ISSN: 0973-7510
E-ISSN: 2581-690X
Air is an important part of human life. However, air can be contaminated with microorganisms such as airborne bacteria and fungi. Temperature and relative humidity in a room can have an impact on the quantity of airborne bacteria and fungi. This study aims to figure out the correlation between the number of airborne bacteria and fungi with temperature and relative humidity. In 15 rooms of Microbiology laboratory, NA and SDA Petri plates were placed, after incubation, the number of colonies in each plate was counted. Pearson test was conducted with SPSS to determine the correlation between temperature and relative humidity to the number of airborne bacteria and fungi. The highest number of airborne bacteria was in the reading room (352 CFU/m3), while the lowest number was in the laundry room (13 CFU/m3) and the highest number of airborne fungi was in the Mycology room (156 CFU/m3), while there were no airborne fungi found in the urine and laundry rooms. Based on the results of the Pearson test, it was found that the value of p = 0.668 (p> 0.5) showed that there was no correlation between temperature and the number of airborne bacteria and fungi. Based on the results of the Pearson test, the value of p = 0.745 (p> 0.5) showed that there was no correlation between relative humidity and the number of airborne bacteria and fungi. There is no correlation between temperature and relative humidity with the number of airborne bacteria and fungi.
Airborne Bacteria, Airborne Fungi, Temperature, Relative Humidity
Indoor air quality is one of the most important variables for a healthy and productive life because humans do more than 90% of their activities indoors and the air inhaled is an average of 15,000 liters of air every day. Reducing exposure to indoor air pollutants becomes an important issue because air pollution is a significant environmental health risk.1,2
More than 10,000 airborne microorganisms, including fungal spores, yeast, bacteria and viruses can be found in hospitals. These microorganisms can infect people indoors via aerosols and in some rare clinical situations, such as skin lesions. These microorganisms can act as a source of airborne particles.3,4 The naturally-produced droplets from humans when breathing, talking, sneezing and coughing can affect a variety of airborne microorganisms.3,5 These microorganisms can survive in the air for a very long time and can travel great distances. The measurement of airborne microorganisms that can infect humans and the assurance of air quality at hospitals are questioned. Until today, there is no universally recognized standard. Regular monitoring of air quality is a guarantee that is needed in the hospital environment to provide a healthy atmosphere for doctors, patient families, hospital employees, and other workers.3,6
Temperature and humidity are two variables that can affect the number of airborne microorganisms.3 The ideal temperature allowed in a laboratory is 20-22°C, and the humidity in a laboratory is 40-60%, according to the Regulation of the Minister of Health concerning Hospital Environmental Health No. 7 of 2019.7 The results of Gumiyarna’s research in 2021 by using statistical tests show that there is a correlation between humidity and lighting with the number of airborne microbes in the rehabilitation rooms owned by community component, but not with room temperature, humidity, or lighting.8 Meanwhile, Siti in 2020 explained that there was a correlation between temperature and humidity with indoor air quality which is related to the number of airborne microbes.9 In another study conducted by Sajaddi in 2016, Hiwar in 2020 and research conducted by Vindrahapsari in 2016, it was found that there was no correlation between relative humidity and the number of airborne microbes.10,11,12 This also happened with research which states that there is no relationship between the number of airborne bacteria and relative humidity. Research conducted by Gumiyarna in 2021 also stated that there was no correlation between temperature and humidity of a room and airborne microbial numbers in rehabilitation rooms owned by government agencies.13
Clinical Microbiology Laboratory has a risk to various types of microorganism exposure which can cause health problems for workers and users. There is a lot of equipment in the microbiology laboratory such as centrifuges and vortexes (tools for mixing microbial suspensions), which are common to be used in microbiology laboratories, which can increase the aerosolization of microorganisms. The measurements of airborne microbiology in clinical microbiology laboratories in hospitals, especially those conducted in Indonesia and the analysis of the correlation between the number of airborne microbes and the indoor temperature and relative humidity have never been done. Therefore, it is important to conduct research on the number of airborne bacteria and fungi and connect the influencing factors, namely temperature and relative humidity.
This research is an analytical observational study with the type of research used is cross sectional which aims to find out the correlation between temperature and relative humidity with the number of airborne bacteria and fungi.
The population and samples in this study were airborne bacteria and fungi in 15 rooms at the Clinical Microbiology Laboratory, Dr. Soetomo Regional General Hospital Surabaya with the number of samples in this study are 60 samples consisting of 30 control Petri plates consisting of 15 control Petri dishes for mushrooms by using Sabouraud Dextrose Agar (SDA) media and 15 control Petri plates using Nutrient Agar (NA) media for bacteria, then 30 treatment Petri dishes consisting of 15 Petri dishes for airborne bacterial cultures using NA media and 15 Petri dishes for airborne fungal cultures using SDA media.
In 15 rooms of the Clinical Microbiology Laboratory of RSUD Dr. Soetomo Surabaya, on August 16, 2022, a study was conducted with closed control Petri plates and open treatment Petri plates 1 meter high from the floor and at least 1 meter from the wall for 1 hour (Figure 1). 14 A measurement of temperature and humidity in each room was also conducted. After 1 hour, the Petri plates were closed and incubated for 24 hours at 37°C for the NA Petri plates used to grow airborne bacteria and the SDA Petri plates were incubated for 5 days at room temperature. After the incubation, the number of each plate was counted and recorded, then converted using the Omeliansky formula to obtain units of CFU/m3. After obtaining the number of airborne bacteria and fungi, a normality test was carried out. Since the data obtained was normally distributed, a Pearson test was carried out to determine the correlation between the number of airborne bacteria and fungi and the indoor temperature and humidity.
From the results of temperature and relative humidity measurements conducted in 15 rooms at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya, we obtained the data summarized in the following Table 1.
Table (1):
The distribution of temperature and relative humidity in the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital, Surabaya.
Rooms |
Temp. (°C) |
Relative humidity (%) |
|---|---|---|
Urine Laboratory |
28.3 |
66 |
Blood Laboratory |
26.5 |
52 |
Pus Laboratory |
28 |
63 |
Sputum Laboratory |
28.1 |
73 |
Tuberculosis Laboratory |
29 |
68 |
Biomolecular 1 Laboratory |
24.4 |
53 |
Biomolecular 2 Laboratory |
29.6 |
65 |
Laundry Laboratory |
28.9 |
62 |
Media room |
26.7 |
66 |
Identification Room |
25.1 |
65 |
Meeting Room |
25.7 |
71 |
Reading Room |
26 |
73 |
Administration Room |
26.3 |
72 |
Hallway |
26.5 |
71 |
Mycology Laboratory |
29.4 |
59 |
The number of airborne bacteria and fungi obtained on each plate is converted to the Omeliansky formula to obtain the number of bacteria/m3 (CFU/m3) with the following formula15
N=5a × 104 (bt)-1
Note:
N = CFU/m3
a = the number of colonies per plate
b = plate size in square centimeters
t = exposure time (minutes)
The distribution of the number of airborne bacteria and fungi in 15 rooms at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya is concluded in the following Table 2.
Table (2):
The distribution of the number of airborne bacteria and fungi at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya.
Rooms |
Airborne bacteria (count/plate) |
Airborne bacteria count (CFU/m3) |
Airborne fungi (count/ plate) |
Airborne fungi count (CFU/m3) |
|---|---|---|---|---|
Urine Laboratory |
25 |
326 |
0 |
0 |
Blood Laboratory |
22 |
286 |
2 |
26 |
Pus Laboratory |
8 |
104 |
4 |
52 |
Sputum Laboratory |
9 |
117 |
9 |
117 |
Tuberculosis Laboratory |
11 |
143 |
3 |
39 |
Biomolecular 1 Laboratory |
3 |
39 |
3 |
39 |
Biomolecular 2 Laboratory |
4 |
52 |
4 |
52 |
Laundry Laboratory |
1 |
13 |
0 |
0 |
Media Room |
4 |
52 |
2 |
26 |
Identification Room |
4 |
52 |
2 |
26 |
Meeting Room |
4 |
52 |
2 |
26 |
Reading Room |
27 |
352 |
3 |
39 |
Administration Room |
14 |
182 |
1 |
13 |
Hallway |
6 |
78 |
4 |
52 |
Mycology Laboratory |
9 |
117 |
12 |
156 |
In the Urine laboratory, the temperature was 28.3°C, the relative humidity was 66%, the number of colonies was 25/Petri plate, which after being converted to the Omeliansky formula, there were 326 airborne bacteria (CFU/m3) found while no airborne fungi were found. In the Blood laboratory, the temperature was 26.5°C, the relative humidity was 52%
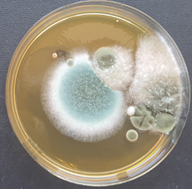
and the number of airborne bacteria was 286 CFU/m3 with the number of airborne fungi 26 CFU/m3. In the Pus laboratory, the temperature was 28°C, the relative humidity was 63% and the number of airborne bacteria was 104 CFU/m3 with the number of airborne fungi 52 CFU/m3. In the Sputum laboratory, the temperature was 28.1°C, the relative humidity was 73% and the number of airborne bacteria was 117 CFU/m3 with the number of airborne fungi 117 CFU/m3. In the tuberculosis laboratory, the temperature was 29°C, the relative humidity was 68% and the number of airborne bacteria was 143 CFU/m3 with the number of airborne fungi 39 CFU/m3. In the Biomolecular Laboratory 1, the temperature was 24.4°C, the relative humidity was 53% and the number of airborne bacteria was 39 CFU/m3 with the number of airborne fungi 39 CFU/m3. In Biomolecular Laboratory 2, the temperature was 29.6°C, the relative humidity was 65% and the number of airborne bacteria was 52 CFU/m3 with the number of airborne fungi 52 CFU/m3. In the washing room, the temperature was 28.9°C, the relative humidity was 62% and the number of airborne bacteria was 13 CFU/m3 and there were no airborne fungi found. In the media room, the temperature was 26.7°C, the relative humidity was 66% and the number of airborne bacteria was 52 CFU/m3 with the number of airborne fungi 26 CFU/m3. In the identification room, the temperature was 25.1°C, relative humidity was 65% and the number of airborne bacteria was 52 CFU/m3 with the number of airborne fungi 26 CFU/m3. In the meeting room, the temperature was 25.7°C, the relative humidity was 71% and the number of airborne bacteria was 52 CFU/m3 with the number of airborne fungi 26 CFU/m3. In the reading room, the temperature was 26°C, the relative humidity was 73% and the number of airborne bacteria was 352 CFU/m3 (Figure 2) with the number of airborne fungi 39 CFU/m3. In the administration room, the temperature was 26.3°C, the relative humidity was 72% and the number of airborne bacteria was 182 CFU/m3 with the number of airborne fungi 13 CFU/m3. In the hallway, the temperature was 26.5°C, the relative humidity was 71% and the number of airborne bacteria was 78 CFU/m3 with the number of airborne fungi 52 CFU/m3. In the Mycology laboratory, the temperature was 29.4°C, the relative humidity was 59% and the number of airborne bacteria was 117 CFU/m3 with the number of airborne fungi 156 CFU/m3 (Figure 3). The highest number of airborne bacteria was found in the reading room, which was 352 CFU/m3 and the least number of airborne bacteria was found in the washroom, which was 13 CFU/m3. The highest number of airborne fungi was found in the Mycology laboratory, namely 156 CFU/m3, while in the Urine laboratory and laundry room there were no airborne fungi found.
After the data normality test with SPSS was conducted, we obtained the results showing that the data was normally distributed, then proceed with the Pearson test. Based on the results of the Pearson test, between temperature and the number of airborne bacteria and fungi, the value of p = 0.668 (p > 0.5) was obtained which indicated that there was no correlation between temperature and the number of airborne bacteria and fungi. Based on the results of the Pearson test, the value of p = 0.745 (p> 0.5) revealing that there was no correlation between relative humidity and the number of airborne bacteria and fungi.
On the results of airborne bacterial culture in 15 rooms at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya, we found a small white colony with 1 mm size in almost every room (the dominant colony). The results of the gram colony were Gram-positive cocci clustered like grapes which is the characteristic of Staphylococcus aureus. Then, a simple identification test was carried out, with catalase test results (+) and positive coagulase test (+) which led to Staphylococcus aureus. After 24 hours, the settle plate repetition was conducted in one of the rooms (Sputum room) and the small white colony was still found with a size of 1 mm which were gram-positive cocci forming clusters like grapes which are characteristic of Staphylococcus aureus. Then, a simple identification test was carried out with the catalase test results (+) and the coagulase test results positive (+) which led to Staphylococcus aureus. The identification of airborne fungi found based on colony morphology and LPCB staining which led to Aspergillus species.
The measurement of the number of airborne microbes was conducted manually after the Petri plates were exposed to free air in 15 rooms at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya for 1 hour, with a distance of 1 meter from the wall and 1 meter from the floor. Then, the Petri plates for bacteria were incubated at 37°C for 1 day meanwhile the Petri plates for fungi were incubated for 5 days at room temperature.14,15 The control Petri plates during the treatment was covered and incubated at the same temperature and time, where there was no growth of bacterial or fungal colonies found in all control Petri plates. This indicated that the growth media used was good and not contaminated.
On the calculation of airborne bacteria and fungi, it was found that the number of airborne microorganisms in the urine laboratory was 326 CFU/m3, the blood laboratory was 312 CFU/m3, the pus laboratory was 156 CFU/m3, the sputum laboratory was 234 CFU/m3, the tuberculosis laboratory was 182 CFU/m3, the Biomolecular laboratory was 1 78 CFU/m3, the biomolecular laboratory 2 was 104 CFU/m3, the laundry room was 13 CFU/m3, the identification room, the media room, as well as the meeting room each contained 78 CFU/m3, the reading room was 390 CFU/m3, the hall was 143 CFU/m3 and the Mycology laboratory was 273 CFU/m3. The number of airborne microorganisms for the areas or units in the laboratory is 200-500 CFU/m3, which is the maximum concentration of microorganisms per m3 of air according to the Regulation of the Minister of Health of the Republic of Indonesia No. 1204 /MENKES/SK/X/2004 concerning Hospital Environmental Health Requirements. Based on this reference, the number of airborne microorganisms in each room was still within safe threshold, yet it is likely that Laboratory Acquired Infections (LAI) or all infections acquired through the laboratory will occur. In Addition, the poor air quality in the hospital environment can cause nosocomial infections.16 One of the mediums in the environment where fungi, viruses and bacteria can live and grow is air. Since the presence of microorganisms in any area is considered indoor air pollution, the rooms with the number of microbes below the fixed threshold must be taken into account. The transmission of nosocomial diseases can occur through indoor air. The presence of germs in the air is one of the variables that determine air quality in the hospital. According to a research, 10-20% of nosocomial infections are caused by poor air quality. A research by Gilang in 2018 showed that air can be a channel for the spread of nosocomial infections.17 Even though there are many evidences showing that the environment can serve as a reservoir for various pathogenic agents, these environmental contaminants are the reason why infections are contracted in hospitals.18
The temperature in every room had been measured according to the reference of the Minister of Health of the Republic of Indonesia No. 1204 /MENKES/SK/X/2004 regarding Hospital Environmental Health Requirements, by using a calibrated tool. The measuring device was turned on and then left for 5 minutes until the temperature was stable, then the numbers listed were read.19 The temperature measured at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya was found in the range of 24.4-29.4°C, in the urine laboratory it was 28.3°C, in the blood laboratory the it was 26.5°C, in the pus laboratory the it was 28°C, in the sputum laboratory it was 28.1°C, in the Tuberculosis laboratory it was 29°C, in the biomolecular laboratory 1 it was 24.4°C, in the biomolecular laboratory 2 it was 29.6°C, in the laundry room it was 28.9°C, in the media room it was 26.7°C, in the identification room it 25.1°C, in the meeting room it was 25.7°C, in the reading room it was 26°C, in the admin room it was 26.3°C, in the hallway it was 26.5°C and in the Mycology laboratory it was 29.4°C, which when compared with the Regulation of the Minister of Health about Health Hospital Environment no. 7 of 2019 the optimum temperature allowed in the laboratory room is 20-22°C and the humidity in the laboratory is 40-60%,7 therefore the temperature in 15 rooms of the Clinical Microbiology laboratory at Dr. Soetomo Regional General Hospital Surabaya did not meet the optimal number.
Based on the analysis results of the correlation between temperature and the number of airborne bacterial and fungal colonies using simple correlation analysis, it can be concluded that there is no correlation between the independent variable, namely temperature, and the dependent variable, namely the number of airborne bacteria and fungi in the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya. This can be caused by the air temperature in the Clinical Microbiology Laboratory room at Dr. Soetomo Regional General Hospital Surabaya ranged from 24.4-29.4°C, while the species/genus found was from the genus of Aspergillus species which can live optimally at temperatures of 35-37°C and are still able to grow at temperatures of 6-60°C. Each species/genus of fungi has different temperature levels to grow optimally in its environment.19
Airborne bacteria and fungi found in the rooms might not be caused by temperature and humidity values, but carried by visitors or room occupants.20 It was proven in the reading room which had the highest number of airborne bacteria and fungi (352 CFU/m3). This room also had a lot of visitors every day compared to the other rooms which caused indoor air bacteria and fungi to stick to indoor furniture. The large number of furniture in the room made dust easy to stick and difficult to clean, therefore the airborne bacteria and fungi were easily carried along with the dust and allowed for an increasing number of airborne bacteria and fungi. This reading room consisted of things such as several books, bookshelves, chairs and cupboards, so that dust easily sticks to them and is difficult to clean. As a result, airborne bacteria and fungi were easily carried away with the dust and allowed for the growth of airborne bacteria and fungi.12 As a comparison, the laundry room had the least number of airborne bacteria and fungi, namely 13 CFU/m3 where this room was rarely visited by laboratory personnel and did not have a lot of furniture.
The measurements in 15 rooms at the Clinical Microbiology Laboratory Dr. Soetomo Regional General Hospital Surabaya showed that there were three rooms that still met the maximum relative humidity standards, namely the Blood Laboratory with a relative humidity of 52%, Biomolecular 1 with a relative humidity of 53% and a Mycology laboratory with a relative humidity of 59%. Meanwhile, the other 12 rooms did not meet the optimum criteria for relative humidity, i.e. the relative humidity in the urine laboratory was 66%, the relative humidity in the pus laboratory was 63%, the relative humidity in the sputum laboratory was 73%, the relative humidity in the TB laboratory was 68%, the relative humidity in the biomolecular laboratory 2 was 65%, the laundry room obtained a relative humidity of 62%, the media room obtained a relative humidity of 66%, the identification room obtained a relative humidity of 65%, the meeting room obtained a relative humidity of 71%, the reading room obtained a relative humidity of 73%, the administration room obtained a relative humidity of 72% and the hallway obtained a relative humidity of 71%, where according to the Regulation of the Minister of Health regarding Hospital Environmental Health No. 7 in 2019, the humidity in the laboratory is 40-60%.7
This study showed that there is no correlation between relative humidity and the number of airborne bacteria and fungi, which is the same as the results of research by Sajaddi in 2016 and Hiwar in 2020, which also reported that there is no relationship between relative humidity and the number of microbes in the air.10,11 Furthermore, research by Vindrahapsari in 2016 found that there is no relationship between the number of airborne bacteria and relative humidity.12 Research by Gumiyarna in 2021 also found that in government rehabilitation therapy rooms, there is no correlation between the number of airborne microbes and room temperature or humidity.13 Research by Vindrahapsari Kamilia in 2016 regarding the factors related to the number of airborne microorganisms in private university classrooms in Jakarta which found a correlation between the physical quality of the rooms such as humidity and the presence of bacteria in the rooms is another study that does not support this research.21 Other research which was conducted by Syahrul in 2018 to figure out the correlation between the physical environment and the quantity of germs in Haji Makassar General Hospital rooms concluded that room humidity is directly related to the quantity of germs. In addition, Research conducted by Arief in 2019 stated that humidity and temperature support the growth of bacteria.22,23
The range of temperature in the Surabaya Clinical Microbiology laboratory rooms which was reached (24.4-29.4°C) was very possible for the viability of the Staphylococcus aureus bacteria to be found, because these bacteria can grow at temperatures of 8-50°C and had an optimal temperature for growth at 37°C.24 Staphylococcus aureus is the most common opportunistic pathogen in humans. In addition, this bacterium is associated with a wide variety of diseases, ranging from mild skin infections to serious conditions that can turn fatal. Folliculitis, furuncles, boils, impetigo, mastitis and wound infections can be caused by this bacterium. There are also a number of additional infections, including toxic shock syndrome, necrotizing fasciitis, sepsis, septic thrombophlebitis, bacteraemia, pneumonia, endocarditis, osteomyelitis, meningitis, urinary tract infection and septic thrombophlebitis.25
In this study, Aspergillus species was also identified. Depending on the species, colonies of Aspergillus species can have different morphology. The majority of species begins as white colonies which turn immediately into green, yellow, orange, black or brown. Colonies develop from downy to velvety in 3-5 days. While some have even colors, some have color patterns that form concentric circles. For microscopic morphology, the hyphae are skeletal and branch at an angle of 45°C in the tissue samples. From the hyphae, long conidiophores arise from the foot cells and end in growing vesicles. Some environmental species produce hyphae that are spiral or zigzag in shape. All can produce vesicles, which can be club-shaped, round, or semi-circular. Phialides, which can be found individually or in pairs, joined end to end covering the vesicles. Conidia, which form chains, come from the phialides. The round conidia may have a smooth or bumpy surface.26 Distal lateral subungual onychomycosis, proximal subungual onychomycosis, otomycosis and cutaneous aspergillosis are a group of superficial and cutaneous mycoses that can be caused by Aspergillus species. Most Aspergillus skin infections are nosocomial in nature, especially in infants and patients with compromised immune systems, as well as after medical procedures such as surgery, catheter insertion or after occlusive dressings on burn victims. However, trauma and skin damage are also significant risk factors for the development of superficial and cutaneous Aspergillosis symptoms.27
The number of bacteria at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya was 13-352 CFU/m3 and airborne fungi was 0-156 CFU/m3 with the temperature distribution in 15 rooms at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya ranged from 24.4-29.9°C and the relative humidity ranged from 52-73%. There is no correlation between temperature and the number of airborne bacteria and fungi at the Clinical Microbiology Laboratory of Dr. Soetomo Regional General Hospital Surabaya. There is no correlation between relative humidity and the number of airborne bacteria and fungi at the Clinical Microbiology Laboratory of Dr. Soetomo correlation Surabaya. There is no correlation between the number of airborne bacteria and fungi with temperature and relative humidity due to the presence of other factors that can affect the number of airborne bacteria such as the wide range of the growth temperature of airborne bacteria and fungi, the number of items found in the rooms, the number of occupants or visitors who can carry airborne bacteria and fungi in the rooms. Even though there is no significant correlation on the results of the study and the number of airborne microorganisms in every room is still within safe threshold, it is still possible for Laboratory Acquired Infections (LAI) to happen or all infections acquired through the laboratory.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Health Research Ethics Committee at Dr. Soetomo Regional General Hospital Surabaya number 1094/LOE/301.4.2/x/2022.
- Bragoszewska E, Bogacka M, Pikon K. Efficiency and eco-costs of air purifiers in terms of improving microbiological indoor air quality in dwellings-a case study. Atmosphere. 2019;10(12):742.
Crossref - Mannan M, Sami GA. Indoor Air Quality in Buildings: A Comprehensive Review on the Factors Influencing Air Pollution in Residential and Commercial Structure. Int J Environ Res Public Health. 2021;18(6): 3276.
Crossref - Pati P. Review on common microbiological contamination found in hospital air. J Microbiol Pathol. 2018;2(1). https://www.hilarispublisher.com/open-access/review-on-common-microbiological-contamination-found-in-hospital-air.pdf. Accessed November 25, 2022.
- Chia CW, Kimberly A, Sznitman J, et al. Airborne transmission of respiratory viruses. Science. 2021;373(6558).
Crossref - Bannadona L, Briancesco R, Maria AC, et al. Microbial Air Quality in Healthcare Facilities. Int J Environ Res Public Health. 2021;18(12): 6226.
Crossref - Peraturan Menteri Kesehatan Nomor 7 tahun 2019 tentang Kesehatan Lingkungan Rumah Sakit. https://peraturan.bpk.go.id/Home/ Details/111721/permenkes-no-7-tahun-2019. Accessed November 13, 2022.
- Jalili D, Dehghani M, Fadaei A, Alimohammadi M. Assesment of airborne bacterial and fungal communities in Shahrekod Hospitals. J Environ Public Health. 2021;8864051.
Crossref - Siti AI, Marlia MH, Ismail M, et al. Laboratory air quality and microbiological contamination in A university building. Arab J Geosci. 2020;13(13):580.
Crossref - Sajjadi SA, Shakeri H, Haghighi MH, Mohammadzade A. Microbial indoor air quality of public places in a semi-dry city in Iran. Int J Trop Med. 2016;11(4):102-107.
Crossref - Hiwar W, Felipe MK, Shuweihdi F, Fletcher LA, Dancer SJ, Noakes CJ. What is the relationship between indoor air quality Parameters and airborne microorganisms in hospital environments? A systematic review and meta-analysis. Indoor Air. 2021;31(5):1308-1322.
Crossref - Vidrahapsari RT. Kondisi fisik dan jumlah bakteri udara pada ruangan AC dan non-AC di Makassar. Fakultas Kesehatan Universitas Muhammadiyah, Semarang. 2016.
- Gumiyarna H. Hubungan suhu, kelembapan dan pencahayaan ruangan dengan mikroba udara di Ruang Perawatan Rehabilitasi Instansi Pemerintah dan Komponen Masyarakat di Lingkungan BNN Kota Cimahi. Jurnal Kesehatan Kartika. 2021;16(2):50-54. http://ejournal.stikesjayc.id/index.php/litkartika/article/ view/171. Accessed November 10, 2022.
- Aniebo MC, Stanley HO, Onwukwe CD. Assessment of the indoor air quality of majors biological laboratories in Ofrima Complex, University of Port-Harcourt, Nigeria. J Pet Environ Biotechnol. 2016;7(4):4.
Crossref - Ashuro Z, Diriba K, Afework A, et al. Assessment of microbiological quality of indoor air at different hospital sites of Dilla University: a cross-sectional study. Environ Health Insights. 2022;16:1-8.
Crossref - Wurtz N, Papa A, Hukic M. Survey of laboratory-acquired infections around the world in Biosafety Level 3 and 4 Laboratories. Eur J Clin Microbiol Infect Dis. 2016;35(8):1247-1258.
Crossref - Gilang LP, Mutmainah N, Rahmiati. Identifikasi bakteri kontaminan udara di ruang Perinatologi RSD Idaman Banjarbaru Tahun 2018. Homeostasis. 2019;2(1):19-24. https://ppjp.ulm.ac.id/journals/index.php/ hms/article/ download/424/415. Accessed November 1, 2022.
- Charles PG. Environmentally Transmitted Pathogens. Environmental Microbiology. 2015: 509-550.
Crossref - Peraturan Menteri Kesehatan Nomor 1204/MENKES/SK/X/2004 tentang Persyaratan Kesehatan Lingkungan Rumah Sakit. https://persi.or.id/wp-content/uploads/2020/11/kmk12042004.pdf. Accesed March 19, 2023.
- Swandi F. Analisis kandungan jumlah bakteri di udara dalam ruang kerja Institusi Pendidikan X di Kota Padang. Universitas Andalas, Padang. 2021. http://scholar.unand.ac.id/82310/. Diakses 03 Januari 2023.
- Kamilia NF, Handayani P, Gisely G. Faktor-faktor yang berhubungan dengan jumlah mikroorganisme udara dalam Ruang Kelas Lantai Delapan Universitas Esa Unggul. Forum Ilmiah. 2016; 13(1). https://ejurnal.esaunggul.ac.id/ index.php/ Formil/ article/ view/1390. Accessed November 6, 2022.
- Syahrul M R. Hubungan keberadaan bakteriologis udara terhadap kondisi ruangan di ruang kuliah mahasiswa S1 Fakultas Kesehatan Masyarakat Universitas Hasanuddin. Universitas Hasanuddin Makassar. 2018. http://digilib.unhas.ac.id/ uploaded_files/ temporary/ DigitalCollection/ MGE5MDQ4ZjE1ZjkyZWU1N mMwMWM4MDI4N zllNTE1NGVlZThkODM0NQ==.pdf. Accessed October 20, 2022.
- Faturrahman MA, Rahmawati, Kurniatuhadi R. Deteksi keberadaan bakteri Staphylococcus di udara dalam ruangan pasar tradisional kota Pontianak. Journal Protobiont. 2019;8(2):30-34.
Crossref - Medvedova A, Havlivoka A, Lehotova I, Valik L. Staphylococcus aureus 2064 growth as affected by temperature and reduced water activity. Ital J Food Saf. 2019;8(4):8287.
Crossref - Zhuli A. Review of Staphylococcus aureus and the emergence of drug-resistant problem. Adv Microbiol. 2018;8(1):65-76.
Crossref - Carmen. Atlas of clinically important fungi. Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada. 2017.
- Merad Y, Derrar H, Belmokhtar Z, Belkacemi M. Aspergillus genus and its various human superficial and cutaneous features. J Pathogens. 2021;10(6):643.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.