ISSN: 0973-7510
E-ISSN: 2581-690X
Infectious gastroenteritis is endemic globally and caused by bacteria, viruses and parasites. The study determined the epidemiological pattern of infectious gastroenteritis within selected urban areas in Malaysia. Analysis of 745 laboratory requests was conducted based on FilmArray gastrointestinal assay and socio-demographic details from suspected cases in KPJ hospitals in Klang Valley, Malaysia, between 2016 to 2020. Descriptive analysis and Fisher-Freeman-Halton Exact testing were performed to ascertain the relationship between socio-demographics with the type of infections. Of 745 requests, 288 (38.7%) were caused by one etiological agent (mono-infection), while the remaining 334 (44.8%) were due to more than one agent (co-infection). Mono-infection was significantly higher among adolescents (n=9; 47.4%) and young adults (n=37; 40.2%) in comparison to adults (n=18; 35.3%). Whereas co-infection was significantly higher in infants (n=216; 49.2%). Mono-infection was mainly caused by bacteria (n=194/288; 67.4%) with Salmonella spp. was prevalent (16.6%), followed by EPEC (12.5%), Campylobacter spp. (11.9%) and Norovirus (10.5%). The co-infections (n=334) were identified with a combination of EPEC and the following bacteria or viruses (Norovirus=38, Campylobacter spp.=30, Salmonella spp.=26, EAEC=22, Rotavirus=19, Adenovirus=10). The month of August to October was recognised as the peak season for infectious gastroenteritis. The present findings may indicate contamination of infectious agents to the food supply and food processing chain as well as reduced hygiene in food sanitation leading to foodborne health issues, particularly among the children in Klang Valley, Malaysia.
Private Healthcare, Gastroenteritis, Film Array Gastrointestinal Assay, Klang Valley, Malaysia
Infectious gastroenteritis is a common disease in children and adults globally caused by enteric pathogens such as bacteria, viruses and parasites.1,2 The typical clinical manifestations are vomiting and watery diarrhoea due to the secretion of enterotoxin, with or without stomach, small and large intestines inflammation.3,4 Infectious gastroenteritis originates from contaminated food, water or person-to-person contact.5,6 Despite firm regulations of food safety and sanitation, the diarrheal disease remains a significant cause of mortality and morbidity in children and adults, mainly from lower-middle-income countries.7
Diarrheal diseases accounted for one in nine child deaths worldwide and became the second leading cause of death among children under five.8 The percentage of deaths caused by diarrhoea in children under five years of age in 2019 was reported to be highest in Nigeria (18%), 10 to 15% in most African countries, 8.0% in Myanmar, 7.5% in Cambodia, 6.5% in India, and 4.8% in Indonesia.7 While in Malaysia, the reported mortality rate due to diarrhoea in children under five years was 0.8% in 2019.7 In contrast, recent data revealed that the positivity rate of diarrheal diseases among children under five was 4.4%.9
Viruses are the primary etiological agents responsible for almost 70% of children’s acute gastroenteritis (AGE) cases.1,10 The common enteric viruses detected globally are Rotavirus, Norovirus, Adenovirus and Sapovirus.1,2,10,11 Rotavirus is the most frequent causative agent of AGE in children less than five years old.12 Meanwhile, Norovirus is a leading etiological agent of infectious gastroenteritis across all age groups globally.2,12,13 Bacteria including Salmonella spp. and Campylobacter1 become the second most common etiological agent of gastroenteritis, with Salmonella spp. being the most common enteric pathogen reported in countries such as Africa,14,15 the USA, Canada16 and Korea.2,6
The etiologic diagnosis of infectious diarrhoea can be challenging because almost all types of enteric pathogens produce similar clinical manifestations, occurrence of multiple infectious agents causing AGE, and the source of contamination, whether from the food, water, environment or animal exposure. Thus, these limit an appropriate and timely epidemiological surveillance report.
Detection of concomitant or multiple enteric pathogens using multiplex molecular assay provides a significant platform to assist the physicians in prescribing pathogen-specific treatment, provision related infection control and public health measures in the event of an outbreak. Hence, this study aimed to determine the epidemiological pattern of infectious gastroenteritis by incorporating the socio-demographic characteristics, etiological agents, type of infections and seasonal peaks based on the private laboratory data within the Klang Valley, Malaysia. The present study’s findings may improve our national surveillance data on infectious gastroenteritis. These will provide insight and aid relevant authorities in formulating the appropriate health policy concerning foodborne illness.
Ethics statement
The ethical approvals were obtained from Universiti Teknologi MARA (UiTM) Research Ethics Committee (REC/654/19) and KPJ Clinical and Research Ethics Review Committee (CRERC/15092020) for a duration of seven years from 2016 until 2022.
Study design and population
A single-centre, retrospective study on identifying the etiological agents causing gastroenteritis from 2016 to 2020 was conducted at the Molecular Diagnostic Laboratory (MDL), KPJ Lablink Central Laboratory, Kuala Lumpur, Malaysia. The MDL provides molecular diagnostic services for infectious diseases for all the KPJ hospitals within the Klang Valley. The data on the etiological agents were retrieved from the FilmArray gastrointestinal panel assay (FAGP) (BioFire Diagnostics-bioMerieux, Italy). The Klang Valley is located in the central of the west coast of Peninsular Malaysia, with an estimated eight million population (Figure 1).17 It is Malaysia’s most urbanised population, a centre for industry and commerce hub. Klang Valley consists of several cities, including Kuala Lumpur, Ampang Jaya, Petaling Jaya, Subang Jaya, Shah Alam, Klang, and Rawang (Figure 1), with seven KPJ hospitals across these cities.
Figure 1. Location of the Klang Valley within the borders of the state of Selangor and the Federal Territory of Kuala Lumpur in the central part of the west coast of Peninsular Malaysia, where the focus area of study for the data catchment area of KPJ hospital. Reprinted from Map of Peninsular Malaysia.17,18
Data collection and definition
A total of 745 laboratory requests for FAGP at KPJ Lablink Medical Laboratory, Kuala Lumpur, were analyzed between January 1st, 2016 to December 31st, 2020. The laboratory requests were from seven KPJ hospitals (Table 1) and the Central Laboratory in Klang Valley, Malaysia (Table 1). Only laboratory requests with complete information of socio-demographic details and result of type of gastrointestinal (GI) infections (mono-infection/co-infection) were included in this study. Mono-infection was defined as gastroenteritis caused by a single agent, virus, bacteria or parasite. While co-infection is characterised by gastroenteritis caused by more than one agent, which involves any combination between virus, bacteria and parasite. The age was categorized as an infant (< 2-year-old), children (2 to 10-year-old), adolescent (11 to 17-year-old), young adult (18 to 40-year-old), adult (41 to 65-year-old), and elderly (> 66-year-old) based on WHO’s classification.19 The laboratory result was grouped into non-infectious gastroenteritis, mono-infection and co-infection accordingly.
Table (1):
The list of KPJ hospitals in Klang Valley with the number of laboratory requests.
KPJ hospitals |
No of Sample (n) |
Distribution (%) |
|---|---|---|
KPJ Ampang Puteri Specialist Hospital |
135 |
18.1 |
KPJ Damansara Specialist Hospital |
162 |
21.7 |
KPJ Kajang Specialist Hospital |
2 |
0.3 |
KPJ Selangor Specialist Hospital |
3 |
0.4 |
KPJ Klang Specialist Hospital |
9 |
1.2 |
KPJ Sentosa KL Specialist Hospital |
1 |
0.1 |
KPJ Rawang Specialist Hospital |
426 |
57.2 |
KPJ Lablink Central Laboratory Kuala Lumpur |
7 |
0.9 |
Total |
745 |
100 |
The FAGP is a multiplex PCR targeted on 22 pathogens commonly associated with gastroenteritis. There are 13 bacterial targets: Campylobacter (C. jejuni, C. coli, and C. upsaliensis), Clostridioides difficile (toxin A/B), Plesiomonas shigelloides, Salmonella spp., Vibrio (V. parahaemolyticus and V. vulnificus), V. cholerae, Yersinia enterocolitica, Enteroaggregative E. coli (EAEC), Enteropathogenic E. coli (EPEC), Enterotoxigenic E. coli (ETEC), Shiga-like toxin-producing E. coli (STEC), E. coli O157, Shigella/Enteroinvasive E. coli (EIEC). There are four parasitic targets: Cryptosporidium spp., Cyclospora cayetanensis, Entamoeba histolytica and Giardia lamblia. In contrast, the viral target pathogens are adenovirus F40/41, astrovirus, norovirus genogroup GI/GII, rotavirus group A, and sapovirus genogroups I, II, IV, and V.
Statistical analysis
Descriptive analysis incorporating the frequency and percentage for the age and gender of the patients with laboratory variables were performed using SPSS Statistics software (SPSS, IBM New York USA; version 21). Statistical differences from the data were assessed using the Fisher-Freeman-Halton Exact Test for categorical variables. A p-value < 0.05 was considered statistically significant. The minimum sample size required to represent the urban area of Klang Valley in the present study is 65, as determined using a formula by Pourhoseingholi et al.20 Therefore, 745 laboratory requests were sufficient to provide significant statistical power.
Gender, age and type of infectious gastroenteritis
Table 2 illustrates the gender, age of the patients and type of infectious gastroenteritis in the Klang Valley, Malaysia, from 2016 to 2020. Majority of the patients were male (n=422; 56.6%), while females accounted for 43.4% (n=323) with the sex ratio male to female of 1:1.3. Infants (n = 439; 58.9 %) were frequently infected in comparison to childhood (n=132; 17.7%) (p<0.05). Both mono and co-infections most commonly occurred in males. Mono-infection was prevalent among adolescents (n=9; 47.4%), young adults (n=37; 40.2%) and adults (n=18; 35.3%) (p<0.05). Conversely, co-infection predominantly occurred in infants (n=216; 49.2%) (p<0.05). The mean age of the studied population was 10.4±17.2 years old, with a median of two years (ranging from one to 82 years).
Table (2):
Distribution of mono-infection and co-infection of infectious gastroenteritis by multiplex RT-PCR assay according to gender, age group and locations of data catchment areas within Klang Valley, Malaysia.
| Gender, age & Distribution Differences, x2 | n (%) | |||
|---|---|---|---|---|
| Mono-infection | Co-infection | Non-infectious gastroenteritis |
Total | |
| Gender | ||||
| Male | 150 (35.5) | 202 (47.9) | 70 (16.6) | 422 (56.6) |
| Female | 138 (42.7) | 132 (40.9) | 53 (16.4) | 323 (43.4) |
| Total | 288 (38.7) | 334 (44.8) | 123 (16.5) | 745 (100) |
| Chi-square: | x2 = 6.3; p>0.05 | |||
| Age Group | ||||
| Infant (<2-year-old) | 162 (36.9) | 216 (49.2) | 61 (13.9) | 439 (58.9) |
| Childhood (2 to 10 y/o) | 59 (44.7) | 59 (44.7) | 14 (10.6) | 132 (17.7) |
| Adolescent (11 to 17 y/o) | 9 (47.4) | 6 (31.6) | 4 (21.1) | 19 (2.6) |
| Young Adult (18 to 40 y/o) | 37 (40.2) | 35 (38.0) | 20 (21.7) | 92 (12.3) |
| Adult (41 to 65 y/o) | 18 (35.3) | 15 (29.4) | 18 (35.3) | 51 (6.8) |
| Elderly (>66-year-old) | 3 (25.0) | 3 (25.0) | 6 (50.0) | 12 (1.6) |
| Mean age ± SD | 10.2 ± 16.6 | 8.1 ± 15.9 | 17.2 ± 22.5 | 10.4 ± 17.2 |
| Median | 2.0 (CL 2-3) | 2.0 (CL 1-4) | 3.0 (CL 1-9) | 2.0 (CL 1-82) |
| Total | 288 (38.7) | 334 (44.8) | 123 (16.5) | 745 (100) |
| Fisher-Freeman-Halton Exact Test: | x2 = 32.2; p>0.05 | |||
| Location | ||||
| APSH | 55 (40.7) | 47 (34.9) | 33 (24.4) | 135 (18.1) |
| DSH | 62 (38.3) | 66 (40.7) | 34 (21.0) | 162 (21.7) |
| KSH | ND | ND | 2 (100) | 2 (0.3) |
| SSH | ND | ND | 3 (100) | 3 (0.4) |
| KLG | 1 (11.1) | 7 (77.8) | 1 (11.1) | 9 (1.2) |
| STS | ND | 1 (100) | ND | 1 (0.1) |
| RSH | 167 (39.2) | 211 (49.5) | 48 (11.3) | 426 (57.2) |
| HQ | 3 (42.9) | 2 (28.6) | 2 (28.6) | 7 (0.9) |
| Total | 288 (38.7) | 334 (44.8) | 123 (16.5) | 745 (100) |
| Fisher-Freeman-Halton Exact Test: | x2 = 44.9; p<0.001 | |||
Abbreviations: GI, Gastrointestinal; y/o, year-old; ND, Not Detected; APSH, KPJ Ampang Puteri Specialist Hospital; DSH, KPJ Damansara Specialist Hospital; KSH, KPJ Kajang Specialist Hospital; SSH, KPJ Selangor Specialist Hospital; KLG, KPJ Klang Specialist Hospital; STS, KPJ Sentosa Specialist Hospital; RSH, KPJ Rawang Specialist Hospital; HQ, KPJ Lablink Headquarters.
The rate of co-infection (n=334; 44.8%) was higher than mono-infection (n=288; 38.7%) (p<0.05) (Table 2). A majority (n=426; 57.2%) of patients were from KPJ Rawang Specialist Hospital, followed by KPJ Damansara Specialist Hospital (n=162; 21.7%) and KPJ Ampang Puteri Specialist Hospital (n=135; 18.1%). Mono-infection was recorded the most in KPJ Ampang Puteri Specialist Hospital (n=55; 40.7%) (p<0.001), with co-infection was highest in KPJ Rawang Specialist Hospital (n=211; 49.5%) (p<0.001).
Distribution of enteric pathogens
Table 3 summarises the distribution of pathogens according to the types of infection, gender, age groups and location of the private hospitals. Bacteria (n=194/288) was the main pathogen for mono-infection across the gender and age groups (p<0.001). In comparison, there was no data on parasites causing infectious gastroenteritis. In co-infection, a combined infection between bacteria and virus was prevalent (n=196/334), followed by co-infection with two different bacteria (n=112/334) (p<0.001). Minimal detection rates for other combinations involving bacteria (n=26/334) and parasites (n=12/334), two different viruses (n=10/334), and multiple co-infection between bacteria, parasites and viruses (n=4/334) were also recorded.
Table (3):
Distribution of enteric pathogens identified in mono and co-infection of infectious gastroenteritis by multiplex RT-PCR assay according to the gender, age and location of KPJ hospitals within Klang Valley, Malaysia, from 2016 to 2020.
| Gender, age & Distribution Differences, x2 |
Mono-infection, n (%) | Co-infection, n (%) | Non-GI infection, n (%) | Total, n (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bac. | Vir. | Par. | Bac. & Bac. |
Bac. & Vir. |
Vir. & Vir. |
Bac. & Par. |
Vir.& Par. | Par. & Par. | Bac. & Par. & Vir. | |||
| Gender | ||||||||||||
| Male | 97 (23.0) | 53 (12.6) | ND | 62 (14.7) | 126 (29.9) | 6 (1.4) | 4 (0.9) | ND | ND | 4 (0.9) | 70 (16.6) | 422 (56.6) |
| Female | 97 (30.0) | 41 (12.7) | ND | 50 (15.5) | 70 (21.7) | 4 (1.2) | 8 (2.5) | ND | ND | ND | 53 (16.4) | 323 (43.3) |
| Total | 194 (26.0) | 94 (12.6) | ND | 112 (15.0) | 196 (26.3) | 10 (1.3) | 12 (1.6) | ND | ND | 4 (0.5) | 123 (16.5) | 745 (100) |
| Chi-square: x2 = 13.9; p>0.05 | ||||||||||||
| Age Group | ||||||||||||
| Infant | 102 (23.2) | 60 (13.7) | ND | 66 (15.0) | 137 (31.2) | 8 (1.9) | 4 (0.9) | ND | ND | 1 (0.2) | 61 (13.9) | 439 (58.9) |
| Childhood | 39 (29.5) | 20 (15.1) | ND | 16 (12.1) | 40 (30.4) | 1 (0.8) | 2 (1.5) | ND | ND | ND | 14 (10.6) | 132 (17.8) |
| Adolescent | 7 (36.8) | 2 (10.5) | ND | 3 (15.8) | 2 (10.5) | ND | 1 (5.3) | ND | ND | ND | 4 (21.1) | 19 (2.6) |
| Young Adult | 28 (30.4) | 9 (9.8) | MD | 15 (16.3) | 13 (14.1) | 1 (1.1) | 4 (4.3) | ND | ND | 2 (2.2) | 20 (21.8) | 92 (12.3) |
| Adult | 15 (29.4) | 3 (5.9) | ND | 10 (19.5) | 3 (5.9) | ND | 1 (2.0) | ND | ND | 1 (2.0) | 18 (35.3) | 51 (6.8) |
| Elderly | 3 (25.0) | ND | ND | 2 (16.7) | 1 (8.3) | ND | ND | ND | ND | ND | 6 (50.0) | 12 (1.6) |
| Mean age ± SD | 11.9 ± 18.2 | 6.8 ± 12.3 | ND | 11.9 ± 18.3 | 5.0 ± 10.5 | 5.1 ± 11.9 | 19.1 ± 19.9 | ND | ND | 21.2 ± 23.4 | 17.2 ± 22.5 | 10.4 ± 17.2 |
| Median | 2.0 | 2.0 | ND | 2.0 | 1.0 | 1.0 | 10.0 | ND | ND | 17.0 | 3.0 | 2.0 |
| Total | 194 (26.0) | 94 (12.6) | ND | 112 (15.0) | 196 (26.3) | 10 (1.3) | 12 (1.6) | ND | ND | 4 (0.5) | 123 (16.5) | 745 (100) |
| Fisher-Freeman-Halton Exact Test: x2 = 73.5; p<0.001 | ||||||||||||
| Locations | ||||||||||||
| APSH | 34 (25.1) | 21 (15.6) | ND | 21 (15.6) | 22 (16.3) | ND | 2 (1.5) | ND | ND | 2 (1.5) | 33 (24.4) | 135 (18.1) |
| DSH | 44 (27.2) | 18 (11.1) | ND | 24 (14.8) | 35 (21.6) | 2 (1.2) | 5 (3.1) | ND | ND | ND | 34 (21.0) | 162 (21.7) |
| KSH | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2 (100) | 2 (0.3) |
| SSH | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3 (100) | 3 (0.4) |
| KLG | 1 (11.1) | ND | ND | 2 (22.2) | 4 (44.4) | ND | ND | ND | ND | 1 (11.1) | 1 (11.1) | 9 (1.2) |
| STS | ND | ND | ND | ND | 1 (100) | ND | ND | ND | ND | ND | ND | 1 (0.1) |
| RSH | 113 (26.5) | 54 (12.7) | ND | 64 (15.0) | 134 (31.5) | 8 (1.9) | 4 (0.9) | ND | ND | 1 (0.2) | 48 (11.3) | 426 (57.2) |
| HQ | 2 (28.6) | 1 (14.3) | ND | 1 (14.3) | ND | ND | 1 (14.3) | ND | ND | ND | 2 (28.6) | 7 (0.9) |
| Total | 194 (26.0) | 94 (12.6) | ND | 112 (15.0) | 196 (26.3) | 10 (1.3) | 12 (1.6) | ND | ND | 4 (0.5) | 123 (16.5) | 745 (100) |
| Fisher-Freeman-Halton Exact Test: x2 = 92.6; p<0.001 | ||||||||||||
Abbreviations: GI, Gastrointestinal; Bac., Bacteria; Vir., Virus; Par., Parasites; y/o, year-old; ND, Not Detected; Infant (<2-year-old); Childhood (2 to 10 y/o); Adolescent (11 to 17 y/o); Young Adult (18 to 40 y/o); Adult (41 to 65 y/o); Elderly (>66-year-old); APSH, KPJ Ampang Puteri Specialist Hospital; DSH, KPJ Damansara Specialist Hospital; KSH, KPJ Kajang Specialist Hospital; SSH, KPJ Selangor Specialist Hospital; KLG, KPJ Klang Specialist Hospital; STS, KPJ Sentosa Specialist Hospital; RSH, KPJ Rawang Specialist Hospital; HQ, KPJ Lablink Headquarters.
Table 4 shows the positivity rates of all the pathogens detected in the infectious gastroenteritis. Among the 22 common enteric pathogens, Salmonella spp. (n=103/622; 16.6%) was the most prevalent pathogen detected, followed by EPEC (n=78/622; 12.5%), Campylobacter (n=74/622; 11.9%) and Norovirus GI/GII (n=65; 10.5%). These pathogens contributed 51.5% of the detected pathogens in KPJ hospitals within Klang Valley, Malaysia, from 2016 until 2020 (Table 4). While other enteric pathogens including C. difficile toxin A/B, Rotavirus A, EAEC, Sapovirus, Astrovirus, P. shigelloides, ETEC, Adenovirus F40/41, Vibrio, STEC, Shigella/ EIEC, V. cholerae, Y. enterocolitica, E. Coli O157, Cryptosporidium, C. cayetanensis and G. lamblia represented only less than 10% (0.3 to 8.5%) of prevalence rate. There were no E. histolytica recorded in the present study (Table 4).
Table (4):
Distribution of enteric pathogens causing mono and co-infection of infectious gastroenteritis detected by multiplex RT-PCR assay in KPJ hospitals within Klang Valley, Malaysia, from 2016 until 2020.
| Pathogens | Mono-infection, n (%) |
Co-infection, n (%) |
Total, n (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bac. | Vir. | Par. | Bac. & Bac. |
Bac. & Vir. |
Vir. & Vir. |
Bac. & Par. |
Vir. & Par. |
Par. & Par. |
Bac. & Par. & Vir. | ||
| Bacteria | |||||||||||
| Campylobacter | 38 (19.6) | ND | ND | 17 (15.2) | 18 (9.2) | ND | 1 (8.3) | ND | ND | ND | 74 (11.9) |
| C. difficile toxin A/B | 23 (11.9) | ND | ND | 12 (10.7) | 17 (8.7) | ND | 1 (8.3) | ND | ND | ND | 53 (8.5) |
| P. shigelloides | 2 (1.0) | ND | ND | 9 (8.0) | 10 (5.1) | ND | 1 (8.3) | ND | ND | ND | 22 (3.5) |
| Salmonella spp. | 73 (37.6) | ND | ND | 14 (12.5) | 15 (7.7) | ND | 1 (8.3) | ND | ND | ND | 103 (16.6) |
| Vibrio | 2 (1.0) | ND | ND | 4 (3.6) | 3 (1.5) | ND | ND | ND | ND | 1 (25.0) | 10 (1.6) |
| V. cholerae | ND | ND | ND | 4 (3.6) | 1 (0.5) | ND | ND | ND | ND | 1 (25.0) | 6 (0.9) |
| Y. enterocolitica | ND | ND | ND | 1 (0.9) | 1 (0.5) | ND | ND | ND | ND | ND | 2 (0.3) |
| EAEC | 10 (5.2) | ND | ND | 16 (14.3) | 19 (9.7) | ND | 1 (8.3) | ND | ND | ND | 46 (7.4) |
| EPEC | 40 (20.6) | ND | ND | 18 (16.1) | 20 (10.2) | ND | ND | ND | ND | ND | 78 (12.5) |
| ETEC | 3 (1.5) | ND | ND | 9 (8.0) | 9 (4.6) | ND | ND | ND | ND | ND | 21 (3.4) |
| STEC | 2 (1.0) | ND | ND | 4 (3.6) | 2 (0.1) | ND | ND | ND | ND | ND | 8 (1.3) |
| E. Coli O157 | ND) | ND | ND | 1 (0.9) | 1 (0.5) | ND | ND | ND | ND | ND | 2 (0.2) |
| Shigella/ EIEC | 1 (0.5) | ND | ND | 3 (2.7) | 3 (1.5) | ND | 1 (8.3) | ND | ND | ND | 8 (1.3) |
| Parasite | |||||||||||
| Cryptosporidium | ND | ND | ND | ND | ND | ND | 2 (16.7) | ND | ND | ND | 2 (0.3) |
| C. cayetanensis | ND | ND | ND | ND | ND | ND | 2 (16.7) | ND | ND | ND | 2 (0.3) |
| E. histolytica | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| G. lamblia | ND | ND | ND | ND | ND | ND | 2 (16.7) | ND | ND | ND | 2 (0.3) |
| Virus | |||||||||||
| Adenovirus F40/41 | ND | 6 (6.4) | ND | ND | 11 (5.6) | ND | ND | ND | ND | ND | 17 (2.7) |
| Astrovirus | ND | 10 (10.6) | ND | ND | 14 (7.1) | ND | ND | ND | ND | ND | 24 (3.9) |
| Norovirus GI/GII | ND | 41 (43.6) | ND | ND | 20 (10.2) | 4 (40.0) | ND | ND | ND | ND | 65 (10.5) |
| Rotavirus A | ND | 30 (31.9) | ND | ND | 17 (8.7) | 3 (30.0) | ND | ND | ND | ND | 50 (8.0) |
| Sapovirus | ND | 7 (7.4) | ND | ND | 15 (7.7) | 3 (30.0) | ND | ND | ND | 2 (50.0) | 27 (4.3) |
| Total | 194 (100) | 94 (100) | ND | 112 (100) | 196 (100) | 10 (100) | 12 (100) | ND | ND | 4 (100) | 622 (100) |
Abbreviations: GI, Gastrointestinal; Bac., Bacteria; Vir., Virus; Par., Parasites; ND, Not Detected; EAEC, Enteroaggregative E. coli; EPEC, Enteropathogenic E. coli; ETEC, Enterotoxigenic E. coli; STEC, Shiga-like toxin-producing E. coli (STEC) stx1/stx2; EIEC, Enteroinvasive E. Coli. The total number of samples subjected to multiplex RT-PCR assay (N) is 745. The total number of confirmed cases of either single GI or co-GI infections is 622.
A total of 334 different co-infection combinations were detected from 622 confirmed cases, as shown in Table 4. The most common combinations were EPEC with virus and EPEC with bacteria (Norovirus GI/ GII, n=38; Campylobacter, n=30; Salmonella spp., n=26; EAEC, n=22; Rotavirus A, n=19; Adenovirus F 40/41, n=10), as well as EAEC in association with Norovirus GI/GII (n=15) (Table 5).
Table (5):
Distribution of co-infection in infectious gastroenteritis consisting of 334 different combinations detected by multiplex RT-PCR assay in KPJ hospitals within Klang Valley, Malaysia, from 2016 to 2020.
Pathogen combinations |
n |
|---|---|
EPEC & Norovirus GI/ GII |
38 |
Campylobacter & EPEC |
30 |
EPEC & Salmonella spp. |
26 |
EAEC & EPEC |
22 |
EPEC & Rotavirus A |
19 |
EAEC & Norovirus GI/GII |
15 |
Adenovirus F 40/41 & EPEC |
10 |
Astrovirus & EPEC |
9 |
C. difficile toxin A/B & EPEC |
9 |
EPEC & Sapovirus |
8 |
C. difficile toxin A/B & Norovirus GI/GII |
7 |
Campylobacter & Rotavirus A |
7 |
Campylobacter & EAEC |
6 |
Campylobacter & C. difficile toxin A/B |
6 |
Campylobacter & Norovirus GI/GII Positive |
6 |
EPEC & P. shigelloides |
6 |
Norovirus GI/GII & Salmonella spp. |
6 |
Norovirus GI/ GII & Rotavirus A |
5 |
P. shigelloides & Norovirus GI/GII |
5 |
Adenovirus F 40/41 & Norovirus GI/GII |
4 |
Campylobacter & Astrovirus |
4 |
Campylobacter & P. shigelloides |
4 |
Campylobacter & Salmonella spp. |
4 |
Campylobacter & Sapovirus |
4 |
P. shigelloides & Salmonella spp. |
4 |
Less frequent co-infection (35 different combinations) with number of cases either three or less |
70 |
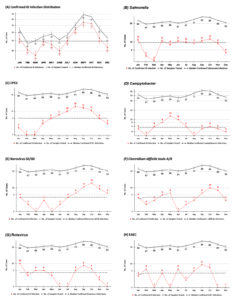
Figure 2. Monthly distribution of infectious gastroenteritis and the most prevalent pathogens detected in 622 confirmed cases by multiplex RT-PCR assay for five years from 2016 until 2020. A: total confirmed infectious gastroenteritis in the study period; B: Salmonella spp.; C: EPEC; D: Campylobacter; E: Norovirus GI/GII; F: C. difficile toxin A/B; G: Rotavirus; H: EAEC
Seasonality of the enteric pathogens
The rate of confirmed infectious gastroenteritis appeared lowest in the year 2016 (n=19/28; 67.9%) and 2020 (n=45/65; 70.3%) as compared to 2017 (n=127/148; 85.8%), 2018 (n=178/210; 84.8%) and 2019 (n=245/295; 85.8), however, the trend was not statistically significant (p>0.05). Figure 2 shows a monthly distribution of confirmed infectious gastroenteritis (Figure 2A) and the most common pathogens recorded (Figure 2B to 2H) for five consecutive years. The peak season for confirmed infectious gastroenteritis was from August until October throughout the five years (2016 to 2020) (Figure 2A). The Salmonella spp. was continuously detected throughout the year with three seasonal peaks: Jan, April, June and October (Figure 2B). The EPEC demonstrated a peak season from May until November (Figure 2C). While the detection rate of Campylobacter spp. was increased above the median positive rate between March and July (Figure 2D). The detection rate for Norovirus GI/GII was raised between July and December (Figure 2E). While C. difficile toxin A/B displayed two peak seasons: April and May, September and November (Figure 2F). Rotavirus had three peak seasons: January, March and April, and August until October (Figure 2G). In contrast, EAEC was detected highest in February, June, August and October (Figure 2H).
We report the five years of data on the epidemiology of infectious gastroenteritis from 745 cases across all age groups involving stool testing using a multiplex molecular array. The multiplex molecular array such as FAGP assists physicians in differentiating bacteria, viruses and parasites as responsible etiological agents. Despite high sensitivity, multiple molecular arrays might detect nucleic acids from non-viable microorganisms unrelated to infectious diarrhoea. However, it provides a prompt diagnosis by detecting 13 bacteria, four parasites and five different viruses simultaneously. Thus, enabling precise patient treatment and reducing the need of antibiotic prescription and health burden due to unnecessary hospitalisation.
In the studied populations, 83.5% (mono-infection, n=288/745; co-infection, n=334/745) of the samples were positive, which was higher than the previously reported study in Malaysia (4.4%)9 and a recent study in Italy with only 56.2%.10 The higher positive rate could be attributed to the utilisation of multiplex molecular arrays from 2016 to 2020 in KPJ hospitals within the Klang Valley, Malaysia. It was noted that there were increased of laboratory requests for FAGP from KPJ Rawang Specialist Hospital, Selangor, owing to the escalation of suspected infectious gastroenteritis cases in Rawang, Selangor, and the availability of the test offered by the laboratory which reflects the advantages of the multiplex molecular detection of enteric pathogens and strain identification. Rawang is a semi-urban area within Klang Valley, with the main economy contributed by agriculture and industrial activities. The intensification of infectious gastroenteritis cases could be attributed to the contamination of infectious agents into the water and food supply and reduced hygiene and sanitation. However, these could not be confirmed as there is no specific epidemiological study on infectious gastroenteritis has been carried out in Rawang.
A higher prevalence of mono-infection in males and among adolescents, young adults and adult patients was observed in contrast to females and infants. The higher rates among adolescents and adults were contributed by the bacteria such as Salmonella spp., EPEC and Campylobacter spp. This probably owing to that Salmonella spp. and Campylobacter spp. were often associated with travelling.20,21 Whereas EPEC has been associated with contaminated food, water, or person-to-person contact.5,6 The median age of the studied population is two years old, which supports infectious gastroenteritis commonly occurring in children below five years of age.8 Besides that, the co-infection cases were significantly higher than mono-infection in the present study, particularly among the infant age group. This could be due to children frequently being exposed to various environmental pathogens at home, daycare, pets, and playgrounds. Thus, children are at risk of acquiring multiple pathogens causing gastroenteritis from an environment and animals, which could cause more severe symptoms.5,22-25
The ability of multiple molecular arrays to distinguish enteric pathogens in positive samples provides insightful and improved epidemiological data for infectious gastroenteritis. Our analysis found that mono-infection is caused mainly by bacteria (67.4%; n=194/288) with Salmonella spp., EPEC and Campylobacter. Our findings are consistent with other previously reported studies in Africa,14,15 the USA and Canada,16 Korea,2,6 Malaysia,26 and Italy.10
Salmonella spp., Campylobacter and Norovirus have been known as common enteric pathogens detected in Africa,14,15 the USA and Canada,16 and Korea.2,6 Interestingly, we observed that there is an emergence of EPEC as one of the prevalent enteric pathogens. For EPEC, an inference is difficult to make as several studies reported an unclear aetiology role of EPEC in infectious diarrhoea.10,27 Recently, EPEC was associated with environmental enteric dysfunction (EED) and affected child growth, thus highlighting the need to formulate a vaccine to reduce the burden and combat childhood malnutrition.27 Furthermore, the National Health and Morbidity Survey 2016 conducted by the Ministry of Health Malaysia on 15,188 children below five years old found that the risk of diarrhoea was higher in children who consumed untreated water and associated with less privileged socio-demographic.9 Nevertheless, our study detected the emergence of EPEC in Malaysia, particularly within the urban area, which notified its clinical importance in Malaysia and required further investigation.
We noted that mixed infection between bacteria and virus is prevalent, with 334 co-infection combinations observed. Our report on the frequent mixed infections between viruses and bacteria was in tandem with other epidemiological reports.10,26,28 Distinctly, we found that the prevalence of co-infection is between EPEC with 38 Norovirus, 30 Campylobacter, 26 Salmonella spp., 22 EAEC, 19 Rotavirus and ten Adenovirus strains (Table 4). Thus, the co-infection between EPEC with several viruses and bacteria species highlights the emergence of EPEC as one of the prevalent enteric pathogens in Malaysia. Hence, detailed epidemiological studies should be performed, and public health measures should be revised as EPEC may cause severe gastroenteritis with a substantial hospitalization rate in the near future.
Enteropathogenic E.coli (EPEC) has been identified as a common pathogen for infectious gastroenteritis in children besides rotavirus.25,29 A comparative study carried out by Ayedun et al.29 revealed that the prevalence of EPEC infection among children in Nigeria, Korea, Sweden and Tanzania was correlated with the risk of limited access to clean water, poor hygiene and sanitation. Thus, there is likelihood for EPEC to spread and contaminate the food supply and food processing chain.30,31 Furthermore, the prevalence of EPEC has been associated with developing nations, as reviewed by Alharbi and colleagues.30 Among the reported possible reasons for high EPEC infection in developing nations such as Malaysia are the consumption of insufficient pasteurized milk, direct contact with animal manure and poor handwashing practice in day care or school for children, exposure to the contaminated swimming pools or fecally contaminated rivers or lake.30
Our time series analysis over the five years detected that the peak season for infectious gastroenteritis was between August until October (Figure 2A). The rise of confirmed infectious gastroenteritis during the peak season was contributed by the higher detection rate of Salmonella spp. (Figure 2B), EPEC (Figure 2C), Norovirus (Figure 2E), Rotavirus (Figure 2G) and EAEC (Figure 2H). The rate of confirmed infectious gastroenteritis increased from 2016 until 2019, showing higher utilisation of multiplex molecular assay in detecting enteric pathogens in KPJ hospitals. Conversely, as observed in Italy, lower testing and detection rates were observed in 2020 due to the reduced referral cases and hospitalisation during the SARS-CoV-2 pandemic.10 It would be tempting to speculate that movement restriction order and social distancing modulated the circulation of enteric pathogens. Therefore, it would be interesting to determine the circulation pattern of the enteric pathogens from 2021 onwards.
Each of the prevalent enteric pathogens reported here has shown a specific seasonality trend where Salmonella spp. was continuously detected throughout the year, EPEC (May until November), Campylobacter spp. (March until July), Norovirus GI/GII (July until December), C. difficile toxin A/B and Rotavirus showed cyclical patterns, respectively. C. difficile toxin A/B had two seasons (April and May, September until November), whereas Rotavirus had three seasons (January, March and April, August until October). Whereas EAEC has shown more dispersal peak seasons in February, June, August and October. Overall, within the peak season of infectious gastroenteritis between August until October over the five years, the highest positive cases were observed in September and October. Salmonella spp., EPEC, Norovirus GI/GII, C. difficile toxin A/B, Rotavirus and EAEC were predominant during these two months.
The highest occurrence of infectious gastroenteritis in the tropical region is reportedly occur during rainy season, while in temperate regions the highest cases were observed during winter.30 There is a possibility to have a correlation between the rainy season in September and October with the peak season of positive infectious gastroenteritis cases in Klang Valley, as gathered in the present study. However, the correlation analysis between the rising cases and rainfall could not be confirmed due to the unavailability of meteorology data between the periods, which is worth exploring. Nevertheless, the spatio-temporal of infectious gastroenteritis in Klang Valley, Malaysia, over five years from 2016 to 2020 has been established.
The present study describes temporal circulating enteric pathogens causing gastroenteritis in Klang Valley, representing the infectious diarrhoea health burden in the Malaysian urban community. The reported predominant Salmonella spp. and the emergence of EPEC emphasize Malaysia’s foodborne health issue, particularly among children.
ACKNOWLEDGMENTS
The authors would like to thank Lablink (M) Sdn. Bhd., KPJ Healthcare Berhad and Universiti Teknologi MARA (UiTM), Malaysia, for their support in data sharing and facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by the Ministry of Higher Education, Malaysia (RACER/1/2019/STG05/UITM//4).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The ethical approvals were obtained from Universiti Teknologi MARA (UiTM) Research Ethics Committee (REC/654/19) and KPJ Clinical and Research Ethics Review Committee (CRERC/15092020).
- Harb A, Abraham S, Rusdi B, Laird T, O’Dea M, Habib I. Molecular detection and epidemiological features of selected bacterial, viral, and parasitic enteropathogens in stool specimens from children with acute diarrhea in Thi-Qar Governorate, Iraq. Int J Environ Res Public Health. 2019;16(9):1573.
Crossref - Sung J, Cheong HK, Kwon HJ, Kim JH. Pathogen-specific response of infectious gastroenteritis to ambient temperature: National surveillance data in the Republic of Korea, 2015-2019. Int J Hyg Environ Health. 2022;240:113924.
Crossref - Hodges K, Gill R. Infectious diarrhea: cellular and molecular mechanisms. Gut Microbes. 2010;1(1):4-21.
Crossref - Gastroenteritis Frequently Asked Question. https://www.cdc.gov/nceh/vsp/pub/faq/faq.htm, Accessed April 29, 2022.
- Hawash YA, Ismail KA, Almehmadi M. High frequency of enteric protozoan, viral, and bacterial potential pathogens in community-acquired acute diarrheal episodes: Evidence based on results of luminex gastrointestinal pathogen panel assay. Korean J Parasitol. 2017;55(5):513.
Crossref - Yang JJ, Lee K. Epidemiologic changes in over 10 years of community-acquired bacterial enteritis in children.Pediatr Gastroenterol Hepatol Nutr. 2022;25(1):41.
Crossref - Diarrhoea. https://data.unicef.org/topic/child-health/diarrhoeal-disease. Accessed April 29, 2022
- Global Diarrhea. CDC 2022. https://www.cdc.gov/healthywater/pdf/global/programs/globaldiarrhea508c.pdf. Accessed April 29, 2022
- Aziz FA, Ahmad NA, Razak MA, et al. Prevalence of and factors associated with diarrhoeal diseases among children under five in Malaysia: a cross-sectional study 2016. BMC Public Health. 2018;18(1):1-8.
Crossref - De Conto F, Di Stefano S, Buttrini M, et al. Detection of potential enteric pathogens in children with severe acute gastroenteritis using the filmarray: Results from a three-years hospital-based survey in Northern Italy. Diagn Microbiol Infect Dis. 2022;102(3):115611.
Crossref - Banyai K, Estes MK, Martella V, Parashar UD. Viral gastroenteritis. The Lancet. 2018;392(10142):175-186.
Crossref - Farahmand M, Moghoofei M, Dorost A, et al. Global prevalence and genotype distribution of norovirus infection in children with gastroenteritis: A meta-analysis on 6 years of research from 2015 to 2020. Rev Med Virol. 2022;32(1):e2237.
Crossref - Fang Y, Zhang Y, Wang H, et al. Molecular epidemiology of norovirus infections in children with acute gastroenteritis in 2017-2019 in Tianjin, China. J Med Virol. 2022;94(2):616-24.
Crossref - Smith SI, Seriki A, Ajayi A. Typhoidal and non-typhoidal Salmonella infections in Africa. Eur J Clin Microbiol Infect Dis. 2016;35(12):1913-22.
Crossref - Gemechu T, Eshetu T, Kassa T, Jarso H. Assessment of Intestinal Parasites, Enteric Bacterial Infections, and Antimicrobial Susceptibility among Street Food Handlers in Jimma Town, Southwest Ethiopia. J Trop Med. 2022.
Crossref - Harrington SM, Buchan BW, Doern C, et al. Multicenter evaluation of the BD max enteric bacterial panel PCR assay for rapid detection of Salmonella spp., Shigella spp., Campylobacter spp.(C. jejuni and C. coli), and Shiga toxin 1 and 2 genes. J Clin Microbiol. 2015;53(5):1639-1647.
Crossref - Department of Statistics Malaysia. Demographic Statistics Second Quarter 2021, Malaysia. https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=430&bul_id=eGtwdjd4amZJb1JmcFFkYXBKNHg3d
z09&menu_id=L0pheU43NWJwRWVSZklWdzQ4TlhUUT09. Accessed April 29, 2022 - Map of Peninsular Malaysia. https://commons.wikimedia.org/wiki/File:Map_PeninsularMalaysia.png, Accessed April 29, 2022.
- World Health Organization (WHO). Definition of key term, 2013. https://www.who.int/hiv/pub/guidelines/arv2013/intro/keyterms/en/ (Updated on June, 2013). Accessed April 29, 2022
- Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14-17. Accessed April 29, 2022
- Centre for Disease Control and Prevention (CDC). Foodborne Germs and Illnesses: Causes of Food Poisoning. 2020. https://www.cdc.gov/foodsafety/foodborne-germs.html. Accessed November 1, 2022.
- Lakhan C, Badrie N, Ramsubhag A, Indar L. Detection of Foodborne Pathogens in Acute Gastroenteritis Patient’s Stool Samples Using the BioFire® FilmArray® Gastrointestinal PCR Panel in the Republic of Trinidad and Tobago, West Indies. Microorganisms. 2022;10(8):1601.
Crossref - Tang YW, Persing DH. Advances in the clinical microbiology of enteric infections. Infections of the Gastrointestinal Tract. Lippincott Williams & Wilkins, Philadelphia, USA. 2002:1185-97. Accessed April 29, 2022
- Akihara S, Phan TG, Nguyen TA, Hansman G, Okitsu S, Ushijima H. Existence of multiple outbreaks of viral gastroenteritis among infants in a day care center in Japan. Arch Virol. 2005;150(10):2061-75.
Crossref - Zheng S, Yu F, Chen X, et al. Enteropathogens in children less than 5 years of age with acute diarrhea: a 5-year surveillance study in the Southeast Coast of China. BMC Infect Dis. 2016;16(1):1-8.
Crossref - Lee WS, Rajasekaran G, Pee S, Karunakaran R, Hassan HH, Puthucheary SD. Rotavirus and other enteropathogens in childhood acute diarrhoea: a study of two centres in Malaysia. J Paediatr Child Health. 2006;42(9):509-514.
Crossref - Beczkiewicz A, Cebelinski E, Decuir M, et al. High relative frequency of Enteroaggregative Escherichia coli among patients with reportable enteric pathogens, Minnesota, 2016-2017. Clin Infect Dis. 2019;69(3):473-479.
Crossref - Park S, Hitchcock MM, Gomez CA, Banaei N. Is follow-up testing with the FilmArray gastrointestinal multiplex PCR panel necessary? J Clin Microbiol. 2017;55(4):1154-1161.
Crossref - Ayedun JS, Buhari OA, Boyejo AO, Makanjuola SO, Azeez IA, Owolabi SL. Prevalence of Enteropathogenic Escherichia coli and Rotavirus Antigen among Diarrheic Children Attending Selected Hospitals in Abeokuta, Nigeria: A Comparative Study. J Adv Biol Biotech. 2022;19:39-45.
Crossref - Alharbi MG, Al-Hindi RR, Esmael A, et al. The “Big Six”: Hidden Emerging Foodborne Bacterial Pathogens. Trop Med Infect Dis. 2022;7(11):356.
Crossref - Hasan H, Nasirudeen NA, Ruzlan MA, et al. Acute Infectious Gastroenteritis: The Causative Agents, Omics-Based Detection of Antigens and Novel Biomarkers. Children. 2021;8(12):1112.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.