ISSN: 0973-7510
E-ISSN: 2581-690X
Plasmodium vivax (P. vivax) remains a major contributor to global malaria morbidity and mortality, particularly outside sub-Saharan Africa. Its unique biological traits including dormant liver stages, low parasitemia, and early gametocyte development pose significant diagnostic and treatment challenges. Despite advancements in malaria control, P. vivax continues to evade elimination efforts. This review examines the current landscape of P. vivax detection, focusing on recent developments in machine learning (ML) and deep learning (DL) techniques applied to thin blood smear image analysis. A systematic selection of peer-reviewed studies from 2010 to 2024, alongside clinical trial data, was analyzed to evaluate the effectiveness, challenges, and future prospects of AI-based diagnostic models. Notably, lightweight convolutional neural networks (CNNs) like MobileNet and detection frameworks such as YOLO have shown promising results in terms of accuracy and computational efficiency. However, limitations related to generalizability, data variability, and model interpretability remain. This review also outlines biological complexities, drug-resistance issues, and the global and Indian epidemiological context of P. vivax. By synthesizing technical, clinical, and biological perspectives, this work aims to guide future research toward more effective, accessible, and scalable AI-assisted malaria diagnostic tools.
Plasmodium vivax, Machine Learning, Deep Learning, Biology, Drug-resistance
The pharmaceutical sector has seen a significant rise in data digitization in recent years; however, this progress brings with it several challenges related to learning, analysing, and applying the data effectively to complex clinical scenarios.1 Despite notable progress in certain regions, malaria continues to pose a serious public health challenge across much of the tropical world, resulting in hundreds of thousands of deaths and hundreds of millions of cases annually.2 Outside of Africa, Plasmodium vivax (P. vivax) is the most prevalent of the five Plasmodium species that infect humans. Its transmission periodicity offers only temporary and weak immune protection, while the presence of dormant liver stages (hypnozoites) hinders effective control and elimination strategies.3
Malaria, caused by Plasmodium parasites and primarily transmitted through the bite of infected female Anopheles mosquitoes, continues to be a significant public health challenge. According to the World Health Organization (WHO, 2023), there were approximately 249 million malaria cases and 608,000 malaria-related deaths globally in 2022, with over 75% of these deaths occurring in children under the age of five, particularly in sub-Saharan Africa. Malaria results in over 600,000 child fatalities and more than 200 million cases each year, these figures vary year by year and are influenced by factors such as climate patterns, funding for control programs, political stability, healthcare access, and the emergence of drug or insecticide resistance.
While the burden remains concentrated in Africa accounting for nearly 95% of global malaria deaths the actual number of child deaths annually due to malaria may fluctuate depending on how well national programs maintain insecticide-treated bed net coverage, access to artemisinin-based combination therapies (ACTs), and early diagnostic efforts.4 In non-African endemic regions like Southeast Asia and parts of South America, the disease burden is significantly lower but still concerning due to emerging resistance to frontline treatments, particularly in P. vivax strains. Furthermore, while the global malaria case count has remained above 200 million annually for the past decade, this statistic alone can be misleading if not contextualized with regional disparities, age-related vulnerability, and differences in surveillance/reporting quality. Thus, broad generalizations should always be paired with geographic, demographic, and temporal qualifiers, supported by the most current surveillance data.
Machine learning (ML) has emerged as a powerful tool in malaria research, effectively addressing various challenges such as detecting evolutionary selection linked to drug-resistance, classifying and identifying Plasmodium parasites in red blood cells (RBCs), and aiding in the discovery of new antimalarial compounds. Deep learning (DL), a specialized branch of ML, leverages large datasets to automatically extract and learn complex, hierarchical data representations. DL algorithms, particularly neural networks, have also been explored in the field of population genetics, extending their utility to malaria and other infectious diseases.5 Malaria diagnosis and monitoring continue to pose significant challenges, particularly in low-resource settings where trained specialists are scarce and computational resources are limited. To ensure timely and accurate diagnosis and treatment, there is a critical need for lightweight, automated technologies that can assist healthcare providers in detecting and monitoring malaria. In recent years, the use of supervised ML techniques for automated malaria detection from thin blood smear microscopy images has shown promising results. These advancements have been enabled by the growing availability of large annotated datasets and the continuous development of computational capabilities.3,6
As predicted by the WHO, there were an estimated 627,000 malaria-related fatalities and approximately 241 million cases of malaria globally in 2020 (WHO, 2021). This represented an increase of 69,000 deaths and 14 million additional cases compared to 2019, highlighting the growing burden of the disease. The surge in cases and fatalities can be attributed to various factors, including disruptions in malaria prevention and treatment programs due to the COVID-19 pandemic, which significantly impacted global healthcare systems.6
Comparing these statistics with prior years, the rise in malaria cases and deaths signals a concerning trend, particularly in sub-Saharan Africa, which remains the region most affected by the disease (WHO, 2021). While the total number of cases in 2020 was slightly below the peak observed in 2017 (approximately 261 million cases), the increase from 2019 underscores the challenges in controlling malaria transmission in the context of both emerging health crises and pre-existing healthcare system gaps. Additionally, the data for 2020 must be interpreted with caution, given that reporting and surveillance efforts in many malaria-endemic regions may have been hindered during the pandemic. As a result, some estimates may not fully reflect the true extent of the malaria burden.7 A more comprehensive assessment will be needed in future years as data collection stabilizes and the impact of the pandemic on malaria transmission is more fully understood.
Recent advancements in the application of DL algorithms, particularly Convolutional Neural Networks (CNNs), have demonstrated high accuracy often exceeding 95% in the automated detection of malarial parasites from stained blood smear images. For example, a study reported a classification accuracy of 96.5% using a pretrained CNN model for distinguishing between parasitized and uninfected cells.8 Similarly, one of the studies achieved 98.6% accuracy using a DL architecture optimized for biomedical imaging tasks. These promising outcomes, showing DL’s potential to augment or even replace manual microscopy, provided the rationale for conducting this study to further explore and evaluate DL methods in malaria diagnosis.9 MobileNet is a type of lightweight deep CNN designed to be faster and more efficient than earlier models, owing to its use of depth wise separable convolutions. This architecture achieves high classification accuracy, particularly for images with consistent background, well-structured composition, and minimal noise.
This review specifically focuses on the application of ML and DL techniques for the diagnosis of malaria using peripheral blood smear images. Blood smear microscopy remains a standard and widely used method for malaria diagnosis due to its ability to detect and differentiate Plasmodium species. Recent advances in image analysis, particularly through CNNs and other DL architectures, have demonstrated significant potential in automating and enhancing the accuracy of blood smear interpretation. By leveraging high-resolution microscopic images, these computational approaches aim to reduce diagnostic errors, minimize reliance on expert microscopists, and increase throughput in both clinical and field settings.
A comprehensive literature search was conducted using reputable databases, including ScienceDirect, PubMed, and Scientific Reports, to identify articles published between 2010 and 2024. The inclusion and exclusion criteria were specifically defined based on the relevance and quality of the articles in addressing the research objectives related to the application of ML and DL techniques in malaria research.
Inclusion criteria
Article type
Only original research articles and review papers were included, as these provide primary evidence and comprehensive overviews of the subject matter.
Language
Articles published in English were included to ensure consistency and to facilitate proper analysis.
Timeframe
Only studies published between 2010 and 2024 were considered, ensuring that the research reflects the most recent advancements in the field.
Focus
Articles specifically addressing ML and DL techniques used in malaria research, including diagnostic methods, predictive modeling, and treatment strategies, were selected.
Exclusion criteria
Non-peer-reviewed content
Articles not published in peer-reviewed journals were excluded to maintain the study’s focus on high-quality, scientifically validated research. This includes thesis, conference papers, and editorials.
Irrelevant subject matter
Articles not directly focused on ML and DL applications in malaria research were excluded. For example, studies solely discussing traditional methods of malaria diagnosis or treatment without integrating ML or DL techniques were not considered.
Insufficient data
Articles that lacked quantitative data or detailed methodologies related to ML/DL techniques were excluded, as they did not contribute to the depth of analysis required for this study.
Outdated or redundant studies
Articles published prior to 2010 were excluded, as this research aims to focus on the most recent developments in the application of ML and DL techniques in malaria.
Inadequate focus on P. vivax
Articles that did not specifically address malaria caused by P. vivax were excluded.
Clinical trial data related to P. vivax were retrieved from ClinicalTrials.gov using the following search parameters:
Search filters: Completed, Interventional, and Observational Studies
Keywords: Plasmodium vivax, malaria, clinical trial
Date of access: 15.11.2024
Biology of P. vivax
Resistance of P. vivax to malaria control and eradication methods is largely attributed to its unique biological characteristics. Firstly, laboratory diagnosis of low-density blood infections of P. vivax, which are common, particularly in regions nearing elimination, remains particularly challenging. Secondly, P. vivax parasites can persist in human hosts for months as hypnozoites-dormant liver stages that can later reactivate and cause relapses. Furthermore, individuals with a deficiency in the enzyme glucose-6-phosphate dehydrogenase (G6PD) are at risk of significant hemolysis when treated with primaquine, the only approved antimalarial drug with hypnozoitocidal activity. This presents a challenge, as effective treatment for vivax malaria requires medications targeting both liver and blood stages of the parasite.
Additionally, P. vivax differs from Plasmodium falciparum (P. falciparum) in its gametocyte development. In P. vivax, early maturation of gametocytes promotes transmission, which is less pronounced in P. falciparum. Diagnosis of P. vivax infection is aided by Giemsa-stained blood smears, which can reveal key morphological differences between P. vivax and P. falciparum. However, because P. vivax typically infects only reticulocytes, parasitemia levels are often low, requiring the use of thick smears to concentrate blood for accurate diagnosis. This can pose a challenge for non-expert microscopists, as the improvement in sensitivity from thick smears may come at the cost of maintaining clear morphological structures.
Morphological identification of Plasmodium species is crucial for accurate diagnosis. For example, P. vivax-infected reticulocytes contain small, dark granules known as Schuffner’s dots parasite-derived caveolae-vesicle complexes that are exported into the reticulocyte’s cytoplasm as the parasite matures. Additionally, P. vivax typically presents with a spherical shape that resembles its asexual stages. Finally, all stages of P. vivax blood-stage development can be found in peripheral blood, further aiding in species identification.10
The female Anopheles mosquito introduces sporozoites into the human bloodstream through its proboscis, depositing them into the dermal layer of the skin. From there, a portion of the inoculum migrates to the liver, where it invades hepatocytes within minutes of transmission. The parasite undergoes a transformation over the next five to eight days into a large exoerythrocytic form, with the specific duration depending on the Plasmodium species. Inside the parasitophorous vacuole membrane (PVM), thousands of merozoites are packed tightly. As the parasite matures, the PVM divides into small vesicular packets containing the merozoites, which are eventually released into the bloodstream. This marks the beginning of erythrocytic invasion.
During the subsequent 48 hours, the parasite undergoes mitotic division and cytoplasmic growth inside the erythrocyte. Depending on the Plasmodium species, it may mature into a gametocyte (sexual stage) or schizont (asexual stage). In P. falciparum, gametogenesis produces the fully transmissible stage V gametocyte, a process that takes 10 to 12 days and requires sequestration in the bone marrow. As a result, P. falciparum sexual stages are typically observed only during blood-stage cycles in the peripheral blood. In contrast, P. vivax sexual stages appear much earlier, though it remains unclear whether sexual commitment in P. vivax occurs at an early stage.
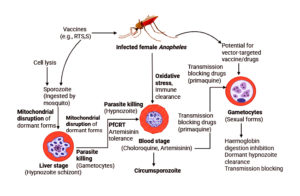
Malaria is primarily caused by single-celled parasites of the Plasmodium genus. Studies have shown that it is most commonly transmitted through the bite of an infected female Anopheles mosquito, with the parasite being injected into the human bloodstream via the mosquito’s saliva. The parasites then migrate to the liver, where they mature and multiply. Figure showing mechanistic insight about the removal perspectives and role in antimalarial effect behaviour.
Figure. Mechanistic insight about the removal perspectives and role in antimalarial effect behaviour
Established Standard Therapy for P. vivax
The clinical management of P. vivax malaria involves a combination of clinical suspicion, accurate blood analysis, and access to effective treatment regimens targeting both liver-stage hypnozoites (for radical cure) and blood-stage schizonts. P. vivax infections in the blood stage are currently treated with either chloroquine or artemisinin-based combination therapy (ACT). Although chloroquine has been the first-line treatment for P. vivax for over 70 years, its reduced efficacy against P. falciparum due to global resistance has led to the adoption of alternative therapies for mixed-species infections or misdiagnosed cases.
The use of ACTs, excluding artesunate-sulphadoxine-pyrimethamine, provides a reliable, unified approach to treating all malaria cases, offering a safety net for undetected P. falciparum co-infections. While chloroquine remains effective against P. vivax in most regions, high-grade resistance has been reported in areas such as Sabah (Malaysia), parts of Indonesia, and Oceania. Despite the higher cost of ACTs compared to chloroquine, their broader efficacy and ability to reduce treatment failure rates can ultimately result in lower overall operational costs for malaria control programs.11
Primaquine remains the only commercially available hypnozoitocidal drug for treating P. vivax malaria. However, its use is constrained by two significant factors: the risk of hemolysis in individuals with G6PD deficiency and the challenge of ensuring adherence to the recommended 14-day treatment regimen. Relapses caused by dormant liver-stage hypnozoites are the primary drivers of P. vivax recurrences. As countries move toward the goal of malaria elimination, particularly for all Plasmodium species, the need for a safe and effective radical cure targeting both the liver and blood stages of the parasite is becoming increasingly urgent.12
Overview of the current P. vivax Malaria situation in India
India bears the highest global burden of P. vivax malaria. In 2014, of the 2.14 million P. vivax cases reported worldwide, approximately 18% occurred in India. That year, the public healthcare sector confirmed around 380,000 P. vivax cases, making up nearly three-quarters of all malaria cases in the country. Historically, P. vivax has accounted for nearly 50% of all malaria cases in India. Notably, children are disproportionately affected: while they make up only 12% of the population, they represent nearly 30% of all P. vivax cases.
India runs the largest national malaria control program in the world. On average, P. vivax accounts for 47% of the country’s malaria cases annually and is the focus of over 40% of global publications related to P. vivax. Research from Gujarat in western India has documented cases of P. vivax malaria with unusually long incubation periods. Similarly, studies conducted in Delhi between 1988 and 1993 identified regional strains of P. vivax associated with delayed onset, suggesting a variety of relapse phenotypes across different regions of the country. Research in Aligarh confirmed the presence of P. vivax strains with prolonged incubation periods in central India. Genotyping studies from Kolkata in eastern India found that 81% of P. vivax infections were locally transmitted, with longer incubation periods, while 19% exhibited shorter incubation.
Complicated cases of P. vivax malaria-once considered rare-have been reported in both Brazil and India, with symptoms such as jaundice, hemoglobinuria, convulsions, and pulmonary edema. In India, fatal outcomes have been linked to a range of clinical complications, including cerebral malaria, anemia, thrombocytopenia, jaundice, renal failure, and acute respiratory distress. For example, hospital data from one Indian center showed that approximately 15% of P. vivax malaria patients experienced severe illness.
Achieving malaria elimination in India requires a thorough understanding of the current incidence and prevalence of the disease. India accounts for approximately 70% of all malaria cases and 69% of malaria-related deaths in Southeast Asia. However, surveillance data from the past decade indicate a steady decline in both reported cases and malaria-related mortality.
To reach the goal of malaria eradication, India classifies states into low, moderate, and high transmission zones based on Annual Parasite Incidence (API). Malaria control efforts are primarily focused at the district level, which is designated as the key planning and implementation unit under India’s National Framework for Malaria Elimination (NFME). The success of malaria eradication will depend significantly on the country’s ability to scale up disease control measures and public health interventions. Accurate and timely diagnosis will be crucial to these efforts. Furthermore, severe cases of P. vivax malaria have been reported across India, with the country accounting for nearly half of all vivax malaria cases in Southeast Asia as of 2018.13
Global endemic spread of P. vivax Infection
P. vivax is a major cause of malaria-related morbidity in endemic regions across Oceania, Asia, Central America, the Horn of Africa, and South America. It significantly impacts maternal and neonatal health, contributing to adverse outcomes such as low birth weight and early pregnancy loss, which in turn increase infant mortality rates. In 2017, approximately 14 million P. vivax infections were reported globally, with the majority occurring in six countries: India, Ethiopia, Pakistan, Afghanistan, Indonesia, and Papua New Guinea. The burden of P. vivax is particularly severe in remote rural areas, where healthcare infrastructure is often inadequate or underfunded. Children, especially those between the ages of two and six, are the most vulnerable, with the highest prevalence observed in this age group.
Historically underrecognized, P. vivax has gained renewed attention due to recent evidence of its presence in Sub-Saharan Africa, where infections have been detected in both Duffy-positive and Duffy-negative individuals challenging previous assumptions about host susceptibility. In India, approximately 97% of all malaria cases are concentrated in high-endemic states, including Andhra Pradesh, Madhya Pradesh, Bihar, Maharashtra, Odisha, West Bengal, and the northeastern regions.14
Leveraging ML and DL techniques for enhanced P. vivax detection
The primary aim of this study was to explore research on machine and DL techniques for detecting malaria caused by P. vivax. This has been more challenging in existing systems due to the typically low parasitemia levels in P. vivax infections compared to P. falciparum. Previous studies relied on conventional image processing methods to segment P. vivax images, often providing manually cropped regions of infected areas.
Various quantitative experimental tests were conducted during the classification stage, with a focus on the cell level. However, the primary objective in diagnosing malaria is to detect and classify every cell in a patient’s sample, including both false-positive cells and parasites. Additionally, strong performance at the cell level does not necessarily guarantee better results at the patient level. Several studies have been conducted to improve the diagnosis of P. vivax. Among them, one15 proposed a novel Stacked CNN framework that improved malaria diagnosis through an automated method, bypassing the need for hand-crafted features. Additionally, the use of fivefold cross-validation led to increased accuracy in parasite detection. The results indicated that convolutional layers can extract various types of information for the classification process by adjusting the depth and filter sizes. This study16 proposed a highly precise, cost-effective, and stable method for identifying apple leaf disease using the MobileNet model. Furthermore, it compared the precision and efficacy of the proposed method with well-established CNN models, such as Inception V3 and ResNet 152. This study5 introduced a robust and rapid detection system for the automatic diagnosis of P. vivax parasites using a cascaded YOLO system. The model utilized the YOLO V2 framework, and its outcomes were compared to those of traditional V2 models. The comparison showed that the proposed method improved the precision of the mean average compared to other traditional models. However, the model faced computational complexities, which impacted its overall accuracy.
This study demonstrates an intelligent framework based on a simple CNN for diagnosing malaria parasites using images of thin blood smears.17 Experimental analysis showed that the proposed model achieved significantly higher sensitivity (97%). Additionally, the study introduced a false-positive reduction technique using grey level co-occurrence matrix (GLCM) features, which effectively minimized false positives. This paper18 presented a method for automated malaria parasite identification using images of thin blood smears. The research performed texture and intensity-based analysis to diagnose blood cell particles in pre-processed images. These extracted blood particles were then passed to a CNN model. The model was trained using a publicly available dataset, which included images of Giemsa-stained blood smears. From this analysis, the method achieved a high Dice score of 0.95.19 This study provides an in-depth analysis of deep learning and transfer learning techniques, such as MobileNet V2, in the detection of malarial diseases. Specifically, the research developed a MobileNet V2 model that incorporated bottleneck identities to diagnose P. vivax infections.
Challenges
Innovative strategies tailored to address the unique biology and epidemiology of P. vivax will be necessary to overcome its challenges. Early diagnosis and prompt treatment could significantly mitigate this major issue. While it requires more time and expertise, the gold standard for diagnosing malaria remains the examination of peripheral blood smears under a microscope.11,20 Developing an ML model that can efficiently count and categorize RBCs in thin blood smear microscopy images for malaria diagnosis presents several challenges. One of the key issues is the significant variability in the appearance of blood smear images. Factors such as hue, contrast, and consistency of the smear images differ due to variations in smear preparation and staining techniques. This variability creates generalization issues when applying ML models to blood smears prepared at different sites. Additionally, overlapping or touching cells pose challenges for detection and segmentation in thin blood smear images. This issue is critical for malaria diagnosis and surveillance, as accurate cell counting is essential for calculating parasitemia, or the parasite burden.21
The distinct characteristics of the P. vivax parasite, which differentiate it from other Plasmodium species, include its high transmission potential due to early and continuous gametocyte production, its tendency to relapse through chronic, dormant liver stages, and its increased infectivity to mosquitoes. Additionally, its shortened vector development cycle contributes significantly to the challenges in managing and eradicating vivax malaria.17
The biology of P. vivax presents several challenges to the control and elimination of vivax malaria. Two key factors contribute to its high transmission potential: (1) its ability to relapse from dormant liver stages (hypnozoites), and (2) its short development cycle in the mosquito vector, high infectivity to mosquitoes, and early, continuous gametocyte production compared to other Plasmodium species. These characteristics allow P. vivax to spread more widely, including into temperate regions, and to be transmitted by migratory mosquito vectors during cooler weather. Once considered a benign, self-limiting infection, emerging evidence suggests that P. vivax is far more serious than previously thought. The disease’s overall burden, economic impact, and severity have been significantly underestimated. People of all ages can suffer from incapacitating effects, including severe anemia, respiratory distress, recurrent fevers, and, in some cases, poor pregnancy outcomes. This growing body of clinically severe reports challenges the notion that vivax malaria is harmless. An additional challenge is the rising resistance to chloroquine, the primary treatment for the disease, which complicates ongoing prevention and control efforts. This highlights the urgent need for the development of new tools specifically targeting P. vivax.10
Resistance to Antimalarial Drugs
P. vivax has shown a more gradual development of chloroquine resistance compared to P. falciparum, with the molecular basis and mechanisms remaining elusive. Over 30 years ago, a case of chloroquine resistance was reported in a traveler returning from Papua New Guinea, and since then, regions such as Indonesia and Oceania have experienced high levels of resistance. As a result, Artemisinin Combination Therapy (ACT) is now commonly used in these areas instead of chloroquine. Highly effective treatments for P. vivax malaria include artesunate-mefloquine, artesunate-pyronaridine, dihydroartemisinin-piperaquine, and artemether-lumefantrine. Although amodiaquine is more effective than chloroquine against resistant P. vivax, it is not as well tolerated as dihydroartemisinin-piperaquine. Additionally, P. falciparum resistance is widespread in regions where P. vivax is endemic, making amodiaquine-containing regimens unsuitable for treating chloroquine-resistant P. vivax. Despite signs of gradual resistance, chloroquine remains effective in the majority of malaria-endemic areas, with low-level resistance often going undetected due to concurrent use of Primaquine for radical treatment, which masks asexual stage activity. The absence of a molecular resistance marker and the limited availability of in vitro tests for P. vivax make the epidemiology of chloroquine resistance poorly understood. P. vivax also exhibits resistance to antifolates, often due to mutations in the Pvdhfr gene, and antifolate resistance is widespread. Furthermore, P. vivax has natural resistance to sulphonamides, which makes the artesunate-sulfadoxine-pyrimethamine ACT unsuitable for its treatment.11
Status of clinical trials for P. vivax
ClinicalTrials.gov is currently the largest accessible clinical trial database.22 To identify and characterize clinical trials for P. vivax, we focused on the active trials registered on ClinicalTrials.gov for this evaluation. Table presents the currently ongoing clinical trials for P. vivax.
Table:
Clinical studies currently in progress are targeting P. vivax infections
No. |
Clinical Trials.gov ID |
Title of the study |
Type of study |
|---|---|---|---|
1. |
NCT05788094 |
ACT vs CQ with Tafenoquine for P. vivax Mono-infection |
Interventional |
2. |
NCT04704999 |
Southeast Asia Dose Optimization of Tafenoquine |
Interventional |
3. |
NCT05044637 |
Study to Evaluate Primaquine for Radical Cure of Uncomplicated P. vivax Malaria in Children |
Interventional |
4. |
NCT05071079 |
A Controlled Human Vivax Malaria Infection Study Through Inoculation of Infected Erythrocytes |
Interventional |
5. |
NCT04083508 |
Vivax Malaria Human Infection Studies in Thailand |
Interventional |
6. |
NCT04411836 |
Effectiveness of Novel Approaches to Radical Cure with Tafenoquine and Primaquine |
Interventional |
7. |
NCT05361486 |
Radical Cure (RC) With Tafenoquine or Primaquine After Semi-quantitative G6PD Testing: A Feasibility Study in Peru |
Observational |
8. |
NCT05540470 |
Radical CURE for Malaria Among Highly Mobile and Hard-to-reach Populations in the Guyanese Shield |
Interventional |
9. |
NCT04706130 |
Rigorous Assessment of P. vivax Relapses and Primaquine Efficacy for Radical Cure |
Interventional |
10. |
NCT05058885 |
P. vivax Among Duffy Negative Population in Cameroon. |
Observational |
11. |
NCT05879224 |
Short Course Primaquine for the Radical Cure of P. vivax Malaria- Indonesia |
Interventional |
12. |
NCT05874271 |
Short Course Primaquine for the Radical Cure of P. vivax– Papua New Guinea |
Interventional |
13. |
NCT04228315 |
Biomarkers of P. vivax Relapse |
Interventional |
14. |
NCT05913973 |
Study of the P. vivax Transmission-blocking Vaccine Pvs230D1- EPA/Matrix-M to Assess Safety, Immunogenicity, and Transmission-blocking Activity in Healthy Malaria-naive Adults |
Interventional |
15. |
NCT03375983 |
Plasmodium Immunotherapy for Advanced Cancers |
Interventional |
16. |
NCT02786589 |
Plasmodium Immunotherapy for Lung Cancer |
Interventional |
17. |
NCT04165590 |
Plasmodium Immunotherapy for Advanced Malignant Solid Tumors |
Interventional |
18. |
NCT03474822 |
Plasmodium Immunotherapy for Breast and Liver Cancers |
Interventional |
NCT05788094
This clinical trial aims to compare three drug combinations for treating P. vivax mono-infections: Dihydroartemisinin-piperaquine + Tafenoquine, Chloroquine + Tafenoquine, and Artemether-Lumefantrine + Tafenoquine. This Phase 4, randomized, parallel assignment study includes 606 participants aged 18 and older. Its primary purpose is treatment, and the trial runs from June 26, 2023, to August 31, 2025. The findings will help evaluate effective regimens and address emerging drug resistance in P. vivax treatment.
NCT04704999
This clinical trial focuses on optimizing the dosage of Tafenoquine in Southeast Asia. It compares the effectiveness of Tafenoquine, Chloroquine, and a combination of Artemether 20 mg-Lumefantrine 120 mg for treating P. vivax. This Phase 4, randomized, parallel assignment study includes 700 participants aged 2 years and older (children, adults, and older adults). The trial is set to run from July 22, 2024, to February 7, 2028, with the primary purpose being treatment optimization. The results will help refine dosing strategies for more effective malaria treatment in the region.
NCT05044637
This clinical trial evaluates the use of Primaquine for the radical cure of uncomplicated P. vivax malaria in children. This Phase 2, randomized, parallel assignment study involves 150 participants aged 6 months to 14 years. The trial is designed to assess the efficacy of Primaquine in curing P. vivax malaria, with a primary treatment goal. The study began on August 26, 2021, and is scheduled to conclude by December 1, 2023. The findings will provide valuable insights into the safe and effective use of Primaquine in paediatric malaria treatment.
NCT05071079
This clinical trial is a controlled human P. vivax malaria infection study that investigates the inoculation of malaria-parasitized red blood cells at varying dilutions. Participants aged 20 to 55 years (adult population) will receive one of four biological inoculums with different blood-stage dilution levels: whole-dose, 1:5 dilution, 1:10 dilution, and 1:20 dilution. The study is designed to assess the effects of these inoculums on P. vivax malaria infection. This interventional study follows a randomized, parallel assignment model and has 48 participants. The trial commenced on May 23, 2022, and is expected to conclude by November 2025. The primary purpose of this study is to further understand the pathogenesis and immune response to P. vivax infection.
NCT04083508
This clinical trial investigates P. vivax malaria human infection studies in Thailand, where participants aged 20 to 55 years (adult population) are exposed to P. vivax through mosquito bites. This interventional study uses a single group assignment model, and the primary purpose is focused on understanding the infection process rather than treatment. A total of 6 participants are enrolled in this study. The trial began on October 5, 2020, and is set to conclude by September 30, 2025. The study aims to gather insights into the natural progression of the disease following mosquito exposure.
NCT04411836
This clinical trial is a Phase 3 study focused on evaluating the effectiveness of novel approaches to radical cure using Tafenoquine and Primaquine for P. vivax malaria. It involves 960 participants aged 18 to 100 years (adult and older adult populations). The study is randomized, following a parallel assignment model, with the primary purpose being treatment. The trial began on April 25, 2021, and is expected to conclude by September 30, 2024. The study aims to compare the efficacy of these two drugs in achieving a radical cure for P. vivax malaria.
NCT05361486
This clinical trial is a feasibility study conducted in Peru to assess the radical cure (RC) of P. vivax malaria using Tafenoquine or Primaquine following semi-quantitative G6PD testing. The study involves 40 participants aged 6 months and older (children, adults, and older adults). This observational study uses an ecologic or community model with a prospective time perspective. The study includes several interventions, such as training on a revised algorithm, enhancing pharmacovigilance, and supervising malaria services in selected facilities. The diagnostic test used in the trial is G6PD testing, and the drugs being studied are Tafenoquine and Primaquine. A follow-up visit is scheduled on Day 3 [+2 days] after the start of treatment. The trial began on August 28, 2023, and is expected to conclude by October 31, 2024.
NCT05540470
This clinical trial is an interventional study titled Radical CURE for Malaria Among Highly Mobile and Hard-to-reach Populations in the Guyanese Shield. It involves 5,000 adults (18 years and older) and uses two drugs-Plasmodium Antigen Rapid Test (PART) and Malaria kit-alongside cross-sectional surveys and a qualitative study. The primary purpose is treatment, using a non-randomized, parallel assignment model. The study began on September 12, 2022, and is expected to conclude by December 12, 2025.
NCT04706130
This is a Phase 4 interventional study titled Rigorous Assessment of P. vivax Relapses and Primaquine Efficacy for Radical Cure. It includes 200 participants aged 15 years and older and evaluates the effectiveness of Primaquine and Artesunate for treatment. The study follows a randomized, parallel assignment model with a primary treatment purpose. It started on April 15, 2021, and is scheduled to end on December 31, 2024.
NCT05058885
This is an observational study titled P. vivax Among Duffy Negative Population in Cameroon. It involves 900 participants aged 1 year and older. The study uses a cross-sectional design with an observational model categorized as “Other”. It began on May 2, 2022, and is expected to conclude on June 30, 2024.
NCT05879224
This is an interventional study titled Short Course Primaquine for the Radical Cure of P. vivax Malaria-Indonesia. It includes 11,250 participants aged 6 months and older. The study evaluates a revised case management package using a single group assignment model. With a primary purpose classified as “Other”, the trial began on August 7, 2023, and is expected to end on July 31, 2025.
NCT05874271
This is an interventional study titled Short Course Primaquine for the Radical Cure of P. vivax -Papua New Guinea. It involves 5,850 participants aged 12 months and older, using a revised case management package. The study follows a single group assignment model with a primary purpose categorized as “Other”. It started on July 10, 2023, and is expected to complete by July 31, 2025.
NCT04228315
This is an interventional study titled Biomarkers of P. vivax Relapse. It includes 100 participants aged 18 years and older and investigates the use of Primaquine for treatment. The study is randomized with a parallel assignment model and a primary purpose of treatment. It began on November 19, 2019, and is scheduled to conclude on December 31, 2024.
NCT05913973
This is a Phase 1 interventional study titled Study of the P. vivax Transmission-blocking Vaccine Pvs230D1-EPA/Matrix-M to Assess Safety, Immunogenicity, and Transmission-blocking Activity in Healthy Malaria-naive Adults. It includes 200 participants aged 18 to 50 years and evaluates the vaccine Pvs230D1-EPA/Matrix-M. The study uses a non-randomized, sequential assignment model with a primary prevention purpose. It began on August 4, 2023, and is expected to end on May 15, 2025.
NCT03375983
This is a Phase 1/2 interventional study titled Plasmodium Immunotherapy for Advanced Cancers. It involves 20 participants aged 18 to 70 years and investigates the use of blood-stage P. vivax infection as a biological intervention for cancer treatment. The study follows a single group assignment model with a primary purpose of treatment. It started on November 23, 2017, and is expected to complete by July 31, 2026.
NCT02786589
This is a Phase 1/2 interventional study titled Plasmodium Immunotherapy for Lung Cancer. It includes 30 participants aged 18 to 70 years and uses blood-stage P. vivax infection as a biological treatment. The study follows a single group assignment model with a primary purpose of treatment. It began on June 27, 2016, and was scheduled to conclude on December 30, 2023.
NCT04165590
This is a Phase 1/2 interventional study titled Plasmodium Immunotherapy for Advanced Malignant Solid Tumors. It involves 60 participants aged 18 to 70 years and investigates Plasmodium immunotherapy as a treatment approach. The study uses a single group assignment model with a primary purpose of treatment. It began on October 24, 2019, and is expected to end on October 31, 2026.
NCT03474822
This is a Phase 1/2 interventional study titled Plasmodium Immunotherapy for Breast and Liver Cancers. It includes 60 participants aged 18 to 70 years and evaluates the use of blood-stage P. vivax infection as a biological treatment. The study follows a single group assignment model with a primary purpose of treatment. It began on August 10, 2018, and is expected to conclude on June 30, 2026.
Recent advancements have demonstrated that AI can significantly enhance drug discovery and streamline virtual clinical trials, reducing the risk of regulatory compliance issues. Companies such as Protai and Netabolics are thriving in their efforts to develop AI-driven drug discovery platforms and predict novel medications by digitizing human cells.23
Benchmarking ML approaches in P. vivax detection
The application of ML in malaria diagnostics has gained momentum over the past decade, particularly for P. falciparum. However, efforts focused specifically on P. vivax remain relatively limited, despite its complex biology and significant global burden. Several studies have benchmarked ML models on P. vivax datasets, with varying methodologies and performance outcomes.
One study demonstrated the use of pre-trained CNNs for detecting malaria parasites in thin blood smears, achieving promising sensitivity levels around 89.2% for mixed P. falciparum and P. vivax samples.8 Their approach emphasized the feasibility of transfer learning in resource-constrained settings. Similarly, a study applied deep CNNs, specifically VGG16, to smartphone-acquired images and reported sensitivities exceeding 91% for P. vivax, highlighting the potential for field-deployable diagnostics.24 Classical machine learning techniques, such as support vector machines (SVMs), decision trees, and random forests, have also been used.25 applied these models to image-based features, reporting diagnostic accuracies between 85% and 88%. While these approaches are computationally efficient, they generally underperform deep learning models when exposed to complex or noisy datasets. Another study examined CNN-based classification of infected erythrocytes, and one study developed automated pipelines combining image segmentation and classification for parasite detection.26,27 Recent studies have identified a clear trend toward ensemble models that integrate image, molecular, and clinical data to improve sensitivity and specificity.28,29 These findings align with the growing interest in hybrid diagnostic platforms capable of compensating for the limitations of single-modality approaches. Despite these advances, relatively few studies have validated ML models in the context of ongoing clinical trials, especially those focusing on P. vivax. highlighted the utility of transfer learning for mobile-based microscopy, but also emphasized the challenge of low-parasitemia detection and the need for curated clinical datasets.30
Moreover, the 2023 World Malaria Report by the WHO emphasized the diagnostic gap in P. vivax detection, particularly in low-transmission or post-elimination settings.4 This has implications for relapse tracking, as outlined in the seminal study which underscores the limitations of current diagnostic tools in identifying dormant hypnozoite stages.31
An international team of dedicated scientists has continued to advance research on P. vivax despite years of neglect, developing tools and models to explore the unique biology of this parasite and, ultimately, create targeted treatments. There are numerous emerging opportunities in this field. Recent progress in animal and human models for studying the parasite’s entire life cycle, as well as advancements in stem cell research and the study of hepatocyte biology, have illuminated key areas to prioritize in order to optimize current resources and maximize our potential. Specifically, efforts should focus on: 1) identifying the host cell that supports invasion and blood-stage development; 2) synthesizing the hepatocyte characteristics required for pre-erythrocytic development and hypnozoite formation in vitro; 3) improving the acquisition of P. vivax single-clonal populations from sustainable sources; and 4) developing reliable techniques for stable parasite transfection through genetic modification. In cases where P. vivax is not available, closely related species such as Plasmodium cynomolgi and Plasmodium knowlesi serve as viable models for studying reticulocyte invasion, hypnozoite production, and reactivation. These efforts aim to enhance existing resources and provide vital new tools to accelerate research and development, driving the global push towards the full eradication of P. vivax. As part of this initiative, the Indian government has outlined a roadmap to eliminate malaria by 2030. It is widely acknowledged that eradicating malaria will improve health outcomes, raise living standards, and help alleviate poverty.32 The WHO’s Global Technical Strategy (GTS) for Malaria aims to eradicate the disease globally by 2030. India, along with other Asia-Pacific nations, has committed to this goal, with a target to reduce malaria-related deaths by 50% worldwide. Collaborating with key partners and stakeholders, the WHO GTS, the Asia Pacific Leaders Malaria Alliance (APLMA), the Malaria Elimination Roadmap, and the National Framework for Malaria Elimination (NFME) 2016-2030 work collectively towards the goal of national malaria eradication by 2030. Additionally, the objective is to reduce the Annual Parasite Incidence (API) to less than 1 by 2024, contributing to improved living standards, better health outcomes, and poverty alleviation.33
Malaria remains a significant global health threat, with high mortality rates. Diagnosing and monitoring malaria continues to be a major challenge, especially in low-resource settings where there are often few specialists to analyze microscopy images and limited computational power to perform automated analyses. To ensure timely and accurate diagnosis and treatment, healthcare providers need access to lightweight automated technologies that can assist in malaria monitoring and detection. Recently, automated malaria detection has begun to integrate supervised ML techniques, driven by the availability of vast amounts of annotated data and advances in processing power. From performance metrics evaluation, it is evident that Plasmodium can be reliably detected in thin blood smear samples using a simple CNN trained with a supplemented small training set. ML technologies hold great promise for various P. vivax diagnosis applications. This discovery aligns with global efforts to eradicate malaria, as there are significant gaps in diagnosing P. vivax. Accurate diagnosis is crucial before starting treatment, as it helps reduce fatality rates, prevent the development of antimalarial drug resistance, and minimize unnecessary side effects from incorrectly prescribed medications.
ACKNOWLEDGMENTS
The authors would like to thank Shri Ramswaroop Memorial University, Lucknow, and Indian Pharmacopoeia Commission for their invaluable support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
VMP conceptualized the study. TQ and SY supervised the study. VMP wrote the original draft. SY wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Paul D, Sanap G, Shenoy S, et al. Artificial intelligence in drug discovery and development. Drug Discov Today. 2020;26(1):80-93.
Crossref - Cui L, Mharakurwa S, Ndiaye D, et al. Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. Am J Trop Med Hyg. 2015;93(3 Suppl):57-68.
Crossref - Rougeron V, Elguero E, Arnathau C, et al. Human Plasmodium vivax diversity, population structure and evolutionary origin. PLOS Negl Trop Dis. 2020;14(3):e0008072.
Crossref - World Health Organization. World Malaria Report 2023. Accessed October 27, 2024. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023.
- Deelder W, Manko E, Phelan JE, Campino S, Palla L, Clark TG. Geographical classification of malaria parasites through applying machine learning to whole genome sequence data. Sci Rep. 2022;12(1):21150.
Crossref - Zawawi A, Alghanmi M, Alsaady I, Gattan H, Zakai H, Couper K. The impact of COVID-19 pandemic on malaria elimination. Parasite Epidemiol Control. 2020;11:e00187.
Crossref - Heuschen A-K, Lu G, Razum O, et al. Public health-relevant consequences of the COVID-19 pandemic on malaria in sub-Saharan Africa: a scoping review. Malar J. 2021;20(1):339.
Crossref - Rajaraman S, Antani SK, Poostchi M, et al. Pre-trained convolutional neural networks as feature extractors toward improved malaria parasite detection in thin blood smear images. Peer J. 2018;6:e4568.
Crossref - Bibin D, Nair MS, Punitha P. Malaria Parasite Detection From Peripheral Blood Smear Images Using Deep Belief Networks. IEEE Access. 2017;5:9099-9108.
Crossref - Adams JH, Mueller I. The Biology of Plasmodium vivax. Cold Spring Harb Perspect Med. 2017;7(9):a025585.
Crossref - Chu CS, White NJ. The prevention and treatment of Plasmodium vivax malaria. PLOS Med. 2021;18(4):e1003561.
Crossref - Commons RJ, Thriemer K, Humphreys G, et al. The Vivax Surveyor: Online mapping database for Plasmodium vivax clinical trials. Int J Parasitol Drugs Drug Resist. 2017;7(2):181-190.
Crossref - Foko LPK, Arya A, Sharma A, Singh. Epidemiology and clinical outcomes of severe Plasmodium vivax malaria in India. J Infect. 2021;82(6):231-246.
Crossref - Kumar P, Pisudde P, Sarthi PP. Meteorological linkage of Malaria cases in the eastern state of India. J Clim Chang Health. 2022;5:100064.
Crossref - Umer M, Sadiq S, Ahmad M, Ullah S, Choi GS, Mehmood A. A Novel Stacked CNN for Malarial Parasite Detection in Thin Blood Smear Images. IEEE Access. 2020;8:93782-93792.
Crossref - Bi C, Wang J, Duan Y, Fu B, Kang JR, Shi Y. MobileNet Based Apple Leaf Diseases Identification. Mobile Netw Appl. 2020;27(10):172-180.
Crossref - Abraham J. Plasmodium Detection Using Simple CNN and Clustered GLCM Features, https://arxiv.org/pdf/1909.13101. IEEE Access 2019.
- Mukherjee S, Chatterjee S, Bandyopadhyay O, Biswas A. Detection of Malaria Parasites in Thin Blood Smears Using CNN-Based Approach. In: Mandal, J.K., Mukherjee, I., Bakshi, S., Chatterji, S., Sa, P.K. (eds) Computational Intelligence and Machine Learning. Advances in Intelligent Systems and Computing, 1276. Springer, Singapore.
Crossref - Sharma R, Anirudhi Thanvi, Goyal D, Kumar M, Singh S, Jangir SK. Malarial Parasite Detection by Leveraging Cognitive Algorithms: A Comparative Study. In: Goyal, D., Gupta, A.K., Piuri, V., Ganzha, M., Paprzycki, M. (eds) Proceedings of the Second International Conference on Information Management and Machine Intelligence. Lecture Notes in Networks and Systems, 166. Springer, Singapore.
Crossref - Iwagami M, Keomalaphet S, Khattignavong P, et al. The detection of cryptic Plasmodium infection among villagers in Attapeu province, Lao PDR. PLOS Negl Trop Dis. 2017;11(12):e0006148.
Crossref - Deniz Kavzak Ufuktepe, Yang F, Kassim YM, et al. Deep Learning-Based Cell Detection and Extraction in Thin Blood Smears for Malaria Diagnosis. PubMed Central. 2021.
Crossref - U.S. National Library of Medicine. Home – Clinical Trials 2019. Accessed November 15, 2024. https://clinicaltrials.gov
- Angrisano F, Robinson LJ. Plasmodium vivax – How hidden reservoirs hinder global malaria elimination. Parasitol Int. 2022;87:102526.
Crossref - Yang F, Quizon N, Yu H, et al. Cascading YOLO: automated malaria parasite detection for Plasmodium vivax in thin blood smears. Medical Imaging 2020: Computer-Aided Diagnosis. 2020.
Crossref - Johann Faouzi, Olivier Colliot. Classic Machine Learning Methods. In: Colliot, O. (eds) Machine Learning for Brain Disorders. Neuromethods, vol 197. Humana, New York, NY.
Crossref - Dong F, Zhang Y, Yang J. Attention-based Recurrent Convolutional Neural Network for Automatic Essay Scoring. ACLWeb. 2017:153-162.
Crossref - Patankar S, Sharma S, Rathod PK, Duraisingh MT. Malaria in India: The Need for New Targets for Diagnosis and Detection of Plasmodium vivax. Proteomics Clin Appl. 2018;12(4):1700024.
Crossref - Mahajan P, Uddin S, Hajati F, Moni MA. Ensemble Learning for Disease Prediction: A Review. Healthcare. 2023;11(12):1808-1808.
Crossref - Ramos-Briceno DA, Alessandro Flammia-D’Aleo, Fernandez-Lopez G, Carrion-Nessi FS, Forero-Pena DA. Deep learning-based malaria parasite detection: convolutional neural networks model for accurate species identification of Plasmodium falciparum and Plasmodium vivax. Sci Rep. 2025;15(1):3746.
Crossref - Ncube NB, Tukulula M, Govender KG. Leveraging computational tools to combat malaria: assessment and development of new therapeutics. Journal of Cheminformatics. 2024;16(1):50.
Crossref - White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malaria J. 2011;10:297.
Crossref - Meghna Maiti, Roy U. Space-time clusters and co-occurrence of Plasmodium vivax and Plasmodium falciparum malaria in West Bengal, India. Malaria J. 2024;23(1):189.
Crossref - Swamy Rakesh Adapa, Taylor RA, Wang C, et al. Plasmodium vivax readiness to transmit: implication for malaria eradication. BMC Syst Biol. 2019;13(1):5.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.