ISSN: 0973-7510
E-ISSN: 2581-690X
Natural products and their derivatives have traditionally been used as a source of therapeutic agents. Their beneficial properties are due to large varieties in their chemical structures and biochemical actions. The discovery of natural products such as phytoconstituents have crucial role in the development of less toxic and more effective drugs. Phytoconstituents have shown to be beneficial in treating viral diseases such as the previous chikungunya virus, hepatitis C virus, SARS, and MERS viral diseases. Flavonoids, alkaloids, terpenoids, and other group of compounds combat against COVID-19 in several ways like by protease inhibition, spike protein inhibition, Nrf2 inhibition. The accumulation of NRF2 inhibits the development of the SARS-CoV-2 virus and stimulates anti-inflammatory action. The present review highlights the therapeutic importance of compounds isolated from medicinal plants and/or herbs, such as crude extracts of Curcumin I-III, Leptodactylone, Ginsenoside-Rb1, Lycorine, Reserpine, Saikosaponin B2, Cepharanthine, Withanoside V, Gingerol, Piperanine, chromans, flavonoids, Amentoflavone etc. against SARS-CoV-2. Natural products are typically safe, stable, and dependable source for finding drugs to control the current pandemic. Antiviral secondary metabolites many medicinal plants have given ingredients that were isolated. The selected plants based phytoconstituents may potentially be used against viruses’ development on anti-SARS-CoV-2 to offer a reference point in this field.
COVID-19, SARS-CoV-2, Natural Products, Alkaloids, Flavonoids, Target Proteins, Pharmacological Activity
The novel coronavirus (SARS-CoV-2 virus) is the main issue of 2nd decade of 21st century in due to its rapid spread across the globe and attacking features. The disease COVID-19 dispatched by contact with infectious, inhalation and incubation duration vary from 2 to 14 days.1-3 SARS-CoV-2 virus is like previous SARS-CoV and MERS (not pandemic) virus with genome modification and WHO declared the COVID-19 as a pandemic on 11 March 2020.4,5 It is noted that viral diseases such as Hepatitis B virus, hepatitis C virus, Zika virus, Ebola virus, malaria, HIV, SARS, and MERS virus have often survived and addressed serious public health challenges in previous days. Although, SARS-CoV-2 virus damages the respiratory system6 but increased rate is with pediatric and adult with cardiovascular diseases7 and diabetes.8 The novel virus represents a global warming and poses a new provocation where vaccine is required for primary treatment and synthetic compound to treat infected patients.9,10 Vaccine (biological preparation) and immunotherapy which boost the body immune system against pathogens and treat various diseases. It has been the most efficient medical method in immunology to minimize death and morbidity of the previous century.11 According to the researchers, synthetic antigens are susceptible to evoke anti-protein immune feedback.12 Macromolecules may contain a massive and variety of antigenic sites but just a specific number of possible antigenic sites are significant.13 The coronavirus family contain huge number of spike proteins which are used as mediator to entry into the epithelial cell in host whereas, the ACE2 enzyme in the human body is the recognition site for spike protein; authorized for entry by this virus into the circulation system of the human being. On the other hand, RNA virus (coronavirus is an RNA virus) manifest RNA polymerase in epithelial cell of human body and approaches new genome sequences or daughter genome sequence using viral RNA template.14 The current study shows phytoconstituents are capable to treat viral diseases 15 such as previous chikungunya virus, hepatitis C virus, SARS and MERS viral diseases and showed effective positive result.16-19 and various guidelines were issued to treat and prevent COVID-19 using herbal medicine in different stages.20 There are around 4000 phytochemicals where more than 150 phytochemicals are studied in detail. From these phytochemicals flavonoids, alkaloids, terpenoids, and miscellaneous compounds are found in most of the plants/herbs which prevent the microbial, fungal and viral infection.21-28 Flavonoids have been depicted to plummet various coronavirus diseases by blocking function of protease and helicase enzyme or interacting with spike protein and suppressing the function of ACE2.29,30 In addition, NRF2 is a transcription factors which conjugate with antioxidant response elements to facilitate factor of transcription in target gene to repair the macromolecular damage and maintain redox homeostasis31,32 as well as decrease the inflammation33 which present in the cytoplasm. Accumulation of NRF2 can inhibit the SARS-CoV-2 virus replication and stimulate the anti-inflammatory activity.34-36 There are available synthetic drugs have been using such as chloroquine, favipiravir, arbidol, remdesivir,37 interferon-alpha 1b, monoclonal antibodies, novaferon and azithromycin as combine therapy although these are not introduced into the international community by FDA.38
The aim of the study is to overview the current strategy and role of phytoconstituents to treat and manage COVID-19 dealing with in silico, in vitro and in vivo experiments.
Effect of phytoconstituents against SARS-CoV-2 (in silico approaches)
Phytocompounds have long been thought to be a source of medicinal substances. With a vast range of options in their chemical compositions and biochemical specificity they have proven to be beneficial properties. The development of natural product drugs is crucial in the pharmaceutical development of less toxic and more effective drugs and having potential. These natural resources have scientific evidence (Table 1).39,40
Table (1):
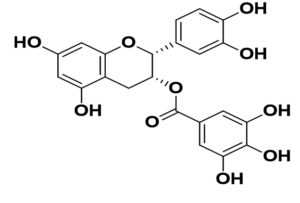
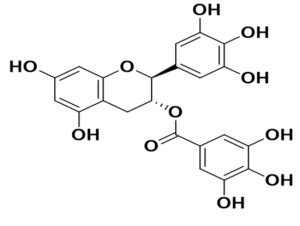
Phytoconstituents against the SARS-CoV-2 (in silico approaches)
Plant Name |
Compounds |
Structures |
Virus acting |
Reported Mechanisms |
Ref. |
|---|---|---|---|---|---|
Boenninghausenia sessilicarpa |
Leptodactylone |
SARS-CoV |
potent antiviral action and protection against virus-infected cells |
56 |
|
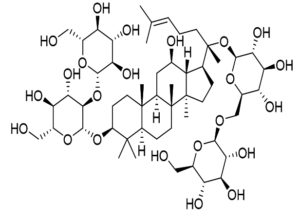
Panax ginseng |
Ginsenoside-Rb1 |
SARS-CoV |
Inhibits glycoprotein activity. |
43 |
|
Lycoris radiate |
Lycorine |
SARS-CoV |
— |
57 |
|
Aesculus hippocastanum |
Aescin |
SARS-CoV |
—– |
57 |
|
Rauwolfia serpentina |
Reserpine |
SARS-CoV |
— |
58 |
|
Stephaniae Tetrandrae Radix |
Tetrandrine |
HCoV-OC43 |
Inhibits p38 MAPK pathway, suppress HCoV-OC replication, |
58 |
|
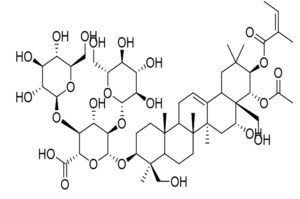
Bupleuri Radix, |
Saikosaponin B2 |
HCoV |
Invasion of cells by viruses and interference with the first stage of viral replication |
59 |
|
Salviae Miltiorrhizae Radix |
Dihydrotanshinone |
MERS- CoV |
viral passage inhibitory effects in MERS-CoV |
58 |
|
Stephania japonica |
Cepharanthine |
SARS-CoV-2 |
ACE inhibitor |
60 |
|
Camellia sinensis |
Epigallocatechin (EGC) |
HCoV, SARS, MERS. |
viral passage inhibitory effects in MERS-CoV |
41 |
|
Camellia sinensis |
Gallocatechin (GC) |
HCoV, SARS, MERS |
viral passage inhibitory effects in MERS-CoV |
41 |
|
Camellia sinensis |
Catechin (C) |
HCoV, SARS, MERS |
viral passage inhibitory effects in MERS-CoV |
41 |
|
Camellia sinensis |
Epicatechin (EC) |
HCoV, SARS, MERS |
viral passage inhibitory effects in MERS-CoV |
41 |
|
Camellia sinensis |
Catechin gallate (CG) |
HCoV, SARS, MERS |
viral passage inhibitory effects in MERS-CoV |
41 |
|
Camellia sinensis |
Epigallocatechin gallate (EGCG |
HCoV, SARS, MERS |
viral passage inhibitory effects in MERS-CoV |
41 |
|
Camellia sinensis |
Epicatechin gallate |
HCoV, SARS, MERS |
viral passage inhibitory effects in MERS-CoV |
41 |
|
Camellia sinensis |
Gallocatechin-3-gallate (GCG) |
HCoV, SARS, MERS |
viral passage inhibitory effects in MERS-CoV |
41 |
|
Clerodendrum spp |
Taraxerol |
HCoV, SARS, MERS |
spike (S) glycoprotein |
61 |
|
Curcuma longa |
Curcumin I-III |
SARS-CoV-2 |
spike protein |
48 |
|
Cupressus sempervirens L |
Amentoflavone |
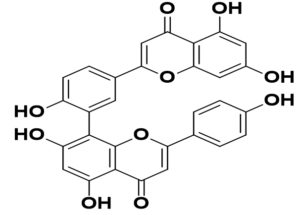
SARS-CoV-2 |
ACE2 to CASP-3 flagging pathway |
43 |
|
Cryptolepis sanguinolenta |
Cryptospirolepine |
SARS-CoV-2 |
the primary protease and the RNA dependent polymerase |
44 |
|
Cryptolepis sanguinolenta |
Cryptoquindoline |
SARS-CoV-2 |
the primary protease and the RNA dependent polymerase |
44 |
|
Cryptolepis sanguinolenta |
Biscryptolepine |
SARS-CoV-2 |
the primary protease and the RNA dependent polymerase |
44 |
|
Withania somnifera |
Withanoside V |
SARS-CoV-2 |
Mpro |
46 |
|
Withania somnifera |
Somniferine |
SARS-CoV-2 |
Mpro |
46 |
|
Zingiber officinale |
(10) Gingerol |
SARS-CoV-2 |
Mpro |
62 |
|
Piper nigrum |
Piperanine |
SARS-CoV-2 |
Mpro |
62 |
|
Piper nigrum |
Piperadine |
SARS-CoV-2 |
Mpro |
62 |
|
Curcuma longa |
Cyclocurcumin |
SARS-CoV-2 |
Mpro |
48 |
|
Curcuma longa |
Curcumin |
SARS-CoV-2 |
Mpro |
48 |
|
Andrographis paniculata |
Andrographolide |
SARS-CoV-2 |
Mpro |
48 |
|
Andrographis paniculata
|
Dihydroxydimethoxy flavone |
SARS-CoV-2 |
Mpro |
48 |
In principle, COVID-19, pedunculagin, tercatain, and punicalin were used to avoid outbreaks. The structural relationship functioning of the plant of hydrolysable tannins as potential antiviral agents & top 3 hit records inhibit COVID-19’s initial protease and hence viral replication.41 Polyphenols, including two anti-HIV medicines (darunavir and lopinavir) which are exposed to subatomic drugs, (brousochalcone A (C2), papyriflavonol A (C4), 30-(3-methylbut-2-enyl)-30, 40,7-trihydroxyflavane (C5), kazineol A (C6), broussoflavane A (C8), kazinol F (C9) and kazineol J (C10). AutoDock Vina’s energy estimates for both these polyphenols were higher than darunavir. Six of them were related to large accumulations of Mpro-reactants (C2, C4, C5, C8, C9 and C10) (His41 The RMSD and RMSF profiles clearly indicate that the buildings of these six Mpro polyphenols are highly stable and conformable. Research from SASA found that all Mpro-polyphenol buildings are slightly smaller and probably larger. The existence of intermolecular hydrogen connections with B in households. The polyphenols of papyrifera (C2, C4, C5, C8, C9 and C10) indicate the security of these polyphenols in the pocket connections of Mpro more strikingly than in the complex of Mpro-darunavir/lopinavir. Both the structures of Mpro-polyphenol were much more stable than the complexes of Mpro-darunavir and Mprolopinavir. The more potent inhibitors of Mpro than previously indicated were T-brousochalcone A 30-(3-methylbut-2, enyl) -30, 40, 7-treihydroxyflavane, and kazinol J. (darunavir and lopinavir).42
In addition, following reputable protein structures, three-dimensional compliance was balanced by the phytocompounds amentoflavone unambiguously connected to the objective proteins. In silico drug likeness and ADMET profiling of the combinations also indicated potential therapeutic activity. In SARS-CoV-2 3CL M-master, compared to COVID-19 remdesivir, oleanolic corrosive has a higher limiting potential. They should be used in conjunction with the official ACE2 to CASP-3 flagging pathway, which needs further research and is meant to offer logical direction, to have an impact on apoptosis. COVID-19 is treated via a variety of organic cycles and routes have been the fundamental goal proteins CASP-3, CASP-9, and XIAP that aid in the management of COVID-19 have been combined into the synthetic blends.43
The different Cryptolepis sanguinolenta alkaloids have shown highly restrictive partiality and thus anticipated inhibitory action against two of the major SARS-CoV-2 prions, the primary protease, and the RNA dependent polymerase. The different Cryptolepis sanguinolenta alkaloids have shown extremely restrictive partiality and thus anticipated inhibitory action against two of the major SARS-CoV-2 prisons, the primary protease, and the polymerase-dependent RNA.44 Eucalyptol has high tilt limits and minimum power constraints. Therefore, it has been recommended that Eucalyptol may be linked to possible treatment options and may be present in therapeutic plants, as predicted by COVID-19 Mpro inhibitors.45
It was predicted that the operation of SARS-CoV-2 (Mpro) was Restorative plants, likely acting as a constraint, and SARS-CoV-2, with high affinity (Mpro), the deterring additional understanding of the viral protein that helps to harm the vital host organs. Certain phytochemicals against COVID-19 may be repurposed. An innocuous ADMET profile has the most effective docked mixtures having drug-like characteristics that can be used to develop more sophisticated, efficient COVID-19 inhibitors. The directions investigating the contemplated buildings showed clear protection during MD runs.46
Glycyrrhizin, tryptanthrin, bicylogermecrene, beta-sitosterol, indirubin, indican, indigo, hesperetin, crysophanic corrosives, rhein, berberin and beta-caryophyllene, which can be considered to be a possible natural competitor hostile to SARS-CoV-2 viral activity. Promising mooring outcomes were carried out that validated the useful essence of these preferred alternatives to combat COVID-19 disease for potential medicine advancement.47
Ginger phytoconstituents such as 10 Gingerol, 8-Gingerol and Piperanine, Piperdardiine is substantially dynamic against COVID-19 with a significant Glide score compared to the second-hand medication Hydroxychloroquine at present (-5.47). Docking results in a similar mode of interaction between its compounds to COVID-19. HIE41, GLN189, SER46, ARG189, MET165, ASP187, THR24, LEU27, THR25, GLY143 and ASN142 Critical function residues attach to ligands. Pepper phytoconstituents such as Piperazine, Piperdardiine and Ginger, such as 10-Gingerol, 8-Gingerol, are significantly opposed to COVID-19.48
The phytoconstituents of turmeric like Cyclocurcumin, Curcumin and similar to andrographolide from Andrographis paniculata, When compared to currently approved COVID-19 medications such hydroxychloroquine (-5.47) nelfinavir, dihydroxy dimethoxy flavone are substantially bound to the active site of the primary SARS-CoV-2 protease (-5.93). Cyclocurcumin from turmeric is significantly more active when compared to common Remdesivir (-6.38). According to the docking results, the drugs interacted with SARS in a similar manner. SARS is brought on by the CoV-2 virus. Important ligand binding residues include THR24, THR25, THR26, LEU27, SER46, MET49, HIE41, GLN189, ARG188, ASP187, MET165, HIE164, PHE181, and THR54.48
From an in silico study, Glu288, Asp289, Glu290, Lys5 are found as the key sites. In addition, Ala285 and Leu286 are found as regulatory sites of interaction. C23 indole-chalcone interacted at Glu288, Asp289 residues with the docking score -10.4 kcal/mol. Quercetin has been found to block the interaction sites of viral spike as well as the main protease’s Glu290 with -9.2 kcal/mol docking score.49 Glycosylated flavonoids: quercetin 3-rhamnoside (-9.7 kcal/mol), myricetin 3-rutinoside (-9.3 kcal/mol) and rutin (-9.2kcal/mol) exhibit higher docking score than the other flavonoids in silico. Compounds result from the substitution at C-3 position of flavonoids with sugar moieties especially have more affinities with main protease active site.50 Kaempferol acts as an inhibitor of 3CLpro and PLpro.51 Based on a study ,52 other flavonoids including luteolin-7-O-glucoside, naringenin, desmethoxycurcumin, curcumin, apigenin-7-O-glucoside, oleuropein, catechin and epicatechin-gallate could potentially inhibit SARS-CoV-2 3CLpro. Taifolin, the other flavonoid has strong inhibitory potentials against SARS-CoV-2 according to a molecular docking study.53 While antiproteases’ key purpose is to inhibit or deactivate proteases, newer research is revealing that they also play a role in regulating excessive inflammation and microbial infection.54 Finding multifunctional plant protease inhibitors may thus provide multistep defense against coronavirus infection. Among all the tropane alkaloids from Schizanthus porrigens schizanthine Z, schizanthine Y expressed binding affinity values -7.5 kcal/mol & 7.1 kcal/mol, respectively. Molecular dynamic simulation, ADME analysis study confirmed that schizanthine Z could be the best drug candidate that blocks papain like protease.55
Phytoconstituents against COVID-19 (in vitro and in vivo)
Natural phytoconstituents have been shown to be beneficial in treating viral diseases such as the previous chikungunya virus, hepatitis C virus, SARS, and MERS viral diseases. Among them, flavonoids and alkaloids have been investigated in vitro and in vivo to combat COVID-19 in many ways like protease inhibition, spike protein inhibition, Nrf2 inhibition. Role of phytoconstituents in the treatment of COVID-19 are given in Table 2.
Table (2):
In vitro and in vivo evidence regarding the use of phytochemicals (alkaloids and flavonoids) in SARS-CoV-2
Phytochemicals |
Class |
Dose/conc. |
Activity |
Ref. |
|---|---|---|---|---|
Baicalin |
flavonoid |
0.04 to 400 µM |
↓ X4 and R5 HIV-1 Env-mediated fusion, CAT activity |
97 |
Baicalin |
flavonoid |
20 µg/mL |
↑ survival rate, IFN-α and IFN-β |
98 |
Baicalin |
flavonoid |
20 µM |
↓ viral replication |
99 |
Baicalin |
flavonoid |
20–80 µg/mL |
↓ virus replication, ↑ cell viability in MDCK cells |
100 |
Baicalin |
flavonoid |
0.5–320 µM |
↓ NP transcription, RIG-1, PKR, NS1 expression, viral replication |
101 |
Baicalin |
flavonoid |
12.5–50 µg/mL |
↑ mTOR phosphorylation, ↓ autophagy |
102 |
Baicalein |
flavonoid |
40–100 µM |
↓ viral replication, IL6, CXCL10, and TNF-α |
103 |
Baicalein |
flavonoid |
200 mg/kg |
↑ respiratory function |
104 |
Taxifolin |
flavonoid |
IC50 = 145.7 µM |
Antimicrobial activities |
105 |
Camellianin A |
flavonoid |
500 µg/mL |
30.2% suppression at EC |
106,107 |
Camellianin B |
flavonoid |
500 µg/mL |
40.7% suppression at EC |
106,107 |
Apigenin |
flavonoid |
500 µg/mL |
30.3% suppression at EC |
106,107 |
EGCG |
flavonoid |
50 µM |
2-fold increase at EC by elevating intracellular Zn2+ level |
108 |
Catechin |
flavonoid |
50 µM |
2-fold increase at EC by elevating intracellular Zn2+ level |
108 |
Purified flavonoids |
flavonoid |
3–30 µg/mL |
↓ IL-6 and MCP-1, ↓ NA activity |
109 |
Purified flavones |
flavonoid |
↓ HIV-1 protease |
110 |
|
Purified flavonol glycosides |
flavonoid |
IC50 = 20–43 µM |
↓ HIV-1 RDDP activity |
111 |
EGCG |
flavonoid |
1–100 µM |
↓ RT activity, protease activity, p24, viral entry, and viral production |
112 |
EGCG |
flavonoid |
25–100 µM |
↓ CD4 expression |
113 |
EGCG |
flavonoid |
6–100 µM |
↓ HIV-1 p24 antigen, ↓ HIV-1 infectivity |
114 |
EGCG |
flavonoid |
1–50 µM |
↓ virus replication |
115 |
EGCG |
flavonoid |
0.2–20 µM |
↓ HIV-1 gp 120 binding to the CD4+ T cells |
116 |
Tetrandrine |
Alkaloid |
10 µM |
Increased endolysosomal pH concentration dependently |
117 |
Dauricine |
Alkaloid |
10 µM |
Increased endolysosomal pH, impaired V-type ATPase activity |
118 |
Daurisoline |
Alkaloid |
10 µM |
Increased endolysosomal pH, impaired V-type ATPase activity |
119 |
Tylophorine |
Alkaloid |
20 nM |
3CLpro inhibitor, block the S and N proteins |
120 |
Quinine |
Alkaloid |
10.7 μM |
Mpro and S proteins inhibitor |
121 |
Neferine |
Alkaloid |
10 μM |
Decreased the levels of viral RNA |
122 |
Lycorine |
Alkaloid |
0.47 μM |
Mpro inhibitor |
123 |
Hernandezine |
Alkaloid |
10 μM |
Blocking the calcium transition |
122 |
Fangchinoline |
Alkaloid |
1.01 μM |
Blocked the expression of S and N proteins |
58 |
Conessine |
Alkaloid |
10.75 μM |
Mpro inhibitor |
124 |
Tetrandrine |
Alkaloid |
2.05 μM |
Mpro inhibitor, block the expression of S and N proteins |
58 |
Oxysophoridine |
Alkaloid |
0.31 μM |
Nucleotide biosynthesis inhibitor |
125 |
Homoharringtonine |
Alkaloid |
0.46 μM |
Blocked S proteins |
126 |
Harmine |
Alkaloid |
13.46 μM |
Mpro inhibitor |
124 |
Emetine |
Alkaloid |
2.55 μM |
Mpro inhibitor |
127 |
Leelamine |
Terpenoids |
3 µM |
Decreased cellular endocytosis |
128 |
Pulsatilla saponin D |
Terpenoids |
1.25 µM |
Downregulated cathepsins |
129 |
Myrtenal |
Terpenoids |
100 µM |
Suppressed the action of V-type ATPase |
130 |
Saikosaponins |
Terpenoids |
0.25–25 µmol/L |
Inhibitory effect on viral attachment and penetration |
59 |
Ferruginol, betulonic acid |
Terpenoids |
0–80 µM |
Reduced SARS-CoV replication substantially |
131 |
Alkaloids
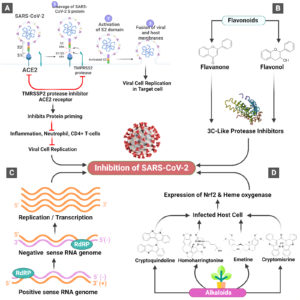
The Nrf2 signaling system regulates anti-inflammatory gene expression and prevents inflammation from progressing.63 Upregulation of Nrf2 signaling, in particular, prevents the overproduction of IL-6, pro-inflammatory cytokines, and chemokines while also limiting NFB activation. Endothelial dysfunction results from a failure to guard against oxidative stress-induced neuronal disruption in cardiovascular disorders and other metabolic syndrome-related pathologies. In cellular redox homeostasis, many antioxidant pathways are involved, with the Nrf2 signaling pathway being one of the most important.64 Against oxidative pulmonary disease, pathological inflammatory and immune reactions, and apoptosis, Nrf2 activates cellular rescue pathways (Figure). The Nrf2 pathway has been shown to defend against acute lung damage and acute respiratory distress syndrome.65 Basically, COVID-19 patients with critical situations present the signs of oxidative stress & systemic inflammation, the main cause of lethality.66,67 Nrf2 controls the antioxidant response participating genes, redox homeostasis genes expressions. It also activates these genes leading to the protection of cells from inflammation 63. In an in vivo test depicted that Nrf2 knockout mice suffered from uncontrolled inflammatory reaction contribute to tissue damage.68 Nrf2 activation also causes the suppression of inflammation through its transcriptional repressor activity- in macrophages it inhibits the expressions of cytokine (IL-1ג, IL-6, TNFב) production, the most pertinent cause of critical illness due to COVID-19.69 The protective role of Nrf2 was shown in numerous animal inflammatory models that Nrf2 inducers decreased the pro-inflammatory cytokines in the bloodstream.70-72 Often, obesity is a significant factor in COVID-19 severity.73 Obesity, diet, and COVID-19 can interact, and this could be attributed to Nrf2.74
Figure. Exploring the potential role of alkaloids and flavonoids against SARS-CoV-2. A. The coronavirus replication loop and main steps for antiviral goals are depicted in this diagram. Antivirals that function extracellularly or intracellularly are shown by white text boxes. Membrane fusion, receptor binding, sub-genomic RNA transcription, viral RNA replication, and translation are all examples of phases in the coronavirus replication cycle. B. Flavonoids activate 3CL-like protease inhibitors which eventually inhibit SARS-Cov-2. C. Flowchart of RNA synthesis by RNA-dependent RNA polymerase (RdRP) of positive-sense and negative-sense ssRNA viruses which inhibit SARS-Cov-2. D. Natural alkaloids enter in the infected host cell and express Nrf2 and heme oxygenase which combat against SARS-Cov-2 and inhibit it
Alkaloids are one of the most diverse groups of natural goods, with members of the Ranunculaceae, Solanaceae, Papaveraceae, Rubiaceae, Fabaceae, and Amaryllidaceae families being the most abundant. The inclusion of the nitrogen atom in their arrangement is the group’s most distinguishing characteristic.75 These are natural compounds with diversified actions against various diseases including COVID-19. Homoharringtonine and emetine with notable anti-herpes activity were reported inhibiting replication of SARS-CoV-2.76 Sinomenine is an isoquinoline alkaloid isolated from Sinomenium acutum (Thunb.) Rehder & E.H.Wilson’s stem and rhizome (Menispermaceae). It decreases lung damage caused by lipopolysaccharides (LPS) and E. coli by regulating the inflammatory signaling cascade, which included downregulation of IL-1, IL-6, NF-B, TNF-a, iNOS, and COX-2, as well as upregulation of the anti-inflammatory adenosine A2A receptor. Sinomenine also blocked oxidative stress indicators, such as superoxide dismutase (SOD) production and malondialdehyde (MDA) production.77,78 Furthermore, 1 hour after causing lung damage in mice with LPS (8 mg/kg), sinomenine [100 mg/kg, i.p.] upregulated the expression of Nrf2 and autophagy-related molecules (Atg5, LC-3II, and Beclin1), as essential mediators in increasing cell tolerance to inflammation and oxidative stress. Furthermore, sinomenine reduced the pulmonary edema, protein leakage, and lung wet/dry (W/D) ratio into bronchoalveolar lavage fluid (BALF), both of which are pathological indicators of lung damage.79 In addition, total alkaloid extraction and six isosteroid alkaloids (verticinone, imperialine, imperialine-3—D-glucoside, verticine, peimisine, and delavine) isolated from bulbs of Fritillaria cirrhosa D.Don (Liliaceae) showed protective effects on lung injury induced by LPS and cigarette smoke, increased the expression of Nrf2 and heme oxygenase (HO-1), and reduced.80,81 Thalimonine and sophaline D showed to be potential drug candidate targeting Mpro of SARS-CoV-2 after performing molecular dynamic simulation and other in silico approaches.82 Toll-like receptor 4 (TLR4) is an inflammatory signalling system whose expression is elevated in patients with acute lung injury.83 Sophocarpine (50 and 25 mg/kg, i.p.), a quinolizidine alkaloid isolated from the seeds of Sophora alopecuroides L. (Fabaceae), inhibited TLR4 expression and thus decreased LPS-induced lung damage in mice.84 In vitro (mouse bone marrow-derived macrophages, 10 M) and in vivo (20 mg/kg, i.p.) and studies found that tabersonine, a monoterpenoid indole alkaloid extracted from the root of Catharanthus roseus (L.) G.Don (Apocynaceae), protected against lung damage caused by LPS. Tabersonine inhibited the activities of p38MAPK-activated protein kinase 2 (MAPK/MK2) and NF-kB by decreasing the expression of TNF receptor-associated factor 6 (TRAF6). The improvement of the above signaling pathways/mediators results in the suppression of proinflammatory mediators and a decrease of pathological indices of lung damage, such as total protein concentrations in BALF.85 Berberine, an isoquinoline alkaloid extracted from Berberis vulgaris L. (Berberidaceae) and Coptis chinensis Franch. (Ranunculaceae), has been shown to shield C57BL/6 mice from LPS-induced lung damage at 10 mg/kg (i.p., 24 and 2 h before injection of LPS, 2.5 mg/kg), as well as in vitro on the hu Berberine was also beneficial to mice suffering from pulmonary edema and protein deficiency in their BALF.86 Matrine (tetracycloquinolizindine),87 antidesmone (tetrahydroquinoline),88 epharanthine (bisbenzylisoquinoline),89 epigoitrin (pyrrolidine),90 isotetrandrine (bisbenzyltetrahydroisoquinoline),91 neferine (bisbenzylisoquinline),92 and oxysophoridine (quinolizidine)93 are other alkaloids that have been found to have anti-lung damage impact in in vitro and in vivo studies. As a result, they regulated pro-inflammatory mediators and oxidative markers which show the chemical compositions of certain alkaloids and other phytochemicals that have defensive properties against lung damage, as well as a graphical diagram of their potential modes of operation. In general, alkaloids, especially quinolines and quinazolines, have shown therapeutic effects on lung injury by inhibiting the MAPK pathway and its interconnected mediators, such as TLR4, as well as inflammatory cytokines including IL-1, TNF-a, and IL-6. These compounds have also been shown to improve antioxidative stress indicators such as the glutathione, Nrf2/HO-1 pathway, and SOD. As a result of this impressive function in lung injury, as well as the alkaloids’ other beneficial functions, especially their antiviral effects, these compounds are now being considered as multitarget agents for the treatment of coronavirus infection and its complications. Lycorine, tylophorine, ouabain, hypericin, myricetin, emodin, mycophenolate mofetil, silvestrol, scutellarein etc. demonstrated strong inhibitory effects against SARS-CoV-2 and other human coronaviruses.94 Indigotica is sometimes used to cure a variety of infectious diseases in the laboratory. Immunomodulatory and antiviral effects were observed in isolated compounds from I. indigotica (indican, isatin, indirubin, and indigotin). Chang et al. found that indigo and indirubin, two alkaloids found in I. indigotica extracts, prevented Japanese encephalitis virus replication in vitro.95 Various clinical trials of using colchicine have been announced are: (i) GRECCO-199 (ClinicalTrials.gov Identifier: NCT04326790) will recruit 180 COVID-19 diagnosed patients with the administration of colchicine for 21 days, (ii) Effects of colchicine in COVID-19 Pneumonia (ClinicalTrials.gov Identifier: NCT04322565) where n = 100, (iii) COLCORONA (ClinicalTrials.gov Identifier: NCT04322682) aims to recruit 6000 high-risk outpatients, (iv) Colchicine co-administration (or not) with lopinavir/ritonavir, ‘The ECLA PHRI COLCOVID’ Trial (ClinicalTrials.gov Identifier: NCT04328480) will recruit 2500 COVID-19 hospitalized patients.96
Flavonoids
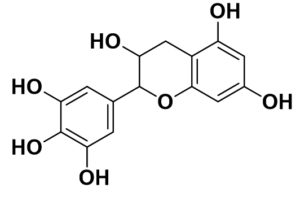
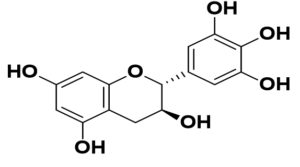
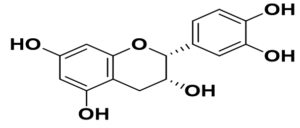
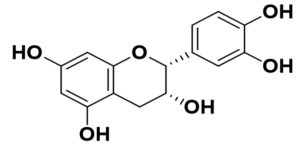
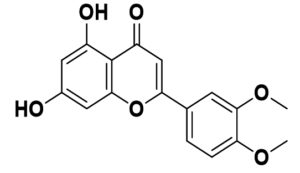
Flavonoids are the common compounds in medicinal plants which containing various anti-viral and anti-bacterial activity132 in pattern of particular chemical structure including hydroxylation, methoxylation and glycosylation.133,134 The flavonoids, a promising group of compounds, have potentials to treat COVID-19. Chalcones, flavonols, flavones, and isoflavones are examples of this essential family of natural compounds.135 Flavonoids have a flavan heart and a 15-carbon skeleton. A heterocyclic pyran ring (B ring) connects the two benzene rings (A and C rings). In hydroxylation, attachment of more hydroxyl group in particular ring of flavonoids decreases the activity such as luteolin has less inhibitory activity compared to apigenin due to presence of more –OH group in B ring whereas dinatin revealed unchanged activity like apigenin. Quercetin and myricetin depicted lower reduction effect comparison to kaempferol due to more –OH group in B ring. These results indicated that, more hydroxyl group in B ring decrease the activity of flavonoids (Figure).136 In the case of methoxylation, addition of methoxy group in flavonoids ring plummated the antiviral activity such as 5,6,7,4′-tetramethoxyflavone and tangeritin exhibited minimum antibiotic activity compared to kaempferol and luteolin but tangeritin showed better antiviral activity than 5,6,7,4′-tetramethoxyflavone because of methoxy group in C-8 position. Although, attachment of methoxy group in flavonoids ring decrease the antiviral activity but in C-8 position.137 As for glycosylation, puerarin revealed greater antiviral activity compared to quercetin and ampelopsin.138 It is estimated that flavonoids group shows grater antiviral effects in contrast to isoflavonoids. The resorcinol molecule, which has two hydroxyl groups in its aromatic ring configuration, and they are positioned at meta-positions with respect to another hydroxyl group, is the most important functional group of flavonoids that could be responsible for ACE2 inhibition. The benzene ring’s behavior is largely determined by the position of these two hydroxyl groups.139 Ring A’s resorcinol moiety may be involved in ACE2 inhibition.140
In vitro studies have shown that flavonoids extracted from Angelica keiskei have a potent inhibitory effect on both 3CLpro and PLpro. Alkylated chalcones were able to inhibit PLpro in a manner that was not competitive. The compounds xanthoangelol E (IC50: 1.2 µM) and xanthoangelol F (IC50: 5.6 µM) proved to be the most effective in this regard. In accordance with the findings of the SAR analysis, the perhydroxyl component of a chalcone is an alkylated chalcone that has a more potent inhibitory action.141 There are a variety of plants that contain the stilbenoid known as resveratrol, including Vaccinium macrocarpon, Vitis vinifera, and Polygonum cuspidatum. Resveratrol’s pharmacological and therapeutic effects include, but are not limited to, hepatoprotective, cardioprotective, and neuroprotective abilities as well as anti-inflammatory and antibacterial activities. Resveratrol has been shown to greatly suppress the growth of MERS-CoV in vitro, as well as diminish MERS-CoV infection. As a direct result of this, resveratrol is an essential anti-MERS medicine and has the potential to be an effective SARS-CoV-2 antiviral.142,143 The SAR analysis conducted on quercetin-3-galactoside and its analogues has revealed several key findings. Firstly, the presence of 4 OH groups on the quercetin moiety is crucial for eliciting biological activity. Secondly, removal of the 7-OH group results in a decrease in inhibitory effect on 3CLpro. Thirdly, the sugar moiety plays a significant role in the compound’s activity. Lastly, alterations to the sugar moiety do not appear to have any impact on the efficacy of the inhibitor.144 Nigella sativa is a source of myricetin and scutellarein, which have been the subject of numerous investigations. Myricetin and scutellarein exhibit inhibitory effects on SARS-CoV 3CLpro at concentrations ranging from 0.01 to 10 µM. Broussochalcone B, broussochalcone A, 4-hydroxyisolonchocarpin, papyriflavonol A, 4,7-trihydroxyflavane, kazinol A, kazinol B, broussoflavan A, kazinol F, and kazinol J are bioactive compounds derived from Broussonetia papyrifera. These compounds have been found to exhibit inhibitory effects against SARS-CoV, as reported in literature sources.145,146
A total of twelve geranylated flavonoids were discovered from Paulownia tomentosa (Thunb.) Steud., a traditional Chinese medicinal (TCM) plant. Among these compounds, five were newly identified as tomentin A-E (8) (2.39-2.43). These flavonoids were found to exhibit mixed-type inhibition against SARS Papain-Like Protease (PLpro), with IC50 values ranging from 5.0 to 14.4 µM. The study found that among the group of inhibitors tested, Tomentin A, B, and E exhibited the highest level of effectiveness in inhibiting PLpro, with IC50 values of 6.2, 6.1, and 5.0 µM, respectively. It was observed that each of the newly synthesised compounds containing dihydro-2H-pyran moiety exhibited superior inhibitory activity compared to their respective precursor compounds.147 The seeds of Cullen corylifolium (L.) Medik. have been found to contain six flavonoids, namely bavachinin, neobavaisoflavone, isobavachalcone, 40-O-methylbavachalcone, psoralidin, and corylifol A. These flavonoids have been observed to exhibit mixed-type inhibition against SARS-CoV PLpro, with IC50 values ranging from 4.2 to 38.4 µM.148 Amentoflavone, a bioflavonoid obtained from Torreya nucifera, has demonstrated noncompetitive inhibition of 3CLpro with IC50 values in the low micromolar range. Amentoflavone (2.6) was identified as the most potent inhibitor (IC50 = 8.3 µM), surpassing the parent compound apigenin (IC50 = 280.8 µM) in terms of inhibitory activity. Luteolin (2.23) and quercetin (2.29), which are flavones containing apigenin, were found to inhibit 3CLpro to a greater extent than the parent compound. The presence of the apigenin moiety at position C-30 of flavones was determined to be essential for their effectiveness. The IC50 values for luteolin and quercetin were 20.2 µM and 23.8 µM, respectively. The primary flavonoid present in honeysuckle, namely luteolin, has been identified as a constituent of Lianhua qingwen, a traditional Chinese medicine utilized for the treatment of COVID-19.149
The compound Quercetin has demonstrated noteworthy inhibition activity against SARS-CoV Mpro, which was expressed in Pichia pastoris, with an IC50 value of 73µM150 The administration of Quercetin in conjunction with vitamin C has demonstrated anti-SARS-CoV-2 and immunomodulatory properties. The combined use of both agents exhibits a synergistic effect and may be utilized for prophylactic purposes in populations at high risk.151 Herbacetin, pectolinarin, and rhoifolin, flavonoids have been found to effectively inhibit the enzymatic function of SARS-CoV Mpro.152 The methanolic extract of Paulownia tomentosa fruits was found to exhibit anti-papain protease activity through fractionation. This activity was attributed to various geranylated flavonoid derivatives, which were identified as potent inhibitors of the SARS-CoV papain protease.153
The evidence mentioned above suggests that certain flavonoids exhibit potential inhibitory activity against SARS-CoV-2 by potentially targeting crucial proteins involved in the virus’s life cycle. Yet only a small number of flavonoids have undergone in vitro testing. Thus, it is necessary to verify the computational investigation by conducting a suitable biological assay.154
Terpenoids
The primary pharmacologically active triterpenoids, commonly in the form of glucosides, are saikosaponins. These are typically derived from traditional Chinese medicine (TCM) sources such as Bupleurum spp., Heteromorpha spp., and Scrophularia scorodonia, and possess antiviral and immunomodulatory properties.155 The antiviral activity of four saikosaponins (saikosaponin A, B2, C, and D) against human coronavirus-229E (CoV-229E) (alphacoronavirus) was investigated. The EC50 values of these saikosaponins were found to be 8.6, 1.7, 19.9, and 13.2 µM, respectively, at concentrations ranging from 5-25 M/L. Additionally, saikosaponin B2 was observed to inhibit viral adherence and penetration stages.59 In 2012, an in vitro study was conducted to evaluate the anti-Human Coronavirus efficacy of Triterpenoids and 3-friedelanol extracted from the leaves of Euphorbia neriifolia. The screening of a triterpenoid in combination with 3-Friedelanol revealed a heightened potential for antimicrobial activity and increased cellular viability following incubation with HCoV. Additionally, 3b-fridelanol exhibited potent inhibitory activity against 3CLpro.156 The active constituents of Glycyrrhiza glabra, namely glycyrrhizin, exhibit antiviral properties against a range of viruses such as hepatitis A, B, and C, varicella-zoster, HIV, and herpes simplex type-1.157 Salvia miltiorrhiza synthesizes tanshinones that possess an abietane diterpene framework. Tanshinones exhibit diverse biological activities such as anti-inflammatory, cardiovascular, and anti-neoplastic effects. The aforementioned compounds exhibit selective inhibition towards the SARS-CoV 3CLpro and PLpro enzymes, with their efficacy being predominantly influenced by the subtype of the enzyme. Several tanshinones have been found to exhibit greater potency in inhibiting PLpro, with IC50 values ranging from 0.8 to 30.0 µM.158
Miscellaneous
Marine microalgae belonging to the phyla Rhodophyta and Phaeophyta were discovered to contain phycocyanin, polysaccharides, lutein, vitamins, and other phenolics, which exhibit significant pharmacological effects such as antibacterial, anticancer, and anti-inflammatory properties.118 The study conducted by Hirata et al. examined the antiviral and antioxidative characteristics of phycocyanobilins, which are a class of tetrapyrrole chromophores present in select marine cyanobacteria.159 The potential application of Griffithsin, a lectin derived from red algae, has been studied and its antiviral properties against HIV-1 and hepatitis C have been demonstrated through testing.160,161 According to a recent in vitro investigation conducted by Millet et al., griffithsin exhibited inhibitory effects against MERS-CoV.162 Axinella cf. corrugate, a marine sponge, has been found to contain esculetin ethyl ester, which has exhibited a significant affinity towards the SARS-CoV-2 protease. This compound has the potential to serve as a viable therapeutic agent for the treatment of COVID-19.163 Carrageenans, a class of sulfated polysaccharides derived from marine sources, have been identified as potential antiviral agents. The mechanism of action involves the inhibition of viral attachment and internalization. Nagle and colleagues postulated that these compounds possess the potential to serve as coating agents on sanitary products for the purpose of impeding COVID-19 infection. The utilization of in silico analyses has recently played a significant role in identifying promising lead compounds for the development of treatments against the COVID-19 pandemic.164
Several herbal extracts and natural products can be useful in treating the symptoms of SARS-CoV-2 infection. Antiviral secondary metabolites have been isolated from a number of medicinal plants and global studies have been conducted to produce antiviral drugs that are effective against SARS-CoV-2. Searching the compounds that modify or disrupt every stage of the virus replication cycle may be the most effective way of preventing COVID-19 infections. Natural products with the ability to inhibit or change the structure of structural proteins (spike glycoprotein), non-structural proteins (3-chymotrypsin-like protease, papain-like protease, helicase, and RdRP), and accessory proteins encoded by the SARS-CoV-2 genome must be investigated. Phytochemicals, which have low toxicity and are used in the pharmaceutical industry for their bioactivity, including antiviral activity, could offer a solution to this problem. SARS-CoV-1 and COVID-19 have a lot in common, which may lead to the discovery of new medicines or even a vaccine. The potential anti-SARS-CoV-2 action of flavonols, flavanones, and flavones, as well as the fact that these metabolites are abundant in angiosperm plants, have given rise to a lot of optimism. Since the majority of current research is theoretical or lacks empirical validation, there is still a long way to go in terms of biological science and optimized extraction and development. Flavonoids and alkaloids combat COVID-19 in several ways like protease inhibition, spike protein inhibition, Nrf2 inhibition. This study initiative would be bolstered by the rigorous review outlined here. As pandemic situation is lasting for a long period, it is crucial to search for best drug candidates that have potentialities against SARS-CoV-2. Therefore, flavonoids and alkaloids may have such kind of potentialities to treat COVID-19. Flavonoids and alkaloids provide capabilities to fight against novel coronaviruses, and researchers can continue to investigate the mechanisms of action in order to develop effective preventions so that the planet will get rid of this deadly viral infection.
ACKNOWLEDGMENTS
The authors would like to thank JSS College of Pharmacy, JSS Academy of Higher Education & Research, Rocklands, Ooty, The Nilgiris, Tamilnadu, India, for their generous research infrastructure and support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
KRAM and KRAJ conceptualized and designed the study, and performed statistical analysis. KRAM, GS and SJ performed acquisition of data. KD, MUK, TBE and HO performed analysis and interpretation of data. KRAM and TBE contributed in administrative, technical, and material support. KRAM, RB and KRAJ drafted the manuscript. KRAM, GS, MUK and HO revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Singhal T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281-286.
Crossref - Kumar R, Yeni CM, Utami NA, et al. SARS-CoV-2 infection during pregnancy and pregnancy-related conditions: Concerns, challenges, management and mitigation strategies–a narrative review. J Infect Public Health. 2021;14(7):863-875.
Crossref - Hossain MJ, Ahmmed F, Rahman SMA, Sanam S, Emran TB, Mitra S. Impact of online education on fear of academic delay and psychological distress among university students following one year of -19 outbreak in Bangladesh. Heliyon. 2021;7(6):e07388.
Crossref - Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157-160.
Crossref - Tareq AM, Emran T Bin, Dhama K, Dhawan M, Tallei TE. Impact of SARS-CoV-2 delta variant (B.1.617.2) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum Vaccines Immunother. 2021;17(11):4126-4127.
Crossref - Grieco DL, Bongiovanni F, Chen L, et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care. 2020;24(1):529.
Crossref - Alsaied T, Aboulhosn JA, Cotts TB, et al. Coronavirus Disease 2019 (COVID-19) Pandemic Implications in Pediatric and Adult Congenital Heart Disease. J Am Heart Assoc. 2020;9(12):e017224.
Crossref - Elbarbary NS, dos Santos TJ, de Beaufort C, Agwu JC, Calliari LE, Scaramuzza AE. COVID-19 outbreak and pediatric diabetes: Perceptions of health care professionals worldwide. Pediatr Diabetes. 2020;21(7):1083-1092.
Crossref - Robson B. COVID-19 Coronavirus spike protein analysis for synthetic vaccines, a peptidomimetic antagonist, and therapeutic drugs, and analysis of a proposed achilles’ heel conserved region to minimize probability of escape mutations and drug resistance. Comput Biol Med. 2020;121:103749.
Crossref - Nainu F, Abidin RS, Bahar MA, et al. SARS-CoV-2 reinfection and implications for vaccine development. Hum Vaccines Immunother. 2020;16(12):3061-3073.
Crossref - Hess KL, Jewell CM. Phage display as a tool for vaccine and immunotherapy development. Bioengineering & Translational Medicine. 2020;5(1):e10142.
Crossref - Arnon R, Shapira M, Jacob CO. Synthetic vaccines. J Immunol Methods. 1983;61(3):261-273.
Crossref - Jefferis R, Lowe J, Ling NR, Porter P, Senior S. Immunogenic and antigenic epitopes of immunoglobulins. I. Cross-reactivity of murine monoclonal antibodies to human IgG with the immunoglobulins of certain animal species. Immunology. 1982;45(1):71-717.
- Huang J, Song W, Huang H, Sun Q. Pharmacological Therapeutics Targeting RNA-Dependent RNA Polymerase, Proteinase and Spike Protein: From Mechanistic Studies to Clinical Trials for COVID-19. J Clin Med. 2020;9(4):1131.
Crossref - Mirzaie A, Halaji M, Dehkordi FS, Ranjbar R, Noorbazargan H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19). Complement Ther Clin Pract. 2020;40:101214.
Crossref - Boozari M, Hosseinzadeh H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phyther Res. 2021;35(2):864-876.
Crossref - Hung TC, Jassey A, Liu CH, et al. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine. 2019;53:62-69.
Crossref - Luganini A, Mercorelli B, Messa L, Palu G, Gribaudo G, Loregian A. The isoquinoline alkaloid berberine inhibits human cytomegalovirus replication by interfering with the viral Immediate Early-2 (IE2) protein transactivating activity. Antiviral Res. 2019;164:52-60.
Crossref - Varghese FS, Thaa B, Amrun SN, et al. The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling. J Virol. 2016;90(21):9743-9757.
Crossref - Ang L, Lee HW, Kim A, Lee MS. Herbal medicine for the management of COVID-19 during the medical observation period: a review of guidelines. Integr Med Res. 2020;9(3):100465.
Crossref - Harborne JB. Phytochemistry of medicinal plants. Phytochemistry. 1996;43(1):317-318.
Crossref - Xu W, Zhang M, Liu H, et al. Antiviral activity of aconite alkaloids from Aconitum carmichaelii Debx. Nat Prod Res. 2019;33(10):1486-1490.
Crossref - Cushnie TPT, Cushnie B, Lamb AJ. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents. 2014;44(5):377-386.
Crossref - Moradi MT, Karimi A, Rafieian-Kopaei M, Fotouhi F. In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against Influenza virus. Microb Pathog. 2017;110:42-49.
Crossref - Khan H, Mubarak MS, Amin S. Antifungal Potential of Alkaloids As An Emerging Therapeutic Target. Curr Drug Targets. 2016;18(16):1825-1835.
Crossref - Abreu AC, Coqueiro A, Sultan AR, et al. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci Rep. 2017;7(1):3777.
Crossref - Wink M. Potential of DNA intercalating alkaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity. 2020;12(5):175.
Crossref - Wang HK, Xia Y, Yang ZY, Morris Natschke SL, Lee KH. Recent advances in the discovery and development of flavonoids and their analogues as antitumor and anti-HIV agents. Adv Exp Med Biol. 1998;439:191-225.
Crossref - Nileeka Balasuriya BW, Vasantha Rupasinghe HP. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Funct Foods Heal Dis. 2011;1(5):172-188.
Crossref - Guerrero L, Castillo J, Quinones M, et al. Inhibition of Angiotensin-Converting Enzyme Activity by Flavonoids: Structure-Activity Relationship Studies. PLoS One. 2012;7(11):e49493.
Crossref - Zinovkin RA, Grebenchikov OA. Transcription Factor Nrf2 as a Potential Therapeutic Target for Prevention of Cytokine Storm in COVID-19 Patients. Biochem. 2020;85(7):833-837.
Crossref - Cuadrado A, Pajares M, Benito C, et al. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol Sci. 2020;41(9):598-610.
Crossref - Checker R, Patwardhan RS, Sharma D, et al. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-kB. Free Radic Biol Med. 2012;53(7):1421-1430.
Crossref - Olagnier D, Farahani E, Thyrsted J, et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun, 2020; 11: 4938.
Crossref - McCord JM, Hybertson BM, Cota-Gomez A, Geraci KP, Gao B. Nrf2 activator pb125® as a potential therapeutic agent against covid-19. Antioxidants. 2020;9(6):1-15.
Crossref - Rabaan AA, Al-Ahmed SH, Garout MA, et al. Diverse immunological factors influencing pathogenesis in patients with covid-19: A review on viral dissemination, immunotherapeutic options to counter cytokine storm and inflammatory responses. Pathogens. 2021;10(5):565.
Crossref - Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14(1):58-60.
Crossref - Mousavi SM, Hashemi SA, Parvin N, et al. Recent biotechnological approaches for treatment of novel COVID-19: from bench to clinical trial. Drug Metab Rev. 2021;53(1):141-170.
Crossref - Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2(2):303-336.
- Tallei TE, Niode NJ, Idroes R, et al. A comprehensive review of the potential use of green tea polyphenols in the management of COVID-19. Evidence-based Complement Altern Med. 2021;7170736.
- Ghosh R, Chakraborty A, Biswas A, Chowdhuri S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors–an in silico docking and molecular dynamics simulation study. J Biomol Struct Dyn. 2021;39(12):4362-4374.
Crossref - Swargiary A, Mahmud S, Saleh MA. Screening of phytochemicals as potent inhibitor of 3-chymotrypsin and papain-like proteases of SARS-CoV2: an in silico approach to combat COVID-19. J Biomol Struct Dyn. 2022;40(5):2067-2081.
Crossref - Borquaye LS, Gasu EN, Ampomah GB, et al. Alkaloids from Cryptolepis sanguinolenta as Potential Inhibitors of SARS-CoV-2 Viral Proteins: An in Silico Study. Biomed Res Int. 2020;5324560.
Crossref - Sharma, A.D. and Kaur I. Eucalyptol (1,8 cineole) from Eucalyptus Essential Oil a Potential Inhibitor of COVID 19 Corona Virus Infection by Molecular Docking Studies. Preprints. 2020;2020030455.
- Shree P, Mishra P, Selvaraj C, et al. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J Biomol Struct Dyn. 2022;40(1):190-203.
Crossref - Soni VK, Mehta A, Ratre YK, et al. Curcumin, a traditional spice component, can hold the promise against COVID-19? Eur J Pharmacol. 2020;886:173551.
Crossref - Narkhede RR, Pise AV, Cheke RS, Shinde SD. Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences. Nat Products Bioprospect. 2020;10(5):297-306.
Crossref - Rajagopal K, Varakumar P, Baliwada A, Byran G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach. Futur J Pharm Sci. 2020;6(1).
Crossref - Vijayakumar BG, Ramesh D, Joji A, Jayachandra prakasan J, Kannan T. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur J Pharmacol. 2020;886:173448.
Crossref - Cherrak SA, Merzouk H, Mokhtari-Soulimane N. Potential bioactive glycosylated flavonoids as SARS-CoV-2 main protease inhibitors: A molecular docking and simulation studies. PLoS One. 2020;15(10).
Crossref - Park JY, Yuk HJ, Ryu HW, et al. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32(1):504-512.
Crossref - Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S. Potential Inhibitor of COVID-19 Main Protease ( M pro ) from Several Medicinal Plant Compounds by Molecular Docking Study. Preprints. 2020:1-14. https://www.preprints.org/manuscript/202003.0226/v1
- Gogoi N, Chowdhury P, Goswami AK, Das A, Chetia D, Gogoi B. Computational guided identification of a citrus flavonoid as potential inhibitor of SARS-CoV-2 main protease. Mol Divers. Published online 2021;25:1745-1759.
Crossref - Meyer M, Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. Am J Physiol – Lung Cell Mol Physiol. 2015;308(12):1189-1201.
Crossref - Alfaro M, Alfaro I, Angel C. Identification of potential inhibitors of SARS-CoV-2 papain-like protease from tropane alkaloids from Schizanthus porrigens: A molecular docking study. Chem Phys Lett. 2020;761:138068.
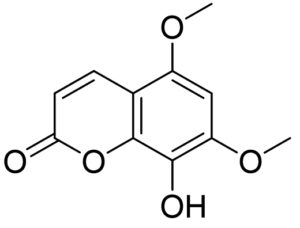
Crossref - QY Y, XY T, WS F. Bioactive coumarins from Boenninghausenia sessilicarpa. J Asian Nat Prod Res. 2007;9(1):59-65. https://pubmed.ncbi.nlm.nih.gov/17365191/
- Lau SKP, Woo PCY, Li KSM, et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A. 2005;102(39):14040-14045.
Crossref - Kim DE, Min JS, Jang MS, et al. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus oc43 infection of mrc-5 human lung cells. Biomolecules. 2019;9(11):696.
Crossref - Cheng PW, Ng LT, Chiang LC, Lin CC. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin Exp Pharmacol Physiol. 2006;33(7):612-616.
Crossref - Fan HH, Wang LQ, Liu WL, et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin Med J (Engl). 2020;133(9):1051-1056.
Crossref - Emirik M. Potential therapeutic effect of turmeric contents against SARS-CoV-2 compared with experimental COVID-19 therapies: in silico study. J Biomol Struct Dyn. 2022;40(5):2024-2037.
Crossref - Rajagopal K, Byran G, Jupudi S, Vadivelan R. Activity of phytochemical constituents of black pepper, ginger, and garlic against coronavirus (COVID-19): An in silico approach. Int J Heal Allied Sci. 2020;9(5):43.
Crossref - Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta – Mol Basis Dis. 2017;1863(2):585-597.
Crossref - Chen B, Lu Y, Chen Y, Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol. 2015;225(3):R83-R99.
Crossref - Zhao H, Eguchi S, Alam A, Ma D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am J Physiol – Lung Cell Mol Physiol. 2017;312(2):L155-L162.
Crossref - Beltran-Garcia J, Osca-Verdegal R, Pallardo FV., et al. Oxidative stress and inflammation in covid-19-associated sepsis: The potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants. 2020;9(10):936.
Crossref - Laforge M, Elbim C, Frere C, et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):515-516.
Crossref - Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47(1):89-116.
- Kobayashi EH, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624.
Crossref - Motterlini R, Nikam A, Manin S, et al. HYCO-3, a dual CO-releaser/Nrf2 activator, reduces tissue inflammation in mice challenged with lipopolysaccharide. Redox Biol. 2019;20:334-348.
Crossref - Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 2008;76(8):967-973.
Crossref - Thimmulappa RK, Scollick C, Traore K, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351(4):883-889.
Crossref - Foldi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes Rev. 2020;21(10):e13095.
Crossref - Mendonca P, Soliman KFA. Flavonoids activation of the transcription factor NRF2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants. 2020;9(8):659.
Crossref - Yang L, Stockigt J. Trends for diverse production strategies of plant medicinal alkaloids. Nat Prod Rep. 2010;27(10):1469-1479.
Crossref - Choy KT, Wong AYL, Kaewpreedee P, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786.
Crossref - Liu S, Chen Q, Liu J, Yang X, Zhang Y, Huang F. Sinomenine protects against E.coli-induced acute lung injury in mice through Nrf2-NF-ךB pathway. Biomed Pharmacother. 2018;107:696-702.
Crossref - Li J, Zhao L, He X, Zeng YZ, Dai SS. Sinomenine Protects against Lipopolysaccharide-Induced Acute Lung Injury in Mice via Adenosine A2A Receptor Signaling. PLoS One. 2013;8(3):e59257.
- Wang X, He P, Yi S, Wang C. Thearubigin regulates the production of Nrf2 and alleviates LPS-induced acute lung injury in neonatal rats. 3 Biotech. 2019;9(12):451.
Crossref - SiMei L, TieChui Y, TseWai M, et al. Isosteroid alkaloids from Fritillaria cirrhosa bulbus as inhibitors of cigarette smoke-induced oxidative stress. Fitoterapia. 2020;140:104434.
Crossref - Wang D, Yang J, Du Q, Li H, Wang S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J Ethnopharmacol. 2016;193:150-158.
Crossref - Garg S, Roy A. In silico analysis of selected alkaloids against main protease (Mpro) of SARS-CoV-2. Chem Biol Interact. 2020;332:109309.
Crossref - Yang Y, Xiu J, Zhang X, et al. Antiviral effect of matrine against human enterovirus 71. Molecules. 2012;17(9):10370-10376.
Crossref - Lu Y, Xu D, Liu J, Gu L. Protective effect of sophocarpine on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2019;70:180-186.
Crossref - Zhang D, Li X, Hu Y, et al. Tabersonine attenuates lipopolysaccharide-induced acute lung injury via suppressing TRAF6 ubiquitination. Biochem Pharmacol. 2018;154:183-192.
Crossref - Liang Y, Fan C, Yan X, et al. Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phyther Res. 2019;33(1):130-148.
Crossref - Li WW, Wang TY, Cao B, et al. Synergistic protection of matrine and lycopene against lipopolysaccharide-induced acute lung injury in mice. Mol Med Rep. 2019;20(1):455-462.
Crossref - Lu XG, Pu YX, Kong WG, et al. Antidesmone, a unique tetrahydroquinoline alkaloid, prevents acute lung injury via regulating MAPK and NF-ךB activities. Int Immunopharmacol. 2017;45:34-42.
Crossref - Huang H, Hu G, Wang C, Xu H, Chen X, Qian A. Cepharanthine, an alkaloid from Stephania cepharantha Hayata, inhibits the inflammatory response in the RAW264.7 cell and mouse models. Inflammation. 2014;37(1):235-246.
Crossref - Luo Z, Liu LF, Wang XH, et al. Epigoitrin, an Alkaloid from Isatis indigotica, Reduces H1N1 infection in stress-induced susceptible model in vivo and in vitro. Front Pharmacol. 2019;10.
Crossref - Liang XM, Guo GF, Huang XH, Duan WL, Zeng ZL. Isotetrandrine protects against lipopolysaccharide-induced acute lung injury by suppression of mitogen-activated protein kinase and nuclear factor-kappa B. J Surg Res. 2014;187(2):596-604.
Crossref - Zhao L, Wang X, Chang Q, et al. Neferine, a bisbenzylisoquinline alkaloid attenuates bleomycin-induced pulmonary fibrosis. Eur J Pharmacol. 2010;627(1-3):304-312.
Crossref - Fu JJ, Wang YT, Zhang JX, Wu W, Chen XY, Yang YR. Anti-inflammatory and anti-apoptotic effects of oxysophoridine on lipopolysaccharide-induced acute lung injury in mice. Am J Transl Res. 2015;7(12):2672-2682.
- Jahan I, Onay A. Potentials of plant-based substance to inhabit and probable cure for the covid-19. Turkish J Biol. 2020;44(3):228-241.
Crossref - Chang SJ, Chang YC, Lu KZ, Tsou YY, Lin CW. Antiviral activity of Isatis indigotica extract and its derived indirubin against Japanese encephalitis virus. Evidence-based Complement Altern Med. 2012;2012:925830.
Crossref - Deftereos S, Giannopoulos G, Vrachatis DA, et al. Colchicine as a potent anti-inflammatory treatment in COVID-19: Can we teach an old dog new tricks? Eur Hear J – Cardiovasc Pharmacother. 2020;6(4):255.
Crossref - Li BQ, Fu T, Dongyan Y, Mikovits JA, Ruscetti FW, Wang JM. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem Biophys Res Commun. 2000;276(2):534-538.
Crossref - Li R, Wang L. Baicalin inhibits influenza virus A replication via activation of type I IFN signaling by reducing miR 146a. Mol Med Rep. 2019;20(6):5041-5049.
Crossref - Zandi K, Musall K, Oo A, et al. Baicalein and baicalin inhibit sars-cov-2 rna-dependent-rna polymerase. Microorganisms. 2021;9(5):893.
Crossref - Ding Y, Dou J, Teng Z, et al. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch Virol. 2014;159(12):3269-3278.
Crossref - Nayak MK, Agrawal AS, Bose S, et al. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J Antimicrob Chemother. 2014;69(5):1298-1310.
Crossref - Zhu HY, Han L, Shi XL, et al. Baicalin inhibits autophagy induced by influenza A virus H3N2. Antiviral Res. 2015;113:62-70.
Crossref - Sithisarn P, Michaelis M, Schubert-Zsilavecz M, Cinatl J. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 2013;97(1):41-48.
Crossref - Song J, Zhang L, Xu Y, et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem Pharmacol. 2021;183:114302.
Crossref - S Sun, S Huang, Y Shi, et al. Extraction, isolation, characterization and antimicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chemistry. 2021;351: 129232.
Crossref - Liu B, Yang J, Ma Y, Yuan E, Chen C. Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of ethanol extract and pure flavonoids from Adinandra nitida leaves. Pharm Biol. 2010;48(12):1432-1438.
Crossref - Nguyen HQ, Nguyen TNL, Doan TN, et al. Complete chloroplast genome of novel Adrinandra megaphylla Hu species: molecular structure, comparative and phylogenetic analysis. Sci Rep. 2021;11(1):11731.
Crossref - Clergeaud G, Dabbagh-Bazarbachi H, Ortiz M, Fernandez-Larrea JB, O’Sullivan CK. A simple liposome assay for the screening of zinc ionophore activity of polyphenols. Food Chem. 2016;197(pt A):916-923.
Crossref - Kang J, Liu C, Wang H, et al. Studies on the bioactive flavonoids isolated from pithecellobium clypearia benth. Molecules. 2014;19(4):4479-4490.
Crossref - Sookkongwaree K, Geitmann M, Roengsumran S, Petsom A, Danielson UH. Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Pharmazie. 2006;61(8):717-721.
- Tewtrakul S, Nakamura N, Hattori M, Fujiwara T, Supavita T. Flavanone and flavonol glycosides from the leaves of Thevetia peruviana and their HIV-1 reverse transcriptase and HIV-1 integrase inhibitory activities. Chem Pharm Bull. 2002;50(5):630-635.
Crossref - Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T. Inhibitory effects of (-)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antiviral Res. 2002;53(1):19-34.
Crossref - Kawai K, Tsuno NH, Kitayama J, et al. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J Allergy Clin Immunol. 2003;112(5):951-957.
Crossref - Nance CL, Siwak EB, Shearer WT. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J Allergy Clin Immunol. 2009;123(2):459-465.
Crossref - Fassina G, Buffa A, Benelli R, Varnier OE, Noonan DM, Albini A. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. Aids. 2002;16(6):939-941.
Crossref - Williamson MP, McCormick TG, Nance CL, Shearer WT. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. J Allergy Clin Immunol. 2006;118(6):1369-1374.
Crossref - Qiu W, Su M, Xie F, et al. Tetrandrine blocks autophagic flux and induces apoptosis via energetic impairment in cancer cells. Cell Death Dis. 2014;5(3):e1123.
Crossref - Sathasivam R, Radhakrishnan R, Hashem A, Abd_Allah EF. Microalgae metabolites: A rich source for food and medicine. Saudi J Biol Sci. 2019;26(4):709-722.
Crossref - Xue L, Liu P. Daurisoline inhibits hepatocellular carcinoma progression by restraining autophagy and promoting cispaltin-induced cell death. Biochem Biophys Res Commun. 2021;534:1083-1090.
Crossref - Li X, Yu HY, Wang ZY, Pi HF, Zhang P, Ruan HL. Neuroprotective compounds from the bulbs of Lycoris radiata. Fitoterapia. 2013;88:82-90.
Crossref - Gendrot M, Andreani J, Boxberger M, et al. Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation. Travel Med Infect Dis. 2020;37:101873.
Crossref - He CL, Huang LY, Wang K, et al. Identification of bis-benzylisoquinoline alkaloids as SARS-CoV-2 entry inhibitors from a library of natural products. Signal Transduct Target Ther. 2021;6(1):131.
Crossref - Li SY, Chen C, Zhang HQ, et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18-23.
Crossref - Shen L, Niu J, Wang C, et al. High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J Virol. 2019;93(12):e00023.
Crossref - Zhang YN, Zhang QY, Li XD, et al. Gemcitabine, lycorine and oxysophoridine inhibit novel coronavirus (SARS-CoV-2) in cell culture. Emerg Microbes Infect. 2020;9(1):1170-1173.
Crossref - Fielding BC, Filho C da SMB, Ismail NSM, de Sousa DP. Alkaloids: Therapeutic potential against human coronaviruses. Molecules. 2020;25(23):5496.
Crossref - STS H. Shedding Light on the Effect of Natural Anti-Herpesvirus Alkaloids on SARS-CoV-2: A Treatment Option for COVID-19. Viruses. 2020;12(4)476.
Crossref - Panigrahi GK, Sahoo SK, Sahoo A, et al. Bioactive molecules from plants: a prospective approach to combat SARS-CoV-2. Adv Tradit Med. 2021.
Crossref - Manli Wang, Ruiyuan Cao, Leike Zhang, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. https://www.nature.com/articles/s41422-020-0282-0
- Martins BX, Arruda RF, Costa GA, et al. Myrtenal-induced V-ATPase inhibition – A toxicity mechanism behind tumor cell death and suppressed migration and invasion in melanoma. Biochim Biophys Acta – Gen Subj. 2019;1863(1):1-12.
Crossref - Wen CC, Kuo YH, Jan JT, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50(17):4087-4095.
Crossref - Wang T yang, Li Q, Bi K shun. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J Pharm Sci. 2018;13(1):12-23.
Crossref - Kim YS, Ryu YB, Curtis-Long MJ, et al. Flavanones and rotenoids from the roots of Amorpha fruticosa L. that inhibit bacterial neuraminidase. Food Chem Toxicol. 2011;49(8):1849-1856.
Crossref - Carvalho O V., Botelho C V., Ferreira CGT, et al. In vitro inhibition of canine distemper virus by flavonoids and phenolic acids: Implications of structural differences for antiviral design. Res Vet Sci. 2013;95(2):717-724.
Crossref - Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutr Sci. 2016;5.
Crossref - Liu AL, Wang H Di, Lee SMY, Wang YT, Du GH. Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorganic Med Chem. 2008;16(15):7141-7147.
Crossref - Wu T, He M, Zang X, et al. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim Biophys Acta – Biomembr. 2013;1828(11):2751-2756.
Crossref - Nguyen TTH, Moon YH, Ryu YB, et al. The influence of flavonoid compounds on the in vitro inhibition study of a human fibroblast collagenase catalytic domain expressed in E. coli. Enzyme Microb Technol. 2013;52(1):26-31.
Crossref - Resorcinol Structure and Physical Properties. Resorcinol. Published online 2005:1-9.
Crossref - Muchtaridi M, Fauzi M, Ikram NKK, Gazzali AM, Wahab HA. Natural Flavonoids as Potential Angiotensin-Converting Enzyme 2 Inhibitors for Anti-SARS-CoV-2. Molecules. 2020;25(17):3980.
Crossref - Park JY, Ko JA, Kim DW, et al. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzyme Inhib Med Chem. 2016;31(1):23-30.
Crossref - Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144.
Crossref - Nassiri-Asl M, Hosseinzadeh H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phyther Res. 2009;23(9):1197-1204.
Crossref - Chen L, Li J, Luo C, et al. Binding interaction of quercetin-3-b-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: Structure-activity relationship studies reveal salient pharmacophore features. Bioorganic Med Chem. 2006;14(24):8295-8306.
Crossref - Pandey P, Khan F, Mazumder A, Rana AK, Srivastava Y. Inhibitory potential of dietary phytocompounds of nigella sativa against key targets of novel coronavirus (Covid-19). Indian J Pharm Educ Res. 2021;55(1):190-197.
Crossref - Yu MS, Lee J, Lee JM, et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorganic Med Chem Lett. 2012;22(12):4049-4054.
Crossref - Cho JK, Curtis-Long MJ, Lee KH, et al. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorganic Med Chem. 2013;21(11):3051-3057.
Crossref - Kim DW, Seo KH, Curtis-Long MJ, et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzyme Inhib Med Chem. 2014;29(1):59-63.
Crossref - Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020;156:104761.
Crossref - NguyenTTH, Woo HJ, Kang HK, et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris . Biotechnol Lett, 2012; 34:831–838.
Crossref - Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front Immunol. 2020;11:1451.
Crossref - Jo S, Kim S, Shin DH, Kim MS. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35(1):145-151.
Crossref - Bבez-Santos YM, St. John SE, Mesecar AD. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21-38.
Crossref - Islam F, Bibi S, Meem AFK, et al. Natural bioactive molecules: An alternative approach to the treatment and control of covid-19. Int J Mol Sci. 2021;22(23):12638.
Crossref - Chikhale R, Sinha SK, Wanjari M, et al. Computational assessment of saikosaponins as adjuvant treatment for COVID-19: molecular docking, dynamics, and network pharmacology analysis. Mol Divers. 2021;25(3):1889-1904.
Crossref - Chang FR, Yen CT, Ei-Shazly M, et al. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat Prod Commun. 2012;7(11):1415-1417.
Crossref - Mahmud S, Paul GK, Afroze M, et al. Efficacy of phytochemicals derived from avicennia officinalis for the management of covid-19: A combined in silico and biochemical study. Molecules. 2021;26(8):2210.
Crossref - Park JY, Kim JH, Kim YM, et al. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorganic Med Chem. 2012;20(19):5928-5935.
Crossref - Mutiawati E, Fahriani M, Mamada SS, et al. Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms – a systematic review and meta-analysis. F1000Research. 2021;10:40.
Crossref - Lusvarghi S, Bewley CA. Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential. Viruses. 2016; 8(10):296.
Crossref - Meuleman P, Albecka A, Belouzard S, et al. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob Agents Chemother. 2011;55(11):5159-5167.
Crossref - Millet JK, Sיron K, Labitt RN, et al. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res. 2016;133:1-8.
Crossref - Vijayaraj R, Altaff K, Rosita AS, Ramadevi S, Revathy J. Bioactive compounds from marine resources against novel corona virus (2019-nCoV): in silico study for corona viral drug. Nat Prod Res. Published online 2020:1-5.
Crossref - Bhatt A, Arora P, Prajapati SK. Can Algal Derived Bioactive Metabolites Serve as Potential Therapeutics for the Treatment of SARS-CoV-2 Like Viral Infection? Front Microbiol. 2020;11.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.