ISSN: 0973-7510
E-ISSN: 2581-690X
Viral-borne diseases have recently gained significant public health importance in the current world. The Viral Research and Diagnostic Laboratory (VRDL) located at Government Theni Medical College (GTMC), Theni, Tamil Nadu, conducts the diagnosis of common virus infections. The purpose of this study is to investigate the seroprevalence of dengue (DENV) and chikungunya (CHIKV) virus infections, as well as their co-infection, in people who have clinical symptoms. From January 2018 to June 2023, serum samples were collected from clinically suspected patients at the tertiary care hospital in Theni, Tamil Nadu. DENV and CHIKV were detected using an enzyme-linked immunosorbent assay (ELISA) in all of the samples. A total of 16,997 cases were enrolled, out of which 11264/2971(26.3%) tested positive for Dengue IgM, 1395/288 (20.6%) for Dengue NS1 Ag, 19/3(15.7%) for IgG, followed by 4319/3388(8.9%) of CHIKV IgM. Fever (n = 16598, 97.6%) was the most prevalent clinical characteristic in all probable dengue and chikungunya patients. Other symptoms were chills (n = 11252, 66.1%), arthralgia (n = 10245, 60.2%), headache (n = 11354, 66.8%), and joint pain (n = 11256, 66.2%). The findings showed a lesser likelihood of acquiring both DENV and CHIKV infections at the same time; however, the risk is still not trivial. This study investigates the clinical presentation of Dengue-Chikungunya patients. The rising prevalence of dengue and chikungunya, as well as their co-infection, need thorough monitoring of endemic areas and good patient care management.
Viral Research and Diagnostic Laboratory, Arbovirus, Dengue, Chikungunya, Seroprevalence
Worldwide, 17% of all infectious diseases affecting people are caused by arthropod-borne viral infections.1,2 The mosquito species Aedes aegypti and Aedes albopictus spread several arboviral species, including those from the genera Alphavirus and Flavivirus.3 Mosquito-borne infections, the most common arboviral diseases, impact millions of individuals and account for a significant fraction of newly emerging and re-emerging human pathogens. Dengue, chikungunya, Japanese encephalitis, yellow fever, and Rift Valley fever have all been linked to a significant increase in disability-adjusted life years.4 One of the most dangerous viral illnesses spread by mosquitoes is dengue fever, which is brought on by DENV and has an increasing global incidence.5 DENV contains four serotypes that cause a variety of diseases: DENV-1, DENV-2, DENV-3, and DENV-4. People who are infected may have mild flu-like symptoms or develop a subclinical illness. Even though it is uncommon, severe dengue can cause major organ damage, haemorrhage, and/or plasma leakage. If severe dengue is not treated successfully, the risk of death increases.6 Dengue is divided into mild dengue (with or without warning signs) and severe dengue by the WHO.7 Chikungunya disease has been documented in non-endemic countries, despite the fact that CHIKV has largely been detected in underdeveloped countries, suggesting the possibility of ongoing transmission to new locations. CHIKV is a positive-sense single-stranded RNA virus of the Togaviridae family.8,9 The onset of a fever, which is frequently accompanied by severe joint pain, is one of the clinical indicators. Joint discomfort is typically intense and only lasts for a few days in CHIKV instances, which can last for weeks, months, or even years.10 The first global statistics on dengue fever were released in 1988, estimating that 80–90 million cases of dengue virus infection had been documented worldwide.11 According to the National Vector Borne Disease Control Programme (NVBDCP), >0.7 million DENV infections (including 0.2 million in 2018-19) and 58,000 CHKIV cases (including 22,000 in 2018-19) will have been documented in India by October 2022.12 Chikungunya fever is a viral infection that is spread by Aedes mosquito bites. It is a tropical disease that causes a severe illness with a high fever, rash, and arthralgia. The vector, symptoms, and geographic distribution of dengue fever and chikungunya are identical.13 In 2006, 13 Indian states had a significant chikungunya outbreak 32 years after the previous outbreak.14 As a result of these new pathogens, public health principles have undergone a paradigm shift.15 Beyond just affecting health, viruses have had an impact on India’s social and economic structure. Because specific antibody reactions last for a long time, seasonality has little impact on serological diagnosis. For the analysis of epidemics and the forecasting of viral outbreaks, long-term data collected at regular intervals is crucial. A national organization called the Integrated Disease Surveillance Programme (IDSP) uses the hospitals’ and medical schools’ already-existing laboratory capabilities to gather disease data.16 It is important to thoroughly research the epidemiology of lethal viral infections. The Viral Research and Diagnostic Laboratory (VRDL) at Government Theni Medical College (GTMC), Theni, conducted the initial diagnosis during the epidemic. The laboratory deals with the diagnoses of all common viruses existing in the region: dengue, chikungunya, Hepatitis A, B, C & E viruses, etc. The purpose of this study was to assess the prevalence and seroprevalence of acute dengue and chikungunya virus infections, as well as their co-infections.
The present study was a retrospective study conducted at the Viral Research and Diagnostics Laboratory (VRDL) located at a tertiary care hospital, Theni, from January 2018 to June 2023. The Ethics Committee of GTMC, Theni, accepted the study after it was carried out in conformity with the Declaration of Helsinki. (Ref No:1515/MEIII/21 dated 11.05.2023).
Study sample and data collection
Patients who may have had dengue or chikungunya, with or without warning signs as defined by WHO/IDSP, and who had a fever of 38°C or higher within the previous 1–14 days were included in the study. Patients who reported upper respiratory sickness symptoms, incomplete data, or refused to fill out laboratory forms were excluded from the study. Each patient had 2-3 ml of blood collected once, centrifuged, marked with their unique lab numbers, and stored at -80÷C for future serological studies. According to the manufacturer’s instructions, each sample was tested using an ELISA kit for dengue NS1 Ag, dengue IgM, dengue IgG, or chikungunya IgM antibodies (all from J.Mitra Pvt. Ltd., NIV Pune, PanBio, India). At 450 nm, the optical density (OD) value was measured, and the result was interpreted in accordance with the manufacturer’s specifications. In this investigation, samples that tested positive for chikungunya IgM antibodies or primary infection (Dengue NS1 Ag) were deemed seropositive.
Statistical analysis
The percentage and proportion of each variable were calculated. For continuous and categorical data, a one-way ANOVA, frequency, and a measure of central tendency were used. Chi-square tests were used to statistically analyze the data (IBM Corp. IBM SPSS Statistics v21).
Participants in this study
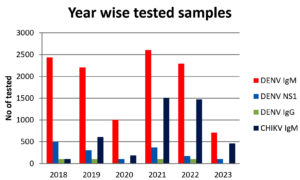
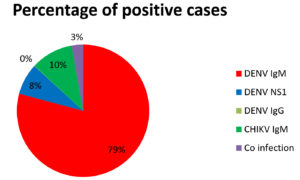
Figure 1 shows the collection of 16997 clinically suspected patient samples from January 2018 to June 2023 to detect DENV, CHIKV, and co-infection. The test was done on samples suspected of having Dengue IgM, IgG, NS1, and Chikungunya viruses. Out of the 16997, about 3753 have been reported to be positive for viral infections (Figure 2). Our ultimate sample size was 16997 people, with 9789 men (57.6%) and 7208 women (42.4%). The ages ranged from 0.1 to 96 (mean = 30.9, median = 28.1, standard deviation = 16.2). Gender distinctions were discovered to be statistically significant (p0.001). The age range 21-40 had the highest number of probable DENV and CHIKV infections. The majority of suspected patients presented to the OPD/IPD within 2-4 days of the onset of illness, with considerable complaints of fever with chills, headache, and body/joint discomfort, according to the study.
Clinical characteristics
We clinically examined 15 symptoms of DENV and CHIKV suspected patients (Table 1). It is significant that dengue is predominant in the districts studied, with a total of 2971/11264 (26.3%) subjects positive for anti-Dengue IgM antibodies (Dengue NS1 288/1395 (20.6%), Dengue IgG 3/19(15.7%)) and the specific difference is statistically significant (p=0.001). This is followed by the Chikungunya virus 388/4319 (8.9%). A total of 103 (3.0%) samples tested positive for both DENV and CHIKV co-infections (DENV & CHIKV positive). (Table 2). DENV male-female ratios were 57.6% and 42.4%, respectively. Patients displayed a wide range of symptoms, including fever (n = 16598, 97.6%), chills (n = 11252, 66.1%), and cough (n=9878, 58.1%). The most common symptoms were arthralgia (n = 10245,60.2%), headache (n = 11354,66.8%), vomiting (n= 6874,40.4%), and abdominal pain (n=2456,14.4%), with haemorrhagic manifestations (n = 1895,11.1%), myalgia (n= 1235, 7.2%), and diarrhoea (n=1098, 6.4%). Rashes (n = 523, 3.0%), sore throats (n = 987, 5.8%), and irritability (n=454,2.6%) were the least common. Fever (97.9% male vs. 97.2% female) and chills (70.0% male vs. 60.0% females) were both gender-specific. However, cough (64.3% female vs. 53.5% male), headache (70.8% female vs. 63.7% male), myalgia (8.0% female vs. 6.7% male), and abdominal discomfort (16.2% female vs. 13.9% male) were more common in females. However, the gender difference was statistically significant (p<0.0001) (data not shown) (Table 1). The age range was 0.1 to 95 years old (mean age = 30.8, median age = 28.0, standard deviation = 16.1). Higher cases of positive results were shown for the Dengue IgM test, especially in the age group 21-40 years and the least were seen in cases above 60 years of age. In the case of Dengue NS1 and IgG, higher cases were seen in 21-40 age group, and no cases were seen in the 60 age group. For CHIK IgM, higher cases were seen in the 21-40 age groups, and the least number of cases in the 60 age group. In cases of co-infection, higher numbers of cases were seen in 21-40 years and the least number of cases in those over 60 years (Table 3). Females outnumbered males in the study. DENV and CHIKV co-infection occurred in 103 patients, 66(64.0%) were male, and 37(36.0%) were female, respectively. A comprehensive serological analysis of DENV-positive patients demonstrates statistical relevance in gender (data not shown) (p<0.001). Only age was shown to be substantially linked with seropositivity for antibodies against both DENV and CHIKV in a multivariate analysis. Participants aged 18-90 years old were around three times more likely to have antibodies against both DENV and CHIKV than younger children aged 0-17 years old. In this study, most dengue-positive individuals had a fever, chills, cough, headache, joint pain, myalgia, and arthralgia, according to their clinical presentation. Fever and arthralgia were more common in chikungunya-positive patients. During the experiment, the majority of participants went to the hospital within 2-4 days of becoming ill.
Table (1):
Clinical characteristics of enrolled symptomatic patients
Clinical Presentation |
n = 16997(%) |
Male (n=9789%) |
Female (n=7208%) |
|---|---|---|---|
Fever |
16598 (97.6) |
9586 (97.9) |
7012 (97.2) |
Cough |
9878 (58.1) |
5243 (53.5) |
4635 (64.3) |
Chills |
11252 (66.1) |
6856 (70.0) |
4396 (60.9) |
Rash |
523 (3.0) |
325 (3.3) |
198 (2.7) |
Headache |
11354 (66.8) |
6244 (63.7) |
5110 (70.8) |
Arthralgia |
10245 (60.2) |
5978 (61.0) |
4267 (59.1) |
Vomiting |
6874 (40.4) |
3972 (40.5) |
2902 (40.2) |
Abdominal pain |
2456 (14.4) |
1282 (13.9) |
1174 (16.2) |
Hemorrhagic manifestation |
1895 (11.1) |
1012 (10.3) |
883 (12.2) |
Breathlessness |
598 (3.5) |
305 (3.1) |
293 (4.0) |
Diarrhea |
1098 (6.4) |
602(6.1) |
496 (6.8) |
Sore throat |
987 (5.8) |
512 (5.2) |
475 (6.5) |
Joint pain |
11256 (66.2) |
6587(67.2) |
4669 (64.7) |
Myalgia |
1235 (7.2) |
658 (6.7) |
577 (8.0) |
Irritability |
454 (2.6) |
248 (2.5) |
206 (2.8) |
Table (2):
Distribution of serologically tested positive cases according to gender
S.No |
Test Name |
No. of Positive |
Male |
Female |
|---|---|---|---|---|
1 |
DENV IgM |
2971 |
1626(54.7) |
1345(45.3) |
2 |
DENV NS1 |
288 |
143(49.7) |
145(50.3) |
3 |
DENV IgG |
3 |
3(100) |
0(0) |
4 |
CHIKV IgM |
388 |
207(53.4) |
181(46.6) |
5 |
Co infection |
103 |
66(64.0) |
37(36.0) |
Total |
3753 |
2045 |
1708 |
Table (3):
The age distribution of serologically positive cases
| S.No | Test Name | 0-20 Years | 21-40 Years | 41-60 Years | >60 Years |
|---|---|---|---|---|---|
| 1 | DENV IgM | 758 | 1538 | 618 | 57 |
| 2 | DENV NS1 | 103 | 108 | 77 | 0 |
| 3 | DENV IgG | 3 | 0 | 0 | 0 |
| 4 | CHIKV IgM | 122 | 149 | 91 | 26 |
| 5 | Co infection | 24 | 45 | 26 | 8 |
| Total | 1010 | 1840 | 812 | 91 | |
The most frequent vector-borne infections in India are dengue and chikungunya fever. Dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) is the most severe form of dengue fever, defined by thrombocytopenia, hemorrhagic symptoms, vascular leakage, hypotension, and shock that can lead to organ failure and death.17 Epidemiological studies reveal that whereas first dengue infections are frequently asymptomatic, they can occasionally manifest as a more serious form of the illness.18 The current study’s findings highlight the widespread distribution of arboviruses among symptomatic individuals, as well as the presence of arboviruses among human populations in nearly all of Theni, Tamil Nadu, implying that the viruses are unlikely to be diagnosed through routine health-care delivery systems. To confirm DENV or CHIKV infection in the early stages of disease, molecular testing is essential. However, as the illness progresses, the sensitivity of molecular methods decreases due to the onset of a rapid immune response and a commensurate drop in viral load. IgM ELISA is currently a more sensitive and accurate diagnostic test.19, 20 Certain studies show that DENV and CHIKV antibodies can be detectable in blood for up to 180 days and 10 months after infection, respectively.21,22 The persistence of these antibodies months after infection will assist in detecting the existence of viruses in non-endemic locations. In the current study, DENV IgM cases (26.3%), DENV NS1 cases (20.6%), and DENV IgG cases (15.7%) outnumbered CHIKV (8.9%), and DENV-CHIKV co-infection was 3%.
We identified people who tested positive for NS1, 26.3% IgM, 15.7% IgG, and 8.9% CHIKV IgM ELISA out of 16997 probable cases. Secondary dengue infection is seen with IgG antibodies in dengue-endemic areas, whereas dengue NS1 and IgM antibodies are known to be positive in initial infection.23 The majority of DENV and CHIKV patients were between the ages of 21 and 40. A similar infection pattern was reported by Kaur et al., Chattopadhyay et al., and Lall et al.24-26 Regarding the co-infection of DENV-CHIKV, all cases came from different ages because of low positivity.
Among those aged 21 to 40, females outnumbered males in dengue IgM, dengue IgG, and chikungunya infections, whereas males outnumbered females in dengue NS1 infections. Because there were more suspected male patients in the trial, there was a higher rate of positivity in men; women stayed at home and were less exposed to the virus. Many Asian countries have low rates of female infection, and it is usually believed that this is because they receive fewer reports to hospitals. When they become unwell or are taken to the hospital when there are no other options, females are likewise less likely to seek medical attention.27 Scientific research has also shown that while viral infections are more common and severe in men, they can also lead to worse illness outcomes in women.28 Female cells showed a 10-fold higher expression rate than male cells, according to in-vitro research.29 However, in females, this can result in the development of an autoimmune illness.
Virus clearance happens more quickly in women because their immune systems are stronger than men’s.28 Because of the rarity of CHIKV positivity and DENV-CHIKV co-infection, the clinical presentation and gender of positive cases are statistically significant. Between August and November, during the monsoon and post-monsoon seasons, infection rates for dengue and chikungunya were noted in many northern Indian states. The month of October saw the highest number of DENV and CHIKV infection cases. According to Ukey PM et al., only 32.1% of patients in Tamil Nadu were serologically positive for dengue infection, compared to 31.3% of patients in Central India.30
In the age groups of 0-20, 21-40, and 41-60, the current study found that arthralgia and headache were the most prevalent clinical features, followed by fever. Similarly, above 21-40 year people had greater DENV prevalence rates than 41-60 year old people, which is consistent with the findings of Malaysian investigations.31 A low DENV prevalence among 15-year-olds has also recently been observed in Cameroon.32 Fever (97.6%), chills (58.1%), arthralgia (60.2%), joint pain (66.2%), headache (66.8%), and cough (58.1%) are the most common complaints among DENV-CHIKV-positive patients (Table 1). The majority of research patients arrived at the hospital within 2-4 days of the onset of their illness, complaining of fever, chills, headaches, and arthralgia. The author believes that the tendency to cure illnesses at home, unwillingness to discuss health-related matters, and disregarding symptoms are the reasons why it took so long to report an illness to the hospital.29 The current study identified 2971 dengue IgM positives, 222 dengue NS1 positives, 3 dengue IgG positives, and 388 chikungunya positives, which shows the viral illness co-circulation in society. DENV and CHIKV are both spread by Aedes mosquitoes. According to the clinical profile, the most common symptoms in patients were fever, chills, arthralgia, joint pain, cough, and headache (Table 1). In one study on DENV-CHIKV dual infection, Singh et al. found co-infection, headache, and arthralgia in DF.33 As a result, the infection may be effectively targeted using clinical symptoms like fever, headache, rash, and arthralgia. A misinterpretation of DENV-CHIKV infection could occur if two or more clinical signs are present, which would impair patient care and lengthen the course of treatment. Because of the ELISA kit’s cross-reactivity, around 3% of the cases in the current investigation tested positive for DENV-CHIKV co-infection. Furthermore, older participants were more likely to be seropositive for CHIKV and/or have prior DENV-CHIKV exposure than younger participants. We found young people who had been exposed to both viruses, indicating recent CHIKV and DENV transmission in the area. More surveillance and testing are needed to ascertain which virus species are circulating in these areas and the true burden of all illnesses in Theni, Tamil Nadu.
This study underlines the need for clinical information in cases of DENV and CHIKV infection and was conducted in Theni, Tamil Nadu. Infected pre-existing medical issues increase the risk of developing major diseases in children and the elderly. Each symptomatic patient should be examined and given a clinical test for DENV and CHIKV infection because both conditions have a deadly outcome if left untreated. This is suggested by several DENV infections, the presence of the CHIKV virus, and their co-infection. Understanding the prevalence of Dengue-Chikungunya infection in this area will be easier with a longer time horizon, a larger sample size, and information on the co-infection status. Taking all of these aspects into account, prompt and effective treatment can aid in the prediction and control of viral epidemics.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Health Research (DHR), the Ministry of Health and Family Welfare, and the ICMR for the Viral Research and Diagnostic Laboratory initiative (Ref.No:VIR/66/2013/ECD-I;30.09.2014).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by DHR-ICMR (Indian Council of Medical Research), New Delhi, India, with No.VIR/66/2013/ECD-I.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was supported by the Institutional Ethics Committee, Government Theni Medical College, Theni, India, with reference number 1515/MEIII/21 dated 11.05.2023.
- Yousseu FB, Nemg FB, Ngouanet SA, Mekanda FM, Demanou M. Detection and serotyping of dengue viruses in febrile patients consulting at the New-Bell District Hospital in Douala, Cameroon. PLoS One. 2018;13(10):e0204143.
Crossref - Kading RC, Brault AC, Beckham JD. Global perspectives on arbovirus outbreaks: a 2020 snapshot. Trop Med Infect Dis. 2020;5(3):142.
Crossref - Vu DM, Banda T, Teng CY, et al. Dengue and West Nile virus transmission in children and adults in coastal Kenya. Am J Trop Med Hyg. 2017;96(1):141-143.
Crossref - Fagbami AH, Onoja AB. Dengue haemorrhagic fever: An emerging disease in Nigeria, West Africa. J Infect Public Health. 2018;11(6):757-762.
Crossref - Rezza G. Dengue and chikungunya: long-distance spread and outbreaks in naive areas. Pathogens and Global Health. 2014;1;108(8):349-55.
Crossref - Roy SK, Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol. 2021;67(10):687-702.
Crossref - Dengue and severe dengue n.d. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed June 18, 2023.
- Fernandez-Salas I, Diaz-Gonzalez EE, Lopez-Gatell H, Alpuche-Aranda C. Chikugunya and zika virus dissemination in the Americas: different arboviruses reflecting the same spreading routes and poor vector-control policies. Curr Opin Infect Dis. 2016;29(5):467-475.
Crossref - WHO, Chikungunya, WHO, https://www.who.int/health-topics/chikungunya#tab=tab_1, 2022 (accessed September 20, 2022).
- da Cunha RV, Trinta KS. Chikungunya virus: clinical aspects and treatment-A Review. Mem Inst Oswaldo Cruz. 2017;112(8):523-31.
Crossref - Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;25;496(7446):504-507.
Crossref - NVBDCP, Chikungunya situation in India: national center for vector borne diseases control(NCVBDC). 2022. https://nvbdcp.gov.in/index4.php?lang=1&level=0&linkid=486&lid=3765. Accessed June 18, 2023.
- Weaver SC, Charlier C, Vasilakis N, Lecuit M. Zika, chikungunya, and other emerging vector-borne viral diseases. Ann Rev Med. 2018;69:395-408.
Crossref - Yergolkar PN, Tandale BV, Arankalle VA, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12(10):1580.
Crossref - Sarma N. Emerging and re-emerging infectious diseases in South East Asia. Indian J Dermatol. 2017;62(5):451-455.
- Ramamurthy M, Sankar S, Nandagopal B, Sridharan G, Risbud AR. Viral Diseases of Public Health Importance in India: Current Priorities with Special Emphasis on Prevention. Journal of Krishna Institute of Medical Sciences (JKIMSU). 2017;6(4).
- World Health Organization. Comprehensive guideline for prevention and control of dengue and dengue haemorrhagic fever. 2011. https://apps.who.int/iris/handle/10665/204894
- Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat Rev Dis Primers. 2016;2(1):16055.
Crossref - Mardekian SK, Roberts AL. Diagnostic options and challenges for dengue and chikungunya viruses. BioMed Res Int. 2015;834371.
Crossref - Neeraja M, Lakshmi V, Teja VD, et al. Unusual and rare manifestations of dengue during a dengue outbreak in a tertiary care hospital in South India. Arch Virol. 2014;159(7):1567-1573.
Crossref - Chelluboina S, Robin S, Aswathyraj S, Arunkumar G. Persistence of antibody response in chikungunya. Virusdisease. 2019;30(3):469-473.
Crossref - Pierro A, Rossini G, Gaibani P, et al. Persistence of anti-chikungunya virus-specific antibodies in a cohort of patients followed from the acute phase of infection after the 2007 outbreak in Italy. New Microbes New Infect. 2015;7:23-5.
Crossref - Jain A, Khan DN, Prakash O, Shukla S, Prakash S, Verma AK. Severity of dengue illness and presence of anti DV IgG in serum of laboratory confirmed dengue cases. J Vector Borne Dis. 2021;58(3):228-231.
Crossref - Kaur M, Singh K, Sidhu SK, et al. Coinfection of chikungunya and dengue viruses: A serological study from North Western region of Punjab, India. J Lab Physicians. 2018;10(04):443-447.
Crossref - Chattopadhyay S, Mukherjee R, Nandi A, Bhattacharya N. Chikungunya virus infection in West Bengal, India. Indian J Med Microbiol. 2016;34(2):213-215.
Crossref - Lall H, Gupta P, Debbarma M, et al. Sero-prevalence of dengue in tertiary care hospital in Delhi. Int J CurrMicrobiol Appl Sci. 2016;5(6):439-445.
Crossref - Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2(1):1-10.
Crossref - Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34(12):1050-1059.
Crossref - Parisius LM, Stock-Schroer B, Berger S, Hermann K, Joos S. Use of home remedies: a cross-sectional survey of patients in Germany. BMC Family Practice. 2014;15(1):1-8.
Crossref - Ukey PM, Bondade SA, Paunipagar PV, Powar RM, Akulwar SL. Study of seroprevalence of dengue fever in central India. Indian J Community Med. 2010;1;35(4):517-519.
Crossref - Mohd-Zaki AH, Brett J, Ismail E, L’Azou M. Epidemiology of dengue disease in Malaysia (2000-2012): a systematic literature review. PLoS Negl Trop Dis. 2014;6;8(11):e3159.
Crossref - Tchuandom SB, Tchadji JC, Tchouangueu TF, et al. A cross-sectional study of acute dengue infection in paediatric clinics in Cameroon. BMC Public Health. 2019;19(1):958.
Crossref - Singh J, Dinkar A, Singh RG, Siddiqui MS, Sinha N, Singh SK. Clinical profile of dengue fever and coinfection with chikungunya. Ci Ji Yi Xue Za Zhi . 2018;30(3):158-163.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.