez JJL, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372(9637):464-474.
Crossref - Biswas A, Das SK, Roy T, et al. A random sample survey for prevalence of major neurological disorders in Kolkata. Ind J Med Res. 2006;124(2):163-172.
- Raina S K, Raina S, Chander V, Grover A, Singh S, Bhardwaj A. Identifying risk for dementia across populations: A study on the prevalence of dementia in tribal elderly population of Himalayan region in Northern India. Ann Indian Acad Neurol. 2013;16(4):640.
Crossref - Banerjee TK, Dutta S, Das S, et al. Epidemiology of dementia and its burden in the city of Kolkata, India. Int J Geriatr Psychiatry. 2017;32(6):605-614.
Crossref - Ravindranath V, Sundarakumar JS. Changing demography and the challenge of dementia in India. Nat Rev Neurol. 2021;17(12):747-758.
Crossref - Baumgart E, Schad A, Grabenbauer M. In Situ hybridization: general principles and application of Digoxigenin-Labeled cRNA for the Detection of mRNAs. Immunocytochemistry and in situ Hybridization in the Biomedical Sciences. 2001;108-137.
Crossref - Choudhary A, Ranjan JK, Asthana HS. Prevalence of dementia in India: A systematic review and meta-analysis. Indian J Public Health. 2021;65(2):152-158.
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.