Postbiotics are an emerging field in gut and gastroenterological research. Despite it being a vast field, limited scientific research has been conducted on this topic. Postbiotics are functional bioactive compounds generated in the cell wall matrix during fermentation that may be used to promote health. Postbiotics play a critical role in human immune development against communicable and noncommunicable diseases. This review focuses on the recent advances and future perspectives of postbiotics in health and food science. The review also discussed the criteria and different types of postbiotics and elucidated the significance of postbiotics. The paper further reviewed the role of postbiotics as preservatives, active ingredients in packaging systems, anti-biofilm agents, and decontaminant agents in food processing industries.
Gut Microbes, Probiotic, Prebiotic, Secondary Metabolites, Cellular Components, Anti- Inflammatory, Immuno-modulation, Bio-preservative
Postbiotics emerged after the terms prebiotic, probiotic, and synbiotic. Postbiotics are metabolic leftovers of probiotic bacteria or the gut microbiota.1 To use a scientific term, they are byproducts of the primary metabolism of probiotics or gut microbes. Non-digestible carbohydrates have a major impact on postbiotics production in the gut, which is highly dependent on the living microbiota of the individual or host. Postbiotics synthesized in the body are metabolized by the liver, kidneys, and other organs. Postbiotics are like magic bullets2 that control immune system effector molecules and signaling pathways.3 Insufficient postbiotics availability in the human system results in the emergence of neurological disorders, damage to the intestinal wall, and unbalanced homeostasis, resulting in metabolic syndromes such as cancer.4 Postbiotics can be readily and thoroughly extracted from the probiotic or gut microbiota. To maximize postbiotics production, it is helpful to provide prebiotics such as resistant starch and different dietary fibers to the gut microbes living in human digestive system.5 Recently, the FDA approved Rebyota, a fecal microbiota, for the prevention of Clostridioides difficile infection.
Major sources of bibliometric information, such as Web of Science, Scopus, PubMed, and Google Scholar, were extensively searched with keywords on postbiotics, health benefits of postbiotics, and their mechanism of formation and actions. From various search engines, a database of 128 papers was obtained. Thirty-two publications met the review criteria. The main purpose of this article is to provide an overview of postbiotics, focusing on their usefulness in the food and pharmaceutical industries as well as their overall quality.
Evolution of the term Postbiotics
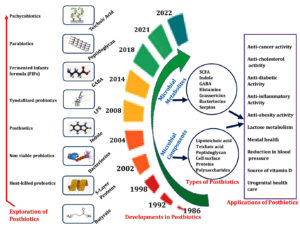
Several hypotheses have been proposed to characterize postbiotics.6 Figure 1 shows the developments in postbiotics.7 According to previous researchers, Postbiotics are non-viable probiotics or non-toxic, and metabolomes of the oral cavity, skin, urogenital tract, and gut.8,9 In contrast, postbiotics are defined by the International Scientific Association for Probiotics and Prebiotics (ISAPP) as “the preparation of inanimate microorganisms and/or their components that confers a health benefit on the host”.1 Inactive microbial cells or their cellular components with or without useful metabolites may also be included as postbiotics.6
Criteria developed by ISAPP for the preparation of Postbiotics
The ISAPP developed the following criteria for the preparation of postbiotics.
- Molecular characterization of the progenitor microbes by sequencing their genes.
- Detailed description of the inactivation methods and the matrix.
- Confirmation that inactivation has occurred.
- Evidence of health benefits in the host from a controlled, high-quality trial.
- Detailed description of the composition of the postbiotic preparation.
- Assessment of safety of the postbiotic preparation in the target host for the intended use.
Production of postbiotics
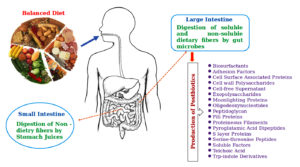
Non-digestible dietary fibers, resistant starches, and non-starch polysaccharides are fermented by colonic microbes under anaerobic conditions in the digestive systems of humans and animals (Figure 2).12 Such a beneficial microbes are known as probiotics,13 where their metabolic products are known as postbiotics.
Types of postbiotics
Many hypotheses have been proposed in previous studies regarding the types of postbiotics. According to Ailioaie et al., postbiotics can be broadly classified as paraprobiotics and Fermented Infant or follow-on formula (FIF).14 Paraprobiotics – otherwise known as ghost probiotics, non-viable probiotics, or inactivated probiotics, are now often defined as non-viable or inactivated microbial cells, which, when administered in sufficient amounts, confer benefits to the host (Salminen et al.). Fermented Infant Formulation (FIF) are infant or follow-on formulae that have been fermented with lactic acid-producing or other bacteria,15 and in most cases do not contain viable bacteria.
However, according to the ISAPP guidelines, postbiotics can be either,
(a) Probiotic organisms and inactive probiotic/gut microbes
(b) Cell-free supernatants (a mixture of compounds produced by microbes)4
(c) Primary and secondary metabolites
Table shows the functional products of microbial metabolites which can act as postbiotics
Table:
List of secondary metabolites (Postbiotics)
Microbial metabolites |
References |
|---|---|
Bioactive peptides |
Cuevas-González et al.16 |
secreted proteins, peptides |
Koleilat17 |
Secreted biosurfactants (Laurostearic acid), Aggregation promoting factor (APF) |
Drolia et al.18 |
Neurotransmitters (GABA) |
Chudzik et al.19 |
polyunsaturated fatty acids (Arachidonic acid, docosahexaenoic acid, linoleic acid, linolenic acids, Omega-3-fatty acids) |
Nazarii et al.20 |
Polypeptides (Bacitracin, Nisin, Reuterin) |
Cabello-Olmo et al.21 |
Bacteriocins: lanthionine-containing bacteriocins (class I) and the nonlanthionine-containing bacteriocins (class II) |
Jastrząb et al.22 |
Vitamin B complex (Biotin, cobalamin, folate, niacin, pantothenic acid, pyridone, riboflavin, thiamine) |
Qing et al.23 |
Aromatic amino acids, Polyamines (Putrescine, cadaverine, spermidine, and spermine) |
Maria et al.,24 |
Lactocepin |
Pandey et al.7 |
Retinoic acid, Long chain fatty acids, Trimethylamine – N-Oxide (TMO), Conjugated linoleic acid (cla) |
Hamid et al.25 |
Superoxide dismutase, catalase, Enterocins, Serpins, Histamine |
Pandey et al.7 |
Functional proteins, teichoic acids, p40 and p75 proteins. |
Walhe et al.26, Lee et al.27, Bauerl et al.28 |
Functional role of commonly known postbiotics (probiotic secretions)
Short-chain Fatty Acids (SCFAs)
Short-chain fatty acids are monocarboxylic acids containing two to six carbon atoms. SCFAs are produced in the large intestine of the digestive system through anaerobic fermentation of indigestible carbohydrates, such as those found in foods high in dietary fiber (which are typically high in cellulose, pectin, hemicellulose, lignin, and resistant starch). It improves the metabolic efficiency of the host microbiome by increasing the transfer of carbon from the diet to the microbiome.29 The movement of SCFAs from colonococci to the bloodstream is typically facilitated by H+-dependent or sodium-dependent monocarboxylate transporters (MCTs and SMCTs).30 Eg: Acetate, Butyrate, Propionate, Formic acid, Valeric acid, Caproic acid, b-hydroxybutyric acids;30 Short-chain galacto-oligosaccharides (scGOS) and long-chain fructo-oligosaccharides (lcFOS).5
Important functions of SCFAs include signaling for TREG (Regulatory T Cells) differentiation to reduce inflammation, which is important in brown tissue activation, functional regulation of liver mitochondria, and regulation of neutral intracellular signal mechanisms in the peripheral nervous system, central nervous system, and gut-associated immune tissues. During the cell cycle, SCFAs play important roles in stimulating the activity of histone deacetylase (HDAC) by increasing the acetylation of lysine residues of nucleosomal histones. Epigenetic cofactors/substrates produced by gut microbiota31 cause DNA methylation and histone modifications. A recent study has summarized the role of SCFAs in inhibiting SARS-CoV-2 surface proteins.11
SCFAs are naturally produced through host metabolic pathways, especially in the liver; however, the colon is the primary site of production and requires the presence of certain colonic bacteria. At concentrations of 500-600 mM, acetate, propionate, and butyrate were the most abundant SCFAs in the feces of healthy humans. Extremely high concentrations (70-140 mM) are released in the proximal colon, with lower concentrations (20-70 mM) in the distal colon and (20-40 mM) in the distal ileum. Non-metabolized SCFAs, except acetate, in colonocytes are utilized in the liver to provide energy to hepatocytes helps to increase gut-brain communications.
SCFAs in the human gut typically cause mucus production in the colorectal region and aid in the protection of the intestinal barrier through their anti-inflammatory effects. Cancer of the colon is present in this area. Irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), obesity, metabolic syndrome, type 2 diabetes, cancer, and autoimmune diseases are all linked to insufficient SCFA availability, which in turn disrupts homeostasis and causes functional disorders. SCFAs inhibit histone deacetylases and function as G protein-coupled receptors.30
Recent research has shown that butyrate and propionate can increase the levels of hormones in the digestive tract, leading to diminished hunger. Bacteroidetes, on the other hand, primarily produce acetate and propionate, and Firmicutes, butyrate.30 Because acetate acts as a lipogenic substrate and propionate inhibits lipogenesis by downregulating fatty acid synthase in the liver, the acetate/propionate ratio is thought to be significant for de novo lipogenesis.32
Loss of body weight and adiposity may be facilitated by propionate and butyrate, which may activate intestinal gluconeogenesis and induce the expression of gluconeogenesis-related genes.3 Both Brown and White adipose tissues respond to acetate by increasing browning activity. The importance of the bacterial production of SCFAs in the physiology of the gastrointestinal tract has led to the hypothesis that alterations in the abundance of acetate, propionate, and butyrate are linked to a decline in health.
Extracellular Vesicles (EVs)
EVs or membrane vesicles are present in the lipid bilayers of cells. It facilitates interkingdom crosstalk and has immunomodulatory and anti-inflammatory responses against pathogens. They are the main therapeutic indicators of functional brain disorders, such as mood disorders33 and neuropathological conditions. It is one of the key segments of probiotic microbes and is involved in TREG (Regulatory T Cells) cell activation. Beneficial bacteroids have been identified in EVs.34 Lactobacillus casei BL23 EVs have been identified to contain lipoteichoic acid and P40 and P75 proteins with proinflammatory activity and induce epidermal growth factor receptors. EVs from Akkermansia muciniphila have been shown to regulate colitis.35
Anti-microbial Peptides (AMPs)
Bacteriocin is a widely studied AMP. They are produced by both Gram-positive and Gram-negative bacteria;36 and have broad-spectrum antimicrobial activity.37 The benefits of bacteriocins are based on six pillars, namely spectrum, stability, bioengineering, diversity, production, and safety.
There are four classes of bacteriocins: Class I: bacteriocins include proteolytic and heat-resistant small peptides substantially modified by specific enzymes at the transcriptional level, lantibiotics, sactypeptides, glycokines, and loop peptides. Class II: bacteriocins are subdivided into four subtypes (at least): pediocin-like, two peptides, circular, and linear non-pediocin-like. They are comprised of small temperature- and pH-resistant peptides without minor or minor post-transcriptional processing, such as disulfide bonds. Class III: Bacteriocins or bacteriolysins incorporate large thermolabile proteins > 10 kDa. Class IV bacteriocins consist of complex proteins conjugated to lipids or carbohydrates.2
Health benefits of postbiotics
Postbiotics can act as effector molecules to trigger molecular signals in the immune system.3 They improve nutrient absorption and release biologically active molecules as needed to maintain metabolic, immune, and neural networks in the body in balance.22 Inadequate postbiotic production disturbs the homeostatic equilibrium of host organisms.38 However, their instigation and potential activity are linked to the host organism.39 With the help of postbiotics, nutrients can be delivered directly to the intestines, where they have the greatest impact, extending their shelf life and reducing logistical burdens. Intestinal microbes and their hosts communicate via molecular interactions following the production of bioactive metabolites (short-chain fatty acids) or via interactions with the host immune cells via cell-surface molecules.40 Postbiotics are effective for treating IBS and related syndromes,41 reducing lactose intolerance, enhancing the absorption of essential amines42 and maintaining steady mucin secretion.43 Postbiotics have the potential to replace antibiotics in the treatment of both communicable9 and noncommunicable.44
Investigations from clinical demonstrations have revealed that a host’s health status and disease complications are significantly associated with lifestyle factors, especially diet.45 Phenolic and bio-active components such as probiotics, prebiotics, synbiotics, and postbiotics, in addition to macro and micro nutrients, play a vital role as health-promoting ingredients in functional foods.46
Figure 3. Assumed mechanism of action of Probiotics, Prebiotics and Postbiotics modified from Michela et al.47
Figure 3 shows the proposed mechanism of action of probiotics, prebiotics, and postbiotics. In maintaining gut homeostasis, organisms/probiotics like Lactobacillus, Bifidobacterium and Clostridiales plays a major role (A) Colonization and proliferation of pathogens is prevented by competing for microenvironment and nutrients. (B) By lowering luminal pH antimicrobial peptides are released with direct bactericidal effect. Probiotics increase of mucin production, expression of tight junctions are enhanced and also promote epithelial restoration. From probiotics prebiotics are derived. (C) By the process of fermentation postbiotics are produced. (D) Gut epithelium directly absorbs oligosaccharides. To relase IL-10, IFN-g they stimulate CD4+ and T-cell. (E) Apoptosis increased, against cancer cells cytotoxicity increased. (F) Inhibition of apoptosis and the level of Ig A, IFN-g and IL-10 increased.47
In addition to optimizing the health benefits attributed to probiotics, a new concept of postbiotics or nonbiotics has been introduced.45 By stimulating the immune system, postbiotics are associated with anti-inflammatory development in the intestine and bowel, as well as immunomodulatory, anti-obesogenic, antihypertensive, anti-proliferative, antioxidative, and hypocholesterolemic activities. It has been reported that the fermentation of intestinal bacteria or its structural fragments generates postbiotics that greatly influence host physiology and have an impact on health. In view of the enzymatic effect on microorganisms, it has been reported that enzymes such as subtilisin-like protease and glutamyl endopeptidase are efficient against degradation and eradication of extracellular polymeric substances and biofilms generated by Serratia marcescens, respectively.48 However, the impacts of macro-and micronutrients from postbiotic sources have also gained significant importance in health. Microbiota-secreted vitamins seem to be protective against Coronary Artery Disease and osteoporosis.49 This implies that the potential mechanism of the effect of postbiotics is anti-inflammatory, immunomodulatory, anti-cancer, anti-tumor, and anti-proliferative activities, which include regulation of the central nervous system and anti-atherosclerotic, hypocholesterolemic, cardioprotective, and anti-hypersensitive effects.50
In addition to the above-mentioned mechanisms, the hepatoprotective and anti-ulcerative properties are also health benefits of postbiotics.50 In addition, intestinal permeability and modulation of microbial activity, along with prebiotic effects, are maintained by the action of postbiotics. As a regulatory mechanism, the inhibition of pathogens, antibiofilm, and anti-adhesion effects are taken care of by the effect of postbiotics to promote health.51
An important emphasis has been placed on the similarity between postbiotics and probiotics in terms of their properties, owing to species and strain specificity. In addition, microbial progenitors that are used for their formulation act as dependency factors for postbiotic actions. In addition, the substrate or matrix, which are the sites for postbiotic production, also turn out to be a deciding factor for the resulting postbiotic. The mechanisms of action of postbiotics must be correlated with the release of a myriad of products, including metabolites and cellular components from resident microbes.52 This adds up to the major significance of the host, while these myriad products behave as messengers in microbiota-host interactions.33 Carbello-Olmo et al. inferred that the gastrointestinal microbiota (GM) could be a major source of postbiotic constituents.53
The function of postbiotics in Diabetes Mellitus (DM)
Resident gastrointestinal microbiota is among the set of environmental factors that are involved in the development and progression of diabetes mellitus (DM).53 A chronic disease such as DM is implicated in an increase in blood glucose levels (hyperglycemia).54 This condition is caused either by beta-cell destruction (Type 1 Diabetes mellitus-T1DM) or the loss of pancreatic function.54 Inadequacy in insulin secretion is attributed to insulin resistivity imposed by peripheral tissues such as the liver, muscle, and adipose tissue (Type 2 Diabetes mellitus-T2D).54
Resident microbes play an interesting role in correlating host energy balance and T2D metabolism, whereas the connectivity between resident microbes and immunity is involved in the development of T1D. However, inflammation is associated with both T1D and T2D.55 Therefore, the reports of Clarke et al. have stated the role of resident microbes in host-energy balance, metabolism, inflammation, and immunity on a widely accepted note.56 Homeostasis in the host occurs through the absorption of nutrients, intestinal permeability, or controlled gene expression. These functions are influenced by gut microbiota.57
A cross-sectional study has inferred the unfriendly composition and activity of diabetic GMs shared among patients with T1D and T2D patients. The actions of this “diabetic microbiota” are associated with the decline of butyrate-producing species, enhancement of opportunistic organisms, and an abundant reduction in gene count attributed to the impairment of metabolic activities, alterations in the transport of nutrients, and enzymatic activity. These fluctuations affect SCFA concentrations and oxidative stress responses.58
In view of animal studies (in rodents) based on microbial exopolysaccharides (EPs) in diabetes mellitus, it was found that the alloxan or streptozotocin (65 mg/Kg BW) induced rodents possessed an increase in serum insulin levels.59 Clinical trials have been performed on T1D models of Sprague-Dawley rats that involved microbial Eps.60 It has also been observed that the levels of HDL increased with a reduction in the levels of Total Cholesterol (TC), Low Density Lipid (LDL), Very Low Density Lipoprotein (VLDL), and Total Glycerides (TG).59 According to Huang et al., an in vitro study conducted on insulin-resistant HepG2 cells, the bioactive EPs component from Lactobacillus plantarum H31-2 showed a decline in the levels of glucose concentration.42 Additionally, inhibition of pancreatic-amylase was also observed, followed by the expression of GLUT-4, Akt-2, and AMPK being upregulated due to the effect of postbiotic component treatment on the model system.
In addition to complications related to DM, a report based on the role of EPs from Bacillus licheniformis in counteracting oxidative stress and preventing diabetic complications by key tissue protection was found to be effective. Tackling diabetes with alterations in GM composition and activity is quite challenging.53 It is very clear that a suitable GM and its postbiotic association with intestinal function would serve as an important factor for the maintenance of proper health and prevention of diabetes in individuals.
Postbiotics’ role in Reactive Oxygen Species (ROS)
Living cells produce reactive oxygen species (ROS) at the molecular level, which are involved in cell signalling by acting as secondary messengers for various metabolic processes.60 Cell and tissue damage occurs because of an imbalance between ROS generation and defenses by anti-oxidant actions (oxidative stress). This could be a result of an increase in ROS production, environmental stressors (UV-B radiation), and xenobiotic compounds (mycotoxins).61 Guerrero Encinas et al. found that rats with Aflatoxin B1-induced oxidative stress had better health when they received intracellular probiotics, such as Lactobacillus casei CRL 431 [IC-431].61 The 2,22 -azinobis (3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) assay and the oxygen radical absorbance capacity (ORAC) assay revealed that IC-431 had antioxidant capacity values of 477 and 282 mol/L, respectively.
Studies suggested that the important anti-oxidant metabolites present in the intracellular fractions of Lactobacillus casei CRL 43162 were saturated and mono-unsaturated fatty acids, as well as glutathione (GSH) and cysteine. Thus, the antioxidant properties of IC-431 were significantly proven by free fatty acids and GSH, as their bioavailability is directly utilized by the cells, which estimates about 10 % of the metabolites from the gut microbiota and is found to be present in the mammalian blood.
Disruptions of bacterial and metabolic by-products undergo physiochemical and physiological processes that have a significant impact on their structure and properties.63 For instance, antioxidant function is affected by the partial oxidation of GSH and cysteine (thiol groups). This reaction is influenced by the pH and partial hydrolysis of GSH molecules that are associated with the disulfide groups of GS-SG and Glu-Cys.64 Lipid damage in cells, tissues, and organs is promoted by uncontrolled biological production of ROS (superoxide anion and hydrogen peroxide).61
Additionally, the mutagenic product of lipid peroxidation is attributed to being malondialdehyde (MDA).61 Mutagenesis and cell death are caused by hydroxide radical reactions. As there is no enzyme specificity against the destruction of hydroxide ions in biological systems, the most effective defense is possible through the reduction of intracellular precursor concentration. Catalases and peroxidases are involved.61 Further dismutation of superoxide radicals into oxygen and hydrogen peroxide is performed by superoxide dismutase (SOD), and this hydrogen peroxide is converted into oxygen and water by the action of catalase (CAT). The final antioxidant activity is expressed in terms of M Trolox equivalents (TE) .61
It was observed that the antioxidant activity was higher and lower malondialdehyde (MDA) levels were observed (TE-dIC-431-Antioxidant capacity of intracellular components of Lactobacillus casei CRL 431 after the digestive process) in the intestinal tissue. This turns out to be a protective mechanism stimulated by IC-431 against oxidative stress.61 This oxidative stress is attributed to mechanical stress caused by the surgical manipulation of the intestine, thereby inducing ROS generation. In rats, Aflatoxin B1 (AFB1) is induced to generate oxidative stress, which is reduced by the intracellular components of Lactobacillus casei (CRL 431).62
It is assumed that part of the antioxidant intracellular components of Lactobacillus casei CRL-431 have a permeability of action through the duodenum intestinal tissue followed by passage into the blood stream to the liver, which is a potential site for participation in different biological processes.61 Based on the ex vivo and in vivo assays conducted by Guerrero-Encinas et al., it was hypothesized that IC-431 showed adaptive responses by modulating mitochondrial function in AFB1-induced oxidative stress conditions in rats.61 According to Guerrero-Encinas et al. the mitochondria are protected naturally by antioxidants and scavengers in an intricate system.61 In this regard, after the observation of lower hydrogen peroxide levels in rats after treating the samples with AFB1 and IC-431, it was revealed that AFB1, which induces mitochondrial damage, was protected after subjecting the samples to IC-431. As IC-431 maintains low levels of hydrogen peroxide, it promotes the expression of antioxidant enzymes in the hepatic mitochondria.61
Further connecting the modulation mechanism of H2O2 with transcription factors in mammalian cells, it was found that low levels of H2O2 act as molecular sensors in the activation and accumulation of the Nrf2 transcription factor in the nucleus, which in turn is responsible for antioxidant element (ARE)-mediated expression.65 Oxygen consumption and isolated AFB1-treated rat livers showed higher mitochondrial membrane potential. This, in turn, shows higher impact of energy capacity on the inner mitochondrial membrane, thereby producing higher ATP synthesis.66
The conclusions drawn from (Guerrero-Encinas et al. states that AFB1 + IC-431 treated mitochondria are responsible for the activation of the physiological uncoupling mechanism for the mitigation of ROS production in the cells occurring through proton leakage induction.61,67 This proton leakage does not affect energy production, as ATP production does not consume all oxygen. Proton leakage induction is involved in controlling oxidative stress via Nrf2 activation.68
The role of postbiotics in the treatment of Cardiovascular Disease (CVD)
Lactobacillus paracasei lives in humans’ mouths and intestines.69 Marra and Svegliati-Baroni reported that a traditional fermented dairy product was the source of this bacterium, which could be credited as a “good” bacterium based on its high lipolytic activity to counteract metabolic syndrome with a bioactive status.70 The lipolytic action of postbiotics limits the formation of complex lipids by influencing the activity of peroxisome proliferator-activated receptors (PPARa), which play a significant role in lipid metabolism control.70
The spectrum of microorganisms producing compounds constitutes postbiotics that act on health through the gut-microbiota-cardiovascular disease (CVD) axis. This occurs by modulation of gut microbiota, elevation of innate immunity, and enrichment of intestinal cells.52 Microbiota-accessible carbohydrates are metabolized by gut microbes to produce SCFAs, which could be involved in host interactions for essential health benefits, especially in the treatment of CVD.71 Endothelial G-protein-coupled receptor 41 is involved in the reduction of blood pressure by an acute microbial SCFA bolus.72
To fight against CVD through modulation of gut microbiota and their metabolites, certain foods and herbs act as postbiotics, such as propionate, which on induction in mice (200 mmol/L) resulted in important findings of alleviated cardiac hypertrophy, fibrosis, vascular dysfunction, and a decrease in cardiac ventricular arrhythmias, aortic atherosclerotic lesion area, and systemic inflammation. Subsequently, magnesium acetate supplementation in mice (200 mmol/L) caused a reduction in systolic and diastolic blood pressures, as well as a decline in cardiac and renal fibrosis, respectively.73 Thus, it can be inferred from the work of Panyod et al. about the kinds of dietary nutrients as CVD risk factor precursors and the preference of food and herb choices to be made as a preventive medicine.73 In addition studies revealed that self-health management tools aimed to reduce CVD-related risks and thereby attain good health and well-being.73
Postbiotics’ role in the treatment of Irritable Bowel Syndrome (IBS)
IBS is a complicated G.I disorder, especially observed among people under 50. This chronic condition is characterized by abdominal pain and bowel dysfunction, presenting as constipation, diarrhea, or alternating periods of both.74 The symptomatic indications for IBS seem to be multifactorial and may include development, persistence following diagnosis, and treatment.74 The features pertaining to The pathophysiology of IBS is based on an increase in intestinal permeability and micro-inflammation, followed by the association of visceral hypersensitivity with gut dysbiosis. In order to promote intestinal homeostasis, postbiotics have been suggested, especially in GI tract inflammation, which involves a mimicking mechanism of probiotics by postbiotics, thereby facilitating the beneficial effects and avoiding the risks of administered live bacteria.
These postbiotic or cell components have shown positive effects on the improvement of host health. Some postbiotic molecules, including acetate, polyP, ACh, and antimicrobial compounds (potentially bacteriocins) produced by probiotic strains, have been used to treat IBS-associated microorganisms.75 The production of acetate relies on the carbon source treated with the strains of L. Plantarum and the efficient results have been observed in the highest acetate concentration production, thereby attributing it to arabinose as its carbon source,75 followed by which large amounts of Ach were also obtained from the strains of Lactobacillus plantarum (12.5 mg/L for CECT7484 and 13.8 mg/L for CECT7485).75 The findings showed that the mechanisms pertaining to the secretion of antimicrobial products from the influence of probiotic bacteria have been recommended for the treatment of diarrhea, lactose intolerance alleviation and reduction of irritable bowel symptoms. In addition, the effect is pronounced in inflammatory bowel diseases by increasing relapse time.
Methods used for the study of postbiotics
Postbiotics can be qualitatively and quantitatively analyzed by several conventional and next-generation techniques, such as flow cytometry, polymerase chain reactions (PCR), enzyme linked immunosorbent assay, Fourier-transform infrared (FT-IR) spectroscopy, NMR, and electron microscopy.1
Potential applications of postbiotics in the food industry
The profound increase in the knowledge of functional foods has resulted in the evolution of novel health products, such as probiotics. The major issue related to probiotics is their antibiotic resistance genes in a few probiotic strains, which have the potential to be transmitted to pathogenic microorganisms through horizontal gene transfer.76 On the other hand, the bacterial viability of food products during processing and storage is another concern for its activity, which may depend on various factors, including pH of the product, temperature, interaction with other microbial species, water activity, nutrient availability, inoculation level, fermentation time, dissolved oxygen level, and formulation techniques (spray drying, freeze drying, spray freeze drying, etc.).77 Therefore, inadequacy of the required microbial load limits its anticipated health benefits and applications in food products.
Conversely, postbiotics, which are metabolic by-products of live bacteria, have been reported to be more stable than probiotics.78 They are employed in different forms of product formulation, such as lyophilization or drying. However, the processing conditions influence its composition and potential activity in food products. Studies showed that freeze drying destroys the hydrogen peroxide level in the product formulation, which is responsible for antimicrobial activity as well as volatile metabolites. Furthermore, antagonistic effects of pH, storage time, and conditions on postbiotics have been documented in the literature.79
Postbiotics as Class-I preservatives
Class-I preservatives refer to compounds obtained from natural sources that are used to enhance the quality and shelf life of food products. A bio-preservative is a Class-I preservative, as it is a remnant or bioactive metabolite derived from bacteria that inhibit foodborne pathogenic microbes.80 The principal advantage of biopreservatives is that they are harmless and do not change the quality of food.81 The antimicrobial properties of postbiotics are mediated by the presence of bacteriocins, organic acids, fatty acids, peptides, vitamins, and hydrogen peroxide. Currently, preservation of perishable foods by the application of postbiotics is a novel technique in the food industry.
Postbiotics as bio-preservatives in dairy products
Milk is considered an excellent vehicle for supplementing consumers with probiotics; however, it is also supposed to be the best medium for microbial contamination, which will spoil dairy products. Furthermore, some external and internal factors negatively affect the viability of probiotics and shelf life of the products.82 In contrast, postbiotics can act as biopreservatives in dairy products to enhance their safety and shelf life. Hamad et al. incorporated a freeze-dried postbiotic mixture obtained from Lactobacillus acidophilus, Lactobacillus plantarum, and Bifidobacterium bifidum into soft cheese.83 Only 1% of postbiotic mixtures of all strains were found to be more effective against pathogenic microorganisms in the product than individual strains.
Garnier et al. demonstrated the antifungal activity of postbiotics in semi-hard cheese and sour cream prepared using three probiotic strains in milk.84 Their results showed a reduction in fungal growth in dairy products without significantly altering the sensory quality. In another study, three different probiotic yoghurts were fortified with postbiotics (0.05–0.15%) prepared from Lactarius volemus.85 Incorporation of postbiotics into the yoghurt samples has presented a significantly higher viable count and essential amino acid content with the best sensory characteristics (at 10% polysaccharide (postbiotics) addition).85 Moradi et al. stated that the postbiotics prepared in MRS media may substantially alter the sensory properties of dairy products, thereby affecting their acceptability in terms of color and appearance.86 Instead, in situ production of postbiotics by co-culturing Lactobacillus species with other probiotic strains has been reported to be the best choice.79
Postbiotics as bio-preservatives in Fish and Meat Products
Microbial contamination can have a significant impact on the nutritional value, taste, and safety of fish and meat, making them even more dangerous to eat.79 Clostridium perfringens and different species of Enterobacteriaceae are the most common microbial contaminants in these commodities.87 Therefore, the application of postbiotics directly to fish, meat, and related products by coating or spraying methods is a superior option for their preservation.
For instance, coating with postbiotic extract is preferred for meat fillets, while spraying techniques are best suited for minced meat.45 Mokhtar et al. demonstrated an extended shelf life of minced meat of up to 3 months when applied with Bifidobacterium lactis Bb-12 and stored at 4 °C.88 Similarly, the application of postbiotics from Lactobacillus rhamnosus EMCC 1105 on minced meat at a concentration of 100 mg/g destroyed Clostridium perfringens during storage at 6°C.89
Furthermore, Moradi et al. reported that the incorporation of postbiotic compounds from Lactobacillus salivarius (35 mg/mL) into minced meat stored at 4 °C minimized psychrotrophic spoilage bacteria.79 Moradi et al. tested the antimicrobial activity of cell supernatants from Lactobacillus salivarious (Ls-BU2) against E. coli in ground beef stored under refrigeration.79 Their results confirmed that cell-free supernatants of Lactobacillus salivarious are effective in controlling the microbial growth and oxidative spoilage of ground beef samples in a dose-dependent manner. Consequently, reports in the literature have confirmed the antimicrobial activity of postbiotics and suggested their role as biopreservatives in meat, fish, and their products.86
Postbiotics as bio-preservatives in fruits and vegetables
The role of postbiotics as antimicrobial agents in fruits and vegetables has been tested in a few studies.90 Postbiotics were applied in a solution form to cut vegetables in the industry. For instance, Lee et al. demonstrated a > 1.5 log reduction in coliforms, aerobic mesophilic bacteria, and mold count in RTE (ready-to-eat) baby leafy vegetables upon the addition of postbiotics (5%) from Leuconostoc mesenteroides WK32 along with 0.1 % grape seed extract.27 In a similar study, a considerably higher reduction (approximately 2.9 log CFU/g) of pathogenic microorganisms, such as Escherichia coli O157: H7 and Listeria monocytogenes was observed in vegetables treated with postbiotics in combination with grape seed extract.
In a recent study, home-processed tomato paste was treated with postbiotics prepared from Lactobacillus plantarum and Lactobacillus acidophilus ATCC 314, and a reduction in the microbial load of Staphylococcus aureus, Aspergillus niger, Escherichia coli, and Aspergillus flavus. This resulted in an enhanced shelf life of tomato paste of up to 25 days at room temperature.91 Another study found that cell-free supernatant (15 mg/mL) of a new Lactobacillus sp. RM1, which is found in traditional Egyptian milk, has antifungal properties against Aspergillus parasiticus in wheat grains.92
Postbiotics in active food packaging systems
Excessive application of preservatives in the food matrix is not recommended, as the spoilage of food starts from the surface due to microbial invasion, particularly fungi.93 The application of postbiotic compounds could be a promising technology to address spoilage and food safety issues in packaging. Despite the effective antimicrobial activity of postbiotic components, their direct incorporation into the food matrix with the aim of enhancing shelf life has few limitations.
For instance, the impact of postbiotic interactions or sequestering with the food components or additives in the food reduces its potential.83 Furthermore, elevated food processing temperatures and pressures adversely affect the stability and concentration of postbiotic components applied to food products. Another drawback is the poor miscibility of certain postbiotic components in the food systems. Hence, the valorization of postbiotics in packaging systems is an ideal choice to extend the shelf life of food. Active packaging is a technique in which the packaging material, food component, and environment positively interact to improve the shelf life and quality of food.94 The advantages of using postbiotics in active packaging systems are as follows:45
- The majority of postbiotics are generally regarded as safe (GRAS) for use in food.
- No adverse effects on eukaryotic cells have been reported.
- They did not affect the intestinal microflora.
- Several postbiotic compounds are resistant to a variety of pH conditions.
- Their antimicobial efficiency has been proven against a wide range of foodborne pathogenic microorganisms, even at low concentrations.
- Some postbiotics are stable at high temperatures and are possible to incorporate into food matrixes by extrusion and spray drying processes.
Postbiotics are used in packaging systems in different forms, such as:
- Inclusion of a coating or adsorbing material in the packaging matrix
- Ionic and covalent linkages are used to immobilise postbiotic components in the polymer matrix.
- Direct incorporation into the packaging system.
- Active material in the packaging systems, which improves the stability and migration.
Among the different forms of postbiotic application in packaging systems, they are directly incorporated into the packaging polymer matrix.
Active packaging films were developed by Beristain-Bauza et al. using whey protein isolate, calcium caseinate, and various concentrations of postbiotics (6, 12, or 18 mg/mL) from Lactobacillus Rhamnosus NRRL B-442.95 Their results showed that a postbiotic concentration of 18 mg/mL presented greater antimicrobial efficiency against Salmonella typhimurium, Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus without altering the properties of the film. Meira et al. prepared a nanocomposite film with corn starch-halloysite clay incorporated with nisin and pediocin.96 The developed nanocomposite films were effective against Listeria monocytogenes and Clostridium perfringens.
The application of halloysite as a nanofiller increased the bacteriocin diffusion rate and enhanced anti-microbial retention in the packaging polymeric matrix. A bacteriocin like substance from Lactobacillus curvatus P99 was used in different concentrations (15.6 µL/mL and 62.5 µL/mL) as an active material in a starch-based edible film. The post-biotic-loaded casing exhibited potential antibacterial activity against Listeria monocytogenes. Furthermore, the storage of sliced cheese in the developed active film for 10 days reduced Listeria monocytogenes levels in packaged cheese without altering its sensory properties.97 In one study, natural and artificial casings were developed using ovine, collagen, porcine, cellulose, and bovine tissues as carriers of postbiotics from L. curvatus ACU-1. Casing was used to wrap sausage meat, and it was found that all postbiotic casings exhibited antimicrobial properties against Listeria innocua and Listeria monocytogenes.98 In another study, a chitosan nanoparticle-based film developed by incorporating postbiotics produced by seven Lactobacillus species, including Lactobacillus plantarum, Lactobacillus helveticus, Lactobacillus rhamnosus, Lactobacillus reuteri, Streptococcus thermophiles, and Enterococcus faecium, showed strong antibacterial and antifungal efficacy in Egyptian cheese.99
Postbiotics as anti-biofilm agents
A biofilm is a network of one or more microorganisms that can develop inside a polysaccharide or protein matrix.100 They are produced by both gram-positive and gram-negative fungi.101 Biofilms are still an important food quality and safety issue in the food industry that requires great attention as they exhibit bacterial resistance to antimicrobials.86 Several studies have demonstrated that postbiotics can be used as antibiofilm agents.88 Generally, two different approaches are used to employ postbiotics as antibiofilm agents. Primarily, postbiotics could be used to prevent biofilm formation and, secondly, to destroy the biofilm developed on the product or material surface. Bacteriocin, biosurfactants and exopolysaccharides are some of the postbiotic compounds with antibiofilm properties.
The antibiofilm property of postbiotics produced by Lactobacillus acidophilus LA5, Lactobacillus casei 431, and Lactobacillus salivarius on the biofilm shaped by Listeria monocytogenes on a polystyrene surface was tested by Moradi et al.79 The results of this study showed that the application of natural acid-based postbiotics effectively damaged biofilm formation. Cui et al. demonstrated the antibiofilm activity of postbiotics derived from Lactobacillus crustorum ZHG 2-1 against biofilm produced by Pseudomonas aeruginosa.102 This ability to hamper biofilm formation is ascribed to the binding ability and disruption of extracellular signal molecules, such as AHL and C4-HSL.
Furthermore, a ZHG 2-1 postbiotic compound was proposed to suppress the regulation and expression of quorum sensing (QS) genes.102 A biofilm developed by Bacillus subtilis BM19 was destroyed by bacteriocins produced by Lactobacillus acidophilus ATCC 4356.103 Petrova et al. reported the antibiofilm potential of postbiotic compounds, Llp1 and L1p2, produced by Lactobacillus rhamnosus GG, against the biofilm matrix developed by S. typhimurium and E. coli.104 This study demonstrated the interaction of postbiotics with pathogenic microbial constituents such as exopolysaccharides, proteins, DNA, and lipids. The target molecules for L1p1 and L1p2 are said to be cellulose and heteropolysaccharide residues of glucose, galactose, glucuronic acid, and fructose in the biofilm matrix, which destabilize the initial biofilm formation.104
Exopolysaccharides are high-molecular-weight polymers produced by bacteria that participate in biofilm development, including bacterial communication and superficial interactions leading to attachment and biofilm formation.105 The exopolysaccharides of Lactobacillus acidophilus A4 suppress genes associated with curli and chemotaxis, which regulate the adhesion capacity of enterohemorrhagic E. coli. Exopolysaccharides from Lactobacillus acidophilus suppress the growth of both Gram positive and Gram negative pathogens and biofilm formation of E.coli O157:H7 with 87% to 94% efficiency depending on the surface.
Postbiotics as decontaminant agents
Recent reports have suggested that postbiotics are a promising area for the decontamination of food from chemicals, such as pesticides, biogenic amines (BA), and mycotoxins. In this context, Mah et al. have focused on the impact of postbitoic compounds on BA levels.106 BAs are a group of low-molecular-weight chemical constituents that have adverse health effects, including tryptamine, tyramine, histamine, cadaverine, and putrescine.107 These compounds are produced in fermented products by the decarboxylation of amino acids, such as lysine, tyrosine, tryptophan, and histidine.106
The role of postbiotics on the destruction of BAs was postulated by Toy and Zogul.108 The following hypothesis was proposed:
- Inhibition of growth of Lactobacillus species producing BAs
- Suppression of BA production by altering the growth conditions such as pH
- Direct destruction of BAs by postbiotics
Postbiotics of Streptococcus thermophiles and Lactobacillus strains at 25% and 50% concentrations efficiently reduced the tyramine production from Staphylococcus aureus. Postbiotics of Lactobacillus acidophilus suppressed the tyramine synthesis at a 50% concentration, as reported by Toy and Ozogul.108 In a study by Xie et al., the influence of postbiotics and heat treatment (100°C, 10 min) from L. plantarum on the growth of Enterobacteria species and diamine production was investigated.109 The results showed a considerable reduction in the growth and diamine production of most Enterobacteria species.109 In another study, Niu et al. had demonstrated the degradation efficiency (40%) of thermostable amine oxidase obtained from L. plantarum CAU 3823 for BAs such as tyramine, histamine, cadaverine, and putrescine.110
Challenges in postbiotics research
As of now, as per the literature survey, postbiotics are believed to have health-promoting activities against various metabolic disorders in animal models. However, there is no scientific evidence available to prove their efficacy against the concerned disease conditions. Furthermore, their metabolic signaling is not well established. Recent research on suckling rats found that a diet supplemented with scGOS and lcFOS resulted in softer feces, changes in microbiota composition, a different SCFA profile, and an increase in Toll-like receptor gene expression.8 But when consuming the direct postbiotics, the healthiness of the vital organs is questionable, especially for children, pregnant women, and heart and kidney patients. Despite the fact that animal models demonstrated efficacy, human trials are required for FDA regulatory approval. So, the toxicity of these products should be well documented and scientifically proven, and clinical trials are mandatory. Also, the dead cells of many microbes have already been used in the form of vaccines. Whether postbiotics are taken as dietary supplements or are prescribed, the inanimate postbiotic molecules can eliminate pathogenicity. As a result, its toxicity can be determined before it is used for human purposes. More research is needed on the viability and shelf life of postbiotic molecules, which must be tested both in vitro and in vivo.
Future perspectives
Postbiotic compounds show promise as potential nutraceuticals for human and animal health enhancement. It can serve as an alternative to existing medications, and the antimicrobial active postbiotic molecules can be administered with available drugs to combat antimicrobial drug resistance problems. The microencapsulated postbiotic molecules can be administered to solve several metabolic syndromes, which can be screened through bioimaging systems. Screening tools can be developed to authenticate gut products. Future techniques must reveal the quantity and quality of gut microbes, as well as their liberation. A new methodology, in particular, must validate the microbiomes of diseased people. There is a live screening system to understand the activity of gut microbes. Imaging systems can be developed to live monitor the number of gut microbes and their secretions on molecular signaling pathways in various disease conditions. There is a model required to monitor the postbiotic molecule secretions during the dysbiosis condition. The beneficial microbes’ interactions with beneficial or pathogenic organisms and their quorum sensing signals can be monitored for the production of postbiotic molecules. Biomarkers must be developed for inanimate products.
Postbiotics are composed of inanimate cells or metabolic products. These secondary metabolites play crucial roles in maintaining metabolic homeostasis. Most of these products can be easily quantified in the fecal matter. In particular, the short-chain fatty acid concentration in the fecal matter helps to analyze the healthiness of an individual. Postbiotics help overcome metabolic syndromes, such as diabetes, irritable bowel disorders (IBD), and different types of cancers. These postbiotic molecules produce immune signals to establish defence systems at the cellular level. The anti-microbial peptide postibiotic establishes strong inhibitory action against various entero-pathogens and neutralizes their toxins in the gut system. Therefore, postbiotics may reduce the incidence of anti-inflammatory and autoimmune disorders. Bioaccumulation of antibiotics and non-antibiotic molecules recommended for various disease conditions may have adverse effects on beneficial intestinal microbes and influence postbiotics liberation. Dietary substrates act as prebiotic sources and are responsible for the metabolic capacity of the gut microbes. These prebiotics are effective sources for aggravating the production of postbiotics. Postbiotic molecules can substitute antibiotics and anti-cancer drugs, which have side effects. To date, their roles in communicable and non-communicable diseases have not been thoroughly studied. New product development with postbiotics is a promising area of research and has an impact on the food and nutrition field. Postbiotic molecule applications in packaging systems in the food industry as Class-I preservatives, biopreservative agents, antimicrobial, and decontaminating agents are areas that need to be explored. Postbiotics molecules studies are still in infancy. It can be further studied for its molecular signalling and quorum activities. Such identified molecules must be transferred from lab to land scale through food industries to common public. In addition, advanced analytical techniques are required to detect postbiotics in the gut.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
ASB and PS conceptualized the study. ASB, PS, AGK, TS and NG wrote the manuscript. AOAS reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not Applicable.
- Salminen S, Maria CC, Akihito E, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Review, Gastroenterolog & Hepatolog. 2021; 18:649- 667.
Crossref - Svetoslav DT, John RT, Iskra VI. Could Probiotics and Postbiotics Function as “Silver Bullet” in the Post-COVID-19 Era? Probiotic and Antimicrob Protein. 2021; 13: 1499–1507.
Crossref - Hoon Kim, Lim JJ, Shin HY, Suh HJ, Choi HS. Lactobacillus plantarum K8-based paraprobiotics suppress lipid accumulation during adipogenesis by the regulation of JAK/STAT and AMPK signaling pathways. J Function Food. 2021; 87:104824.
- Oh BS, Choi WJ, Kim JS, et al. Cell-Free Supernatant of Odoribacter splanchnicus Isolated From Human Feces Exhibits Anti-colorectal Cancer Activity. Frontiers in Microbiolog. 2021;12: 736343.
- Dorna D-D, Manica N, Iman K, et al. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Food. 2019; 8(3):92.
Crossref - Collado MC, Vinderola G, Salminen S. Postbiotics: facts and open questions. A position paper on the need for a consensus definition. Benefic Microb. 2019; 10(7): 711-719.
Crossref - Pandey M, Bhati A, Priya K, Sharma KK, Singhal B. Precision Postbiotics and Mental Health: the Management of Post-COVID-19 Complications. Probiotic and Antimicrob Protein. 2021; 14: 426-448.
- Morales-Ferré C, Azagra-Boronat I, Massot-Cladera M, et al. Effects of a Postbiotic and Prebiotic Mixture on Suckling Rats’ Microbiota and Immunity. Nutrient. 2021; 13(9): 2975.
Crossref - Aziz HR, Leili AM, Hossein SK, Hamideh FZ, Amin A. Postbiotics as Promising Tools for Cancer Adjuvant Therapy. Advance Pharmaceutica Bullet. 2021; 11(1): 1-5.
Crossref - Bourebaba Y, Krysztof M, Malwina, Lynda Bourebaba. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomedicine& pharmacotheraphy. 2022; 153:113138.
Crossref - Muskan P, Archana B, Kumari P, Sharma KK, Barkha. Precision Postbiotics and Mental Health: the Management of Post-COVID-19 Complications. Probiotic and Antimicrob Protein. 2022; 14: 426-448.
Crossref - Sharma JK, Sihmar M, Santal AR, Prager L, Carbonero F, Singh NP. Barley Melanoidins: Key Dietary Compounds with Potential Health Benefits. Frontier in Nutrit. 2021; 8:708194.
Crossref - Belkina TV, Averina OV, Savenkova EV, Danilenko VN. Human Intestinal Microbiome and the Immune System: The Role of Probiotics in Shaping an Immune System Unsusceptible to COVID-19 Infection. Biology Bulletin Review. 2021; 11(4): 329–343.
Crossref - Ailioaie LM, Litscher G. Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges. Internat J Molecul Sci. 2021; 22(9): 4942.
Crossref - Fabiano V, Indrio F, Verduci E, et al. Term Infant Formulas Influencing Gut Microbiota: An Overview. Nutrient. 2021; 13(12): 4200.
Crossref - Cuevas-González PF, Liceaga AM, Aguilar-Toalá JE. Postbiotics and paraprobiotics: From concepts to applications. Food Research International. 2020; 136:109502.
- Koleilat A. Beyond probiotics the Postbiotics. Gastroenterol Hepatology. 2019; 10(6):324-326.
Crossref - Drolia, R, Amalaradjou MAR, Ryan V. et al. Receptor-targeted engineered probiotics mitigate lethal Listeria infection. Nat Communications. 2020; 11: 6344.
Crossref - Chudzik A, Orzyłowska A, Rola R, Stanisz GJ. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain–Gut–Microbiome Axis. Biomolecules. 2021; 11 (7): 1000.
Crossref - Nazarii K, Tetyana F, Galyna M, et al. Probiotic and omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance, improves glycemia and obesity parameters in individuals with type 2 diabetes: A randomised controlled trial. Obesity Medicine. 2020; 19: 100248.
Crossref - Cabello-Olmo, M, Araña M, Urtasun R, Encio IJ, Barajas M. Role of postbiotics in diabetes mellitus: current knowledge and future perspectives. Foods. 2021;10(7):1590.
- Jastrząb R, Graczyk D, Siedlecki P. Molecular and Cellular Mechanisms Influenced by Postbiotics. International Journal of Molecular Sciences, 2021; 22(24): 13475.
Crossref - Qing, G, Ping, L. Chapter 7: Biosynthesis of Vitamins by Probiotic Bacteria. In Probiotics and Prebiotics in Human Nutrition and Health. Intech, 2016; 135-148.
Crossref - Maria do CGP, Alfredo M, Fermin IM. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends in Food Science & Technology, 2021; 108:11-26.
Crossref - Hamid P, Amir AA, Rezvan P, Hamed A. Increase in conjugated linoleic acid content and improvement in microbial and physicochemical properties of a novel kefir stored at refrigerated temperature using complementary probiotics and prebiotic. Food Science and Technology (Campinas). 2021; 41(1): 254-266.
Crossref - Walhe R, Khan H, Kumari S. From Probiotics to Postbiotics: Key to Microbiome and Health. In Microbiome-Gut-Brain Axis (pp. 367-381);2022.
- Lee KJ, Park HW, Choi EJ, Chun HH. Effects of CFSs produced by lactic acid bacteria in combination with grape seed extract on the microbial quality of ready-to-eat baby leaf vegetables. Cogent Food & Agricultur. 2016; 2(1):1268742.
- Bäuerl C, Abitayeva G, Sosa-Carrillo S, et al. P40 and P75 Are Singular Functional Muramidases Present in the Lactobacillus casei/paracasei/rhamnosus Taxon. Frontiers in Microbiol. 2019; 10:1420.
Crossref - Paulina M, Katarzyna Ś. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020; 12(4): 1107.
Crossref - Parada VD, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in Immunology. 2019; 10: 277.
Crossref - Naser AA. The Role of Short-Chain Fatty Acids in the Interplay between a Very Low-Calorie Ketogenic Diet and the Infant Gut Microbiota and Its Therapeutic Implications for Reducing Asthma. Internat J Molecul Sci. 2020; 21(24): 9580.
Crossref - Sara D, Kathleen M, Jeroen R, Kristin V, Severine V. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EbioMedicin. 2021; 66: 103293.
Crossref - Haas-Neill S, Forsythe P. A budding relationship: Bacterial extracellular vesicles in the microbiota-gut-brain axis. Internat J of Molecul Science. 2020; 21(23):8899.
Crossref - Ahmadi BS, Moshiri A, Ettehad MF, Mojtahedzadeh M, Kazemi V, Siadat SD. Extraction and evaluation of outer membrane vesicles from two important gut microbiota members, Bacteroides fragilis and Bacteroides thetaiotaomicron. Cell. 2020; 22(3): 344–349.
Crossref - Ottman N, Reunanen J, Meijerink M, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PloS ONE. 2017; 12(3): p.e0173004
- Sharma V, Harjai K, Shukla G. Effect of bacteriocin and exopolysaccharides isolated from probiotic on P. aeruginosa PAO1 biofilm. Folia Microbiol (Praha). 2018; 63(2):181-190.
Crossref - Brink LR, Chichlowski M, Pastor N. Thimmasandra Narayanappa A, Shah N. In the Age of Viral Pandemic, Can Ingredients Inspired by Human Milk and Infant Nutrition Be Repurposed to Support the Immune System? Nutrien. 2021; 13(3):870.
Crossref - Iyer N, Sinéad CC. Gut Microbial Metabolite-Mediated Regulation of the Intestinal Barrier in the Pathogenesis of Inflammatory Bowel Disease. Nutrients. 2021; 13(12): 4259.
Crossref - Li H-Y, Zhou D-D, Gan R-Y, et al. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients. 2021; 13(9):3211.
Crossref - Ciabattini A, Garagnani P, Santoro F, Rappuoli R, Franceschi C, Medaglini D. Shelter from the cytokine storm: Pitfalls and prospects in the development of SARS-CoV-2 vaccines for an elderly population. Seminar in Immunopatholog. 2020;42(5):619–634.
- Marta P, Erola A, Pol H, Cristina A, David B, Jordi. Derived Postbiotics of a Multi-strain Probiotic Formula Clinically Validated for the Treatment of Irritable Bowel Syndrome. FASEB J. 2020; 34(S1):1-1.
Crossref - Huang Z, Lin F, Zhu X, Zhang C, Jiang M, Lu Z. An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway. Internation J of Biologica Macromolecul. 2020; 143: 775-784.
- Pothuraju R, Sanjib CSR, Sukhwinder K, Hemant KR, Michael B, Surinder KB. Mucins, gut microbiota, and postbiotics role in colorectal cancer, Gut Microbe. 2021; 13(1): 1974795.
Crossref - Puccetti M, Xiroudaki S, Ricci M, Giovagnoli S. Postbiotic-Enabled Targeting of the Host-Microbiota-Pathogen Interface: Hints of Antibiotic Decline? Pharmaceutic. 2020; 12(7): 624.
Crossref - Rad AH, Abbasi A, Kafil HS, Ganbarov K. Potential pharmaceutical and food applications of postbiotics: a review. Curren Pharmaceutica Biotechnolog. 2020; 21(15): 1576-1587.
- Manach C, Milenkovic D, Van de Wiele T, et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Molecul Nutrition & Food Resear. 2017; 61(6):1600557.
- Michela R, Catia C, Micaela C, et al. The Challenge of ICIs Resistance in Solid Tumours: Could Microbiota and Its Diversity Be Our Secret Weapon? Frontiers in Immunolog. 2021; 12:704942.
- Mitrofanova O, Mardanova A, Evtugyn V, et al. Effects of Bacillus serine proteases on the bacterial biofilms. BioMed Research Internation. 2017; 8525912.
Crossref - Beulens JW, Booth SL, van den Heuvel EG, Stoecklin E, Baka A Vermeer C. The role of menaquinones (vitamin K2) in human health. Brit J Nutrit. 2013; 110(8):1357-1368.
- Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics—a step beyond pre-and probiotics. Nutrient. 2022; 12(8): 2189.
- Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microbia Cell Factorie. 2020; 19(1):1-22.
- Peluzio MDCG, Martinez JA, Milagro FI. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trend in Food Scienc & Technolog. 2021; 108: 11-26.
Crossref - Carbello-Olmo M, Araña M, Radichev I, Smith P, Huarte E, Barajas M. New insights into immunotherapy strategies for treating autoimmune diabetes. Internat J Molecul Sci. 2019; 20(19): 4789.
- American Diabetes Association (ADA). 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care, 43(Supplement_1), 2020; pp.S14-S31.
- Tan SY, Wong JLM, Sim YJ, et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabete & Metabolic Syndrome: Clinical Research & Rev. 2019; 13(1): 364-372.
- Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Molecul Endocrinolog. 2014; 28(8): 1221-1238.
- Blandino G, Inturri R, Lazzara F, Di Rosa M, Malaguarnera L. Impact of gut microbiota on diabetes mellitus. Diabetes & Metabolism. 2016; 42(5),303-315.
- Adachi K, Sugiyama T, Yamaguchi Y, et al. Gut microbiota disorders cause type 2 diabetes mellitus and homeostatic disturbances in gut-related metabolism in Japanese subjects. J Clin Biochem Nutr. 2019; 64(3): 231-238.
Crossref - Ghoneim MA, Hassan AI, Mahmoud MG, Asker MS. Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats. BMC Complement and Alternativ Medicin. 2016; 16 (1):1-12.
- Sies H. Oxidative stress: Concept and some practical aspects. Antioxidant. 2020; 9(9): 852.
- Guerrero-Encinas I, González-González JN, Santiago-López L, et al. Protective Effect of Lacticaseibacillus casei CRL 431 Postbiotics on Mitochondrial Function and Oxidative Status in Rats with Aflatoxin B1–Induced Oxidative Stress. Probiotic and Antimicrobia Protein. 2021; 13(4):1033-1043.
- Aguilar-Toalá JE, Astiazarán-García H, Estrada-Montoya MC, et al. Modulatory effect of the intracellular content of Lactobacillus casei CRL 431 against the aflatoxin B1-Induced oxidative stress in rats. Probiotic and Antimicrob Protein. 2019;11(2): 470-477.
- Effinger A, McAllister M, Tomaszewska I, et al. Investigating the Impact of Crohn’s Disease on the Bioaccessibility of a Lipid-Based Formulation with an In Vitro Dynamic Gastrointestinal Model. Molecul Pharmaceutic. 2021; 18(4): 1530-1543.
- Bertoni S, Albertini B, Facchini C, Prata C, Passerini N. Glutathione-Loaded Solid Lipid Microparticles as Innovative Delivery System for Oral Antioxidant Therapy. Pharmaceutics. 2019;11(8): 364.
Crossref - Covas G, Marinho HS, Cyrne L, Antunes F. Activation of Nrf2 by H2O2: de novo synthesis versus nuclear translocation. In Methods in Enzymol. 2013; 528: 157-171.
- Zorova LD, Popkov VA, Plotnikov EJ, et al. Functional significance of the mitochondrial membrane potential. Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biolog. 2018; 12(1): 20-26.
- Cheng J, Nanayakkara G, Shao Y, et al. Mitochondrial proton leak plays a critical role in pathogenesis of cardiovascular diseases. Mitochond Dynamic in Cardiovascul Med. 2017; 359-370.
- Trinchese G, Cavaliere G, Canani RB, et al. Human, donkey and cow milk differently affects energy efficiency and inflammatory state by modulating mitochondrial function and gut microbiota. J Nutrition Biochemist. 2015; 26(11): 1136-1146.
- Nocerino R, Paparo L, Terrin G, et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: a randomized controlled trial. Clinical Nutrition. 2017; 36(1): 118-125.
- Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatolog. 2018; 68(2): 280-295.
- Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018; 362(6416):776-780.
- Natarajan N, Hori D, Flavahan S, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiologic Genomic. 2016; 48(11): 826-834.
- Panyod S, Wu WK, Chen CC, Wu MS, Ho CT, Sheen LY. Modulation of gut microbiota by foods and herbs to prevent cardiovascular diseases. J Tradition and Complement Medicin. 2023;13(2):107-118.
Crossref - Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clinic Epidemiolog. 2014; 6: 71-80.
- Loureiro AI, Rebouta J, Bonifacio MJ, Soares-da-Silva P. In vitro Species Different Metabolism and CYP Phenotyping of Zamicastast. FASEB J. 2020; 34(S1):1-1.
- Imperial IC, Ibana JA. Addressing the antibiotic resistance problem with probiotics: re-ducing the risk of its double-edged sword effect. Front in Microbiol. 2016; 7:1983.
- Shah A, Gani A, Ahmad M, Ashwar BA, Masoodi FA. β-Glucan as an encapsulating agent: Effect on probiotic survival in simulated gastrointestinal tract. Internation J Biologica Macromolecule. 2016; 82: 217-222.
- Venema K. Foreword: prebiotics that modulate the endogenous microbiota are also very important. Beneficial Microbe. 2013; 4(1):1-2.
- Moradi M, Mardani K, Tajik H. Characterization and application of postbiotics of Lactobacillus spp. on Listeria monocytogenes in vitro and in food models. LWT- Food Science & Technolog. 2019; 111: 457-464.
- Amiri S, Aghamirzaei M, Mostashari P, Sarbazi M, Tizchang S, Madahi H. The impact of biotechnology on dairy industry. In Microbial biotechnology in food and health, 2021; 53-79.
- Rai M, Pandit R, Gaikwad S, Kövics G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J Food Science and Technolog. 2016; 53(9): 3381-3394.
- Barros CP, Guimaraes JT, Esmerino EA, et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr Opin in Food Scien. 2020; 32: 1-8.
- Hamad G, Botros W, Hafez E. Combination of probiotic filtrates as antibacterial agent against selected some pathogenic bacteria in milk and cheese. Internat J of Dair Scien. 2017; 12: 368-376.
- Garnier L, Mounier J, Lê S, et al. Development of antifungal ingredients for dairy products: From in vitro screening to pilot scale application. Food Microbiol. 2019; 81:97-107.
- Huang Y, Zhao S, Yao K, et al. Physicochemical, microbiological, rheological, and sensory properties of yoghurts with new polysaccharide extracts from Lactarius volemus Fr. using three probiotics. Internat J Dair Technolog. 2020; 73(1):168-181.
- Moradi M, Tajik H, Mardani K Ezati P. Efficacy of lyophilized cell-free supernatant of Lactobacillus salivarius (Ls-BU2) on Escherichia coli and shelf life of ground beef. In Veterinary Research Forum. 2019; 10(3):193. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
- Manson AL, Van Tyne D, Straub TJ, et al. Chicken meat-associated enterococci: influence of agricultural antibiotic use and connection to the clinic. Appl and Environment Microbiolog. 2019;85(22): e01559-19.
- Mokhtar M, Mostafa GA, Eldeeb GS, Taha RA. Effect of bacteriocins (from Bifidobacterium spp.) on prevalence of some Aeromonas and Pseudomonas species in minced meat during cold storage. J of Food and Nutritional Disord. 2016; 5(1).
- Hamad GM, Abdelmotilib NM, Darwish AM, Zeitoun AM. Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerobe. 2020; 62:102181.
- Tenea GN, Olmedo D, Ortega C. Peptide-based formulation from lactic acid bacteria Impairs the pathogen growth in Ananas comosus (Pineapple). Coatings. 2020; 10(5): 457.
- George-Okafor U, Ozoani U, Tasie F Mba-Omeje K. The efficacy of cell-free supernatants from Lactobacillus plantarum Cs and Lactobacillus acidophilus ATCC 314 for the preservation of home-processed tomato-paste. Scientif Afric. 2020; 8: e00395.
- Shehata MG, Badr AN, El Sohaimy SA, Asker D, Awad TS. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Annal of Agricultur Sci. 2019; 64(1): 71-78.
- Vilela C, Kurek M, Hayouka Z, et al. A concise guide to active agents for active food packaging. Trend in Food Scienc & Technolog. 2018; 80: 212-222.
- O’bryan CA, Crandall PG, Ricke SC, Ndahetuye JB, Taylor T. Lactic acid bacteria (LAB) as antimicrobials in food products: Analytical methods and applications. Handbook of Natural Antimicrobials for Food Safety and Quality. 2015; 137-151.
- Beristain-Bauza SC, Mani-López E, Palou E, López-Malo A. Antimicrobial activity and physical properties of protein films added with cell-free supernatant of Lactobacillus rhamnosus. Food Cont. 2016; 62:44-51.
- Meira SMM, Zehetmeyer G, Werner JO, Brandelli. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocolloid. 2017; 63:561-570.
- Marques J, Funck GD, da Silva Dannenberg G, et al. Bacteriocin-like substances of Lactobacillus curvatus P99: characterization and application in biodegradable films for control of Listeria monocytogenes in cheese Food Microbiol. 2017; 63:159-163.
- Rivas FP, Cayré ME, Campos CA, Castro MP. Natural and artificial casings as bacteriocin carriers for the biopreservation of meats products. J Food Safet. 2018; 38(1): p.e12419.
- Sharaf OM, Al-Gamal MS, Ibrahim GA, et al. Evaluation and characterization of some protective culture metabolites in free and nano-chitosan-loaded forms against common contaminants of Egyptian cheese. Carbohydrat Polymer. 2019; 223: 115094.
- Urish KL, DeMuth PW, Kwan BW, et al. Antibiotic-tolerant Staphylococcus aureus biofilm persists on arthroplasty materials. Clinic Orthopaedic and Related Resear. 2016; 474(7): 1649-1656.
- Miao J, Liang Y, Chen L, et al. Formation and development of Staphylococcus biofilm: with focus on food safety. J Food Safe. 2017; 37(4): e12358.
- Cui T, Bai F, Sun M, et al. Lactobacillus crustorum ZHG 2-1 as novel quorum-quenching bacteria reducing virulence factors and biofilms formation of Pseudomonas aeruginosa. LWT-Food Science & Technolog. 2020; 117: 108696.
- Sarikhani M, Kermanshahi RK, Ghadam P, et al. The role of probiotic Lactobacillus acidophilus ATCC 4356 bacteriocin on effect of HBsu on planktonic cells and biofilm formation of Bacillus subtilis. Internat J Biologica Macromolecule. 2018; 115: 762-766.
- Petrova MI, Imholz NC, Verhoeven TL, et al. Lectin-like molecules of Lactobacillus rhamnosus GG inhibit pathogenic Escherichia coli and Salmonella biofilm formation. PloS ONE. 2016; 11(8): e0161337.
- Wang K, Niu M, Song D, et al. Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J Bioscienc and Bioengineerin. 2020; 129(2): 206-214.
- Mah JH, Park YK, Jin YH, Lee JH, Hwang H. Bacterial production and control of biogenic amines in Asian fermented soybean foods. Food. 2019; 8(2): 85.
- Czajkowska-Mysłek A, Leszczyńska J. Risk assessment related to biogenic amines occurrence in ready-to-eat baby foods. Food and Chemic Toxicolog. 2017; 105: 82-92.
- Toy N, Özogul F, Özogul Y. The influence of the cell free solution of lactic acid bacteria on tyramine production by food borne-pathogens in tyrosine decarboxylase broth. Food Chem. 2015; 173: 45-53.
- Xie C, Wang HH, Deng SL, Xu XL. The inhibition of cell-free supernatant of Lactobacillus plantarum on production of putrescine and cadaverine by four amine-positive bacteria in vitro. LWT-Food Science and Technolog. 2016; 67: 106-111.
- Niu T, Li X, Guo Y, Ma Y. Identification of lactic acid bacteria to degrade biogenic amines in Chinese rice wine and its enzymatic mechanism. Food. 2019; 8(8):312.
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.