One of the most important health problems in the world, especially in developing countries, is the spread of multidrug-resistant foodborne pathogens. Such resistant pathogens can spread as an epidemic due to enhanced resistance against therapeutic antibiotics and/or the antibiotics used for growth promotion in livestock. Salmonella enterica strains can cause gastroenteritis leading to more complicated disorders in humans. Contaminated food can quickly spread the pathogen to a larger population, resulting in an epidemic. This review summarizes the antibiotic susceptibility patterns of Salmonella enterica strains isolated from food.
Salmonella, Antibiotic Susceptibility, Epidemic, Food
Public health and food safety issues due to drug-resistant microorganisms have long been a great concern.1 A rise in the consumption of food has been observed in recent years, which leads to a rise in diseases triggered by non-typhoidal Salmonella (NTS).2 Food can act as the main medium for NTS because aquatic animals excrete them in enormous quantities without revealing evident differences in their condition; consequently, they may potentially be a venue for the multiplication of resistant microorganisms.3 There have not been studies performed on drug-resistant Salmonella isolated from foods in some individual countries, and hence the problem in these vicinities is not clear, but the results of this article can provide some references.4 The present paper provides a precise, dependable, and broad review of the research that has discussed the susceptibility of subsequently taken out Salmonella isolated from foods, as the objects of this study and also provides a reliable evaluation of the present state, facilitates the outlook for the above-discussed research, and promotes the enhancement of this study.5
Salmonella seems to be one of the most common kinds of zoonoses worldwide, and its protection and treatment are becoming challenging due to the incident of multidrug-resistant (MDR) strains of this microbe.6 Increased usage of antibiotics in the farming and food industries of many nations has led to the introduction of microbes that are highly resistant to drugs.7 Antibiotics have been extensively used for the treatment of S. enterica infection in human beings and economically significant domestic animals.8 Surveillance is of central importance in the control of infectious diseases. Surveillance schemes in both affected and unaffected bodies may be used as an early warning for rising resistance-devastating resistance.6 Since early 2018, drug resistance in both S. Enteritidis and S. Typhimurium has been increasing.9 The purpose of this study is to provide an up-to-date evaluation of the resistance of Salmonella enterica strains retrieved from foods implanted for eight antimicrobial drugs utilized in the treatment of salmonellosis, using the available strategies for the monitoring of protection.10 The study stated herein is important since, to the best of the knowledge of the authors, it is the first comprehensive review of the antibiotic resistance profiles of Salmonella enterica strains taken from foods.11
Salmonella enterica: Characteristics and Pathogenicity
Antibiotic susceptibility patterns in Salmonella enterica strains from food should be taken seriously, as multiple Salmonella enterica strains have evolved extensively and still pose serious threats to public health.2 This review provides an overview of the distribution, classification, and pathogenic mechanism of Salmonella enterica.8 By analyzing the pathogenic mechanism of Salmonella enterica, this study reviews the microbial toxicity and abuse of antimicrobials in the aquatic products industry, briefly summarizes the sources of S. enterica in aquatic products and the means of transmission through the food chain, and thoroughly reviews the antibiotic resistance of bacterial series in aquatic products in recent years, providing a reference basis for the development of prevention and treatment strategies for Salmonella enterica infections.12
Salmonella enterica is classified under the Enterobacteriaceae family and includes two subtypes: typhoid serotype (enterica) and non-typhoid serotype.13 The strains of enterica group are further classified as named serotypes by Kauffman-White-LeMinor scheme.3 In addition, more than 2,500 serovars of Salmonella enterica are subdivided into different serotypes from A to Z.14 Generally, contaminated feeds and companion animals are considered as the main sources of Salmonella enterica in animal production.7 Their serotypes originate from several notifiable Salmonella serovars frequently isolated from companion animals, such as Salmonella oranienburg and Salmonella derby.4 Salmonella species are gram-negative, non-sporulating facultative anaerobic bacilli, usually appearing organized in short, motile, peritrichous flagella.15 An infectious dose of as few as 15 to 20 cells may cause death.16 Adhesion to enterocyte surfaces and transcytosis of the M cells are essential steps for the pathogenic forms.2
Taxonomy and classification
Salmonella enterica is a facultative intracellular bacterium that can cause disease in humans and animals, and it is found worldwide in aquatic and soil environments.7 This bacterium is one of the most frequent sources of foodborne bacterial illnesses and may be responsible for a variety of pathologies, including gastroenteritis, septicemia, and localized infections.2 The study will concentrate on food and detect Salmonella strains in seafood that are immune to antibacterials as a fundamental aspect of UK and international food security.17
Salmonella enterica belongs to the Enterobacteriaceae and is a Gram-negative rod bacteria that is facultative and motile.12 There are approximately 2,600 serotypes, which are divided into six subspecies and one undefined subspecies. It is usual for serovars to have multiple various names, including serovar, serogroup, and, strangely, subspecies.2 Only one Salmonella species has been established in one of the three existing subspecies and ssp, with two Salmonella species additionally indexed.15 Other than all the above sounds a bit complicated, it can be assured that Salmonella enterica has a lot of room for exploration.18 All three Salmonella enterica subspecies belong to the genus Salmonella, which includes Salmonella enterica, Salmonella bongori, and Salmonella subterranean.6 Salmonella enterica, plus the other 6 subspecies, are responsible for the vast majority of all human and animal infections.14 To put it another way, in this study, it is only the bacteria of this particular faction that are being examined.8
Morphology and biochemical properties
Salmonella enterica is a rod-shaped, gram-negative, non-sporulating, motile, facultative anaerobic, and oxidase-negative bacterium.13 S. enterica produces smooth (S) and rough (R) colonies on Salmonella-Shigella agar.2 S. enterica does not swallow the dye when subjected to the Gram stain and decolorizes with alcohol.15 The bacterial cell walls of serotype S. enterica are comprised of two sugars, glucosamine and muramic acid, plus an amino acid, L-alanine.7 In addition, a single cluster of N-acetylglucosamine further extends the peptidoglycan composition of bacterial cell walls.6
When it comes to the microscopic stuff, in both drying and wet preparations, S. enterica exhibits the motility feature.16 Arable soil contaminated by animal waste, water, and raw sewage, as well as living organs of healthy and sick animals, supply a large amount of Salmonella belonging to the Enterobacteriaceae family.4 The H2S production, citrate utilization, indole production, phenylalanine deaminase, lysine decarboxylase, ornithine decarboxylase, lactose fermentation, trehalose diacetate, D-sorbitol, melibiose, and dulcitol tests, as well as results for the Voges/Proskauer test, all play an incredible and critical role in the identification of Salmonella.8 Aeromonas, Citrobacter, Edwardsiella tarda, Escherichia, Hafnia, Morganella, Proteus, Shewanella, Vibrio, and Yersinia are all closely related species that must be separated from Enterobacteriaceae.18
Pathogenicity mechanisms
Salmonella enterica can cause food and blood poisoning called foodborne salmonellosis.1 This bacterium has many virulence factors (ViFs) that allow migration and spread in the host.3 In the process of lipid metabolism, reactive oxygen species (ROS) are produced, which may damage the bacteria’s lipids, proteins, and DNA.10 Lipids play an important role in the process of infection and colonization for Salmonella.2 The modification of fatty acids changes the membrane fluidity, thus affecting the bacterial virulence.14 The relationship between fatty acid degradation and Salmonella pathogenicity still remains unclear.7
This understanding is necessary for the comprehensive review of antibiotic susceptibility in food.12 This is because different VFs are required in differently infected tissues when causing diseases.4 Our review found that most of the studies focused on adaptive virulence factors.15 In contrast, VFs encoding genes that are involved in pathogenic pathways, such as SisA (adherence, invasion, and replication), were analyzed and summarized with related mechanisms such as how the pathogen interacts with the host, and the characteristics of infective and adopted intracellular lifestyle.8
Food contamination with Salmonella enterica
Salmonella enterica is the leading bacterial cause of foodborne disease outbreaks worldwide.1 This is largely due to the numerous sources and high prevalence of Salmonella enterica contamination in foods of animal origin.14 In aquatic animals and their derived products, their presence is associated with geographical areas and various pre-harvest and post-harvest handling factors.4 The frequency with which the bacteria occur in seafood around the globe varies from 0 to 44%, with the incidence up to 50% and 100%.5 In ocean waters, the major sources of environmental contamination with Salmonella enterica are most likely, paradoxically, the warm-blooded vertebrates themselves, particularly birds and reptiles.7 After depositing their feces, they leave the beaches with the bacteria that they carry.2 Human density, seabirds, and domestic dogs were indicated as factors contributing to the presence of the bacteria in coastal areas.6
Once reaching saltwater, Salmonella enterica can easily survive and live up to 70 and 4900 days, which is the expected time of coastal water renewal.18 The bacteria concentration levels are higher near the coast than in the open sea but, in any case, after filtration by shellfish, it can be transported and accumulated within their tissues, exoskeleton, or intestines.4 The most frequently reported Salmonella enterica serotypes in aquacultural environments and derived products are S. enterica serotype Agona, Anatum, Brandenburg, and Typhimurium, often reported as the main serotype also in other contexts.2 Salmonella enterica should not be present in any type of food in the ready-to-eat state, nor in fishery products for which it is not possible to carry out further treatment before entering the market.12 Therefore, new technologies to increase consumer confidence are required, and the application of the Hazard Analysis and Critical Control Points (HACCP) has become a legislative obligation.3 Of course, one of the fundamental aspects of the HACCP approach is the assessment of microbiological hazards and their susceptibility to antibiotics.15
In this respect, surveillance activities have several purposes, including the monitoring of foodborne pathogens and establishing preventive actions promoting health protection and ensuring a high level of food safety.9 Given the above, the proposed wide and comprehensive review work has been published according to this antimicrobial’s publication.10 The objective of this systematic literature review was to examine the available data on the prevalence and serotype profile of Salmonella enterica from food that is commercially available around the world.11
Sources of contamination
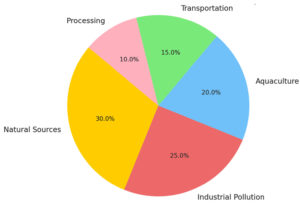
Food sources, including the water bodies that support the lifestyles of terrestrial animals and plants and contain terrestrial microorganisms, are also important sources of contamination (Figure 1).2 Salmonella is resistant to aquatic sources as an environmental reservoir.13 The main sources of S. enterica contamination include natural processes and activities of various microorganisms and personnel.4 In addition to contamination from natural processes, industrial production is also an important source of food origin contamination.12 Physical, chemical, and biological hazards may compromise seafood safety.7 Pollution sources include heavy metal pollution of marine and freshwater ecosystems, aquaculture, and industrial pollution.5
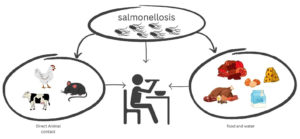
There are many sources of S. enterica pollution, including natural sources (e.g., birds, mammals) and anthropogenic sources such as pathogen carrier animals, human activities, and environmental composting.6 The ability of wild aquatic animals to carry S. enterica is an important factor in salmonellosis in geographically distinct wild aquatic animals, mainly through the health care of food sources and the accumulation of naturally contaminated surface or aquifer (Figure 2).12 A water source with body fluids and feces, for example, irrigation water, and airborne dust attached to food contact surfaces.7 Bacterial pollution can occur at any time during food production in two main steps: aquaculture and aquatic product processing.4 Food sources can be contaminated with S. enterica using various instruments, including physical means (e.g., direct contact with pets, birds, reptile habitats, and contaminated soils or feces) and transport vehicles (e.g., water, equipment) and foodborne microbiota (e.g., contaminated feed, water, and buildings).1
Prevalence and incidence
Salmonella enterica is an important foodborne pathogen that causes food-related illnesses worldwide.12 This section presents the prevalence (proportion of contaminated samples) and incidence (proportion of positive samples) of Salmonella enterica identified in food.6 The prevalence of Salmonella enterica may represent the frequency of its occurrence, indicating the distribution of this pathogen in specific aquatic and food environments.14 The incidence may also reflect the temporal trends in Salmonella enterica contamination.3 Salmonella sources, geographical variation, and other obvious factors can affect the prevalence of Salmonella in various food sources.2 The category of aquatic can be diverse. We elaborate on the prevalence and incidence of Salmonella enterica in a selected kind of food (e.g., groundwater, fresh fish, and oyster).15
The interval of published studies is another important factor that can influence the prevalence and incidence of Salmonella.11 This review includes articles published from 2009 to October 2021 that focus on the occurrence of Salmonella enterica in “food”.4 To collate results from studies that have a similar diagnostic approach, this study also included publications where some strains were found by rapid techniques (such as PCR) and then confirmed by a full microbiological method (ISO, MLG, etc.) to maintain the interest in “pathogenicity”.1
Antibiotic susceptibility testing methods
Hosts’ responses to antibiotics are not only determined by the biological activity of the compounds in action, but also affected by the interactions between the antibiotics, the hosts, and the pathogens, including the susceptibility, diffusion, and even degradation properties.10 It is the result of the three-dimensional comprehensive action.2 Correspondingly, the amount of the minimal antibacterial drugs, the minimum inhibition concentration, and the critical values of bacterial susceptibility can be supposedly determined after the pathogen has been isolated and identified.8 It is of great significance for further exploration of the mechanisms involved in antibiotic resistance.14
The antibiotics, resistance rates, mechanism research, etc., are closely related to the drug-sensitivity test, as well as the drug-resistance gene identification.7 In view of the current domestic and international circumstances of the drug sensitivities of bacteria isolated from foods, the majority of the research on the drug sensitivities of these bacteria is determined by the two main methods: the phenotypic and the genotypic testing, which provides an essential reference for the sensitivity of bacterial pathogens.4 Phenotypic methods have the purpose of directly empowering the study of antibiotics distributed and the drug resistances of different serotypes of Salmonella.6 Due to their simplicity, reliability, ability to gain direct information, and above all, their low cost, these kinds of methods are more and more common in clinical food inspection and are widely accepted in vitro. 12 The latter method, on the other hand, needs too many references and requires perfecting, and so imposes essentially higher economic and human cost.1 It is primarily used for the complementation of drug susceptibility testing in the context of drugs that are difficult to perform on the plate.5
Phenotypic methods
Antibiotics are classified by the World Health Organization (WHO) according to their effect on specific types of organisms.1 The review considers susceptible, resistant, and reduced antibiotic susceptibility patterns.7 The minimum inhibition concentration (MIC) range was used as the criterion for defining the susceptibility of the antimicrobials.2 Three major methods to assess antibiotic susceptibility are recognized: phenotypic, genotypic, and direct detection.10 Currently, there are two main phenotypic methods: disk diffusion and broth microdilution.6
Phenotypic methods assess the susceptibility of Salmonella enterica bacteria to antibiotics based on measurable qualities such as observable reactions to antibiotics or bacteria growth dynamics.15 The most frequently used method is the disk diffusion technique.4 This method is cheap and simple to manage, and it can be adapted to accommodate a wide range of antibiotics.11 On the other side, the direct cost of BD includes the price of the microplate, the price of the meals, and the expense of the pharmaceutical substances required to produce the dilution series.12 The broth microdilution method can be used to provide accurate MIC data in relatively short periods of time.3 Additional variations of microdilution, miniaturized to levels of 96 microdilution plates, have been developed and are primarily used for high-throughput testing.8 These systems allow for the testing of many samples at the same time.2 The broth microdilution test is known for its small sample size and low sensitivity.7
Genotypic methods
In the last few decades, the molecular tools used for the detection of resistant Salmonella strains have shown some significant developments compared to those applied in the 1980s.12 Generally, these methods are based on PCR with specific primer pairs or the hybridization of resistance gene probes to total DNA of the bacteria (like a DNA microarray assay), or in a more advanced study, primers targeting mostly mobile resistance gene sequences (e.g., integrase sequences or conserved direct repeats flanking these included).10 The amplification of the desired gene is often visualized using agarose gel electrophoresis.8 Similar in principle to PCR, these newly developed molecular assays include DNA microarray, multiplex real-time PCR, whole genome microarray attainment, genome-scale antimicrobial resistance diagnostics (GARD) assay, and others.1 Sequencing is also an alternative method sought out for in detecting resistance because it is capable of identifying the sequence type, a tool used for evading methodological and epidemiological doubts when detecting MDR Salmonella amongst other genera.4
In the next section, I will try to elaborate on genetic molecular techniques in order to provide a detailed insight into the molecular methods used.6 Detecting the molecular basis of resistance is indeed the most reliable means to delve into understanding infectious organisms, overcoming time constraints.7 Detection of more than one gene provides a reliable basis for the investigation of the mechanisms of resistance.10 Moreover, without the study of the genetic basis of resistance, clinicians and health caregivers may treat patients with an incorrect antibiotic in response to AMR bacteria.5
Antibiotic resistance mechanisms in Salmonella enterica
Intrinsic resistance mechanisms are important to consider because they are involved in resistance patterns towards several antibiotics, including first-line drugs such as ampicillin and the quinolone family (ciprofloxacin, ofloxacin) and the last-resort drug cefotaxime.2 However, the main mechanism of resistance in Salmonellae is the acquisition of resistance genes.4 In Gram-negative bacteria, the primary mechanism of dissemination of resistance arises through the acquisition of extrachromosomal elements.6 Specific genes associated with resistance are located in these extrachromosomal elements such as plasmids and virulence islands.7 The acquisition of these elements mainly occurs via horizontal gene transfer.12 Plasmids have a high prevalence in different isolates of Salmonella enterica, although some genes are encoded in the chromosome.14
The most frequent mechanism of acquisition of resistance is via plasmids.15 The first antimicrobials displaying resistance in this bacterial genus were sulphonamide and streptomycin in the 1950s.1 One important intrinsic resistance factor is based on the acquisition of efflux systems (that expel the drug) that may remove the drug from the bacterial cell.10 In addition, the main mechanism by which an intrinsically susceptible Salmonella mutates towards resistance is through genes located in the bacterial chromosome.4 Due to exposure to the antibiotic, some bacterial cells may produce a mutation in their DNA, in the part encoding for the target protein within the bacterial cell (enzymes, ribosomes).8 These point mutations can make antibiotics unable to correctly recognize the target protein as a result of changes in the 3D protein structure.3 This point mutation in the DNA can also confer efflux pumps activity by negative regulation, allowing those bacteria to be resistant by selective pressure.12
Plasmid-mediated resistance
Potential implications of our findings
Notwithstanding the low antimicrobial resistance rate displayed by the isolates included in our survey, our results reveal that, although recovery of antibioresistant bacteria from seafood is a rare event, Salmonella strains are equipped with extrachromosomal DNA elements responsible for coding drug resistance, particularly to tetracycline.7 Our study does not allow us to deduce how resistance genes could be transferred from one strain to another, but it has been suggested that the transfer of resistance to strains of other serovars might be due to the transposition of Tn916-like elements.10 Whether the resistance genes we have detected are located on plasmids or on transposons within the chromosome has not been clarified.14 In this research, no effort was made to isolate plasmid DNA by alkali lysis, and therefore, certain limitations of our methods should be kept in mind.6
The stability of a plasmid, however, is highly influenced by environmental and bacterial factors.5 Plasmid evolution can be driven by many mechanisms, and plasmids can change by mutation, deletion, and rearrangement every time they are introduced into new hosts or receive genes by recombination or gene conversion.2 Such studies, which are cost-effective and provide reliable data that are more informative than resistance patterns, should be extended to a larger number of Salmonella strains isolated from food.4 In fact, it is supposed that the ability to acquire and maintain resistance plasmid(s) could be considered as one of the main megavirulence axes, and comparing the antibiotic resistance patterns of isolates obtained from different aquatic environments, namely fresh water, cultured or seawater, could provide insight into the influence of PCB bioaccumulation on horizontal transfer of resistance generally, and on the potential development of drug-resistant strains.7 Moreover, the development of resistance to pediatric dosed antibiotics in foodborne pathogens could render current treatment strategies ineffective.14
Chromosomal mutations
Chromosomal mutations could be included in the modification of targets, the limitations of drugs entering cells, the removal of drugs out of cells, and all the other effects that could reduce antibiotic activity in an integrative manner.10 In this part, mutations that lead to ESBLs production and the AmpC β-lactamase overexpression in Salmonella are involved in the genetic changes of the expression profiles, indicating functional resistances.6 Mutational mechanisms related to fosfomycin, which include mutations in chromosomal acrB, uhpA, glpT, and uhp genes, also constitute certain selection pressure, finally leading to chromosomal alterations and development of resistant strains.15,19 Mechanically, certain processes may lead to resistance phenotype via a mutational way, such as lipopolysaccharide (LPS) mutations of LPS biosynthesis genes fimH, dedA, pgnP, wabG, and gmhA, drug targets, dsbA, fri, rne, ptsl NAD, ptsI, pmrK, pmrH, and ugd, etc.2 On the whole, these mutations which reduce drug susceptibility could have significant and far-reaching implications for the clinical treatment of Gong meat products polluted with S. enterica infectious patients.4
Resistance of Salmonella to clinically important drugs can be conferred by chromosomal point mutation.1 Usually, resistant development related to chromosomal point mutations could be viewed as the evolutionary result and lead to reduced fitness.18 Point mutations that could lead to a reduction of mutant fitness are more deleterious than the point mutations conferring resistance that gives the bacteria a selective advantage, resulting in the selection of strains with mutations that contain increased fitness and do not only apply to ciprofloxacin but also levofloxacin.7 With the introduction of ciprofloxacin and third-generation cephalosporins for treating Salmonella infections in humans, this possible selection strategy of inactivating chromosomal mutations has been suggested.15 In addition, chromosomal mutations, which lead to the reduction of antibiotic susceptibility and clinical treatment failure, have become a serious public health problem.6
Efflux Pumps
Antibiotic resistance is mostly mediated by several mechanisms, including the production of hydrolytic enzymes, modification of target molecules, reduced cell permeability, and increased efflux of antibiotics out of the bacterial cell.12 Efflux pumps are transport systems that expel structurally and/or functionally unrelated substances out of bacterial cells, including antibiotics.2 All efflux systems are transmembrane proteins and can be classified into five super families, according to the sequence of amino acids that make them up or due to the energy source they require.10 Among them, the major facilitator system (MFS)-typically H+-coupled-, the resistance-nodulation division family (RND)-that require energy provided by the proton motive force-and the small multidrug resistance (SMR) family of efflux pumps are overexpressed in the cells of microorganisms belonging to family Enterobacteriaceae, including Salmonella spp., E. coli, Shigella spp., and Klebsiella spp.7 This upregulation mainly occurs in MDR strains.1 Both structural and functional differences and similarities exist among individual families of efflux pumps.4
The primary role of efflux pumps consists in the export of toxic molecules; however, many strains overexpressing efflux mechanisms have been found to be involved in other processes, including self-maintenance and stress responses.14 An important clinical aspect is that some efflux proteins are responsible for resistance to a specific antibiotic or to antibiotics belonging to the same family, whereas others confer resistance to compounds ensuring different targets and/or used to treat a different range of infections.2 Therefore, efflux pumps expressed by microorganisms are involved in both the increased survival of bacterial cells carrying the pumps and the decreased chances for an effective infection therapy.15 In Salmonella enterica infections, efflux pumps need to be currently investigated because of their increasing importance.3 Therefore, in this review, we analyzed scientific papers covering the study of the antibiotic susceptibility patterns in different serotypes of Salmonella enterica isolated from different aquatic animal species and published from 2011 to 2021.6,20
Global trends in antibiotic resistance in Salmonella enterica
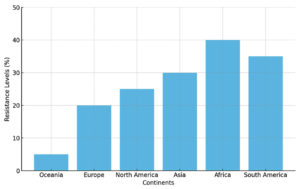
These results show a steady resistance trend to widely used antibiotics in Salmonella isolated from aquatic products.12 The proportion of resistant Salmonella from aquatic products showed great variation in different continents.7,21 These findings might again act as a baseline drug sensitivity data for microbial resistance monitoring and control programs.14 Scientific opinion points to the extensive selective pressure exerted by antimicrobial agent misuse and abuse on the emergence and development of antibiotic resistance.4 Once resistance appears, it can be further spread via horizontal transfer.15 According to these reports, the prevalence of resistance in various geographic areas is becoming similar, irrespective of where the resistance first emerged.2 This regional convergence of resistance is a dangerous emergent trend, as it is important for food safety and public health to take effective strategies to control antibiotic resistance.3 The following section of this review is to describe in more detail the situation in aquatic products in various parts of the world1 (Figure 3).
Increasing evidence seems to suggest that molecular adaptation and phenotypic plasticity in bacteria can result in the emergence of resistance to a specific compound or class of agents, from which an almost unlimited number of new resistance developments can arise.10 There is therefore a dangerous risk of not being able to control the overall increasing resistance found in different geographical regions.9 In this review, we examined antibiotic susceptibility patterns and resistance trends as described in scientific articles and public reports.12 The search was based on the discrimination of the isolation sources of the bacteria, according to the following species: S. Senftenberg, S. Typhimurium, or only Salmonella enterica, as they are the most common isolates from food specimens.2 The following is an examination of the current situation regarding antibiotic-resistant strains of the genus, isolated from food, in various regions of the world.14
Regional variations
There is variation in the bacterial resistance patterns based on the geographic location.10 Because of disparities in antimicrobial usage, national differences vary, as well as in susceptibility testing and isolation, which varies between countries and the level of surveillance.4 And since the aquatic products mainly are imported, obtaining background knowledge and data that can be in many ways accurate is required.2 In this review, we have carefully explained the presence and range of antibiotic resistance from strains of Salmonella enterica isolated from fish, molluscs, and crustaceans in 12 different parts of six big regions across the globe.7
In this review, we detected diverse antibiotic resistance frequency patterns among S. enterica isolated from food of 12 different parts of six substantial regions in the planet.12 We attribute these disparities to several inconsistencies in factors among different parts, including variations in antibiotic usage, especially when considering that foods are often imported from elsewhere.6 Regarding national differences in antibiotic use, continent differences in antibiotic resistance were also detected.9 Oceania exhibited the lowest levels of S. enterica resistance against each antibiotic, while the other regions usually presented levels in the middle, especially in Europe and North America.1 Antimicrobial stewardship comparable to the well-run program of the UK, and commonly China, exhibited to a limited extent, can significantly alleviate or resolve this antimicrobial resistance issue.15 The level of surveillance may also contribute to the difference.4 These disparities may also be attributed to ecological determinants, as seen.10
Emerging resistance patterns
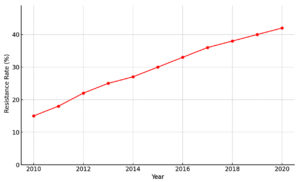
Emerging patterns of resistance to first-line antibiotics are a serious and concerning issue.7 These patterns could derive from the global trade and travel of infected humans and animals, spreading resistant bacteria, or from random mutation and selection caused by the selective pressure of antibiotics.12 Zonja et al. showed that Salmonella serovar Heidelberg strains isolated from pork were characterized by amoxicillin-clavulanic acid and colistin resistance.15 That same study reported that temocillin, a specific penam that is not yet registered in the United States, was tested against four resistant amoxicillin-clavulanic-acid isolates belonging to MLST ST15 and was found to be effective in eliminating the strains.4 Zonja and coworkers have shown that Salmonella Heidelberg strains isolated from pork show resistance to first-line antibiotics, and such strains can be responsible for extra-intestinal infections in humans.10 In the Netherlands, a recent study showed increasing amoxicillin and tetracycline resistance in Salmonella ser. Paratyphi B, while decreased ciprofloxacin resistance was observed concurrently.2 This study stresses the need to continuously monitor resistance levels in order to give early warning signs when resistance rates change and to increase awareness of both clinicians and microbiologists for possible treatment failure in invasive salmonellosis cases 3 (Figure 4).
Consumption of foods has increased annually, offering food companies great opportunities for business development.1 Among microbial pathogens, Salmonella has been considered a principal source of foodborne illness among foodborne-related outbreaks.5 In general, most of the isolated strains were susceptible to the five drugs, and less than 0.5% of each year’s formula was resistant to ceftazidime.6 The fluoroquinolone resistance rates of ciprofloxacin and levofloxacin also showed an increasing trend.7 This study aimed to provide a basic outline for choosing treatment regimens for epidemic trends in human and animal salmonellosis.15 The surveillance for emerging resistance is critical for public health problems to implement positive intervention strategies.12 Antimicrobial stewardship should be performed through continuous monitoring based on the drug susceptibility profile of the gastroenterology series.4,22
Public health implications of antibiotic resistance in Salmonella enterica
Isolation of Salmonella enterica strains from foods, such as fish, shrimp, and crab, was reported worldwide.2 Eating foods that are contaminated by this bacteria is one of the potential risk factors for acquiring salmonellosis.1 Currently, usage of antibiotics for non-therapeutic applications (growth promotion, disease prevention, and disease treatment) in animals is the major concern in food safety.7 Development of antibiotic resistance in S. enterica strains contributes to reduced efficacy of antimicrobial treatment, causing illness that can be more severe, last longer, and be more likely to spread to others.15 Therefore, the antimicrobial resistance (AMR) present in S. enterica strains is a potential public health concern.4
The entire set of bacterial, animal, and human aspects of antibiotic resistance should not be underestimated.6 This broader perspective forms the basis of the integrated approach that recognizes the multifaceted nature of AMR.12 Whether the origin is in the animal or human environment, the resistance mechanism is specific to a given bacterium.1 To understand the mechanisms of transmission and resistance, it is also important to integrate expertise related to human medicine and the environment into surveillance and knowledge systems.10 Consequently, this surveillance should ideally explore the interconnections between human, animal, and environmental health, so-called One Health.2 In summary, quantitative projections of the burden of infections and partnerships between human and veterinary health services will be useful both to explore their relationships and to prioritize resources in regions or epidemiological contexts where they differ.7
Treatment challenges
Foods are one of the important components of a healthy diet, with the supply and consumption of foods steadily growing globally.5 However, aquatic animals and environments form a major habitat for different microorganisms, including pathogenic bacteria.4 Among these bacteria, Salmonella enterica is a serious concern for foodborne infectious diseases.2 Although some extrinsic factors, such as pathogen intake concentrations and pre-harvest interventions, impact the rate of infection, other factors related to the development and spread of fluoroquinolone resistance in Salmonella enterica are linked to therapy.15 With the increasing prevalence of antibiotics to enteric and systemic infections, empiric therapy can be delayed or incorrect and result in higher patient mortality.14 In advanced or bloodborne disease, a combination therapy course of antibiotics may be required, with limited treatment options due to multidrug resistance in some parts of the world.12 These are becoming significant challenges to the effective treatment of systemic nonspecies zoonotic salmonellosis.7
From an international perspective, nontyphoidal salmonellosis is estimated to result in 93.8 million public health cases and 155,000 deaths annually.18 The trend of resistance to fluoroquinolones in enteric and typhoidal Salmonellae has recently been increasing.6 Salmonella is increasingly found to be an active source of antibiotic resistance and impairs the current abundant therapeutic armamentarium available for Salmonella infections, increasing treatment failure and overall healthcare expenditure.10 Resistance to quinolones causes Carroll’s or Hagens’ gene mechanisms of action; whatever the mechanism is, resistance to FQ is caused by point mutations primarily in the QRDR of GyrA and occasionally in the ParC gene.15 Several efforts must be made to deal with such treatment constraints as easy entry of resistance (internal species as well as contemplating the wider aquatic environment), reducing measures via the food chain from animal to human affecting sustainable exports, and increasing systemic infections and delayed clinical failure.2,23
One health approach
The cause of the antibiotic resistance situation is due to the historical (probably) misuse of antibiotics, over-prescribing by physicians, and even the availability of antibiotics for sale to farmers and anyone else in certain countries.7 Indeed, antibiotic resistance is not a problem only in medicine; it is also a problem of public health.10 A common approach should come in where human, animal, and environmental health must work together.4 This area of research is generally referred to as One Health.15 Members of the medical, veterinary, and environmental professions, as well as many other occupations, generally take part in the One Health community.2 In recent years, there has been increased awareness of the importance of interdisciplinary research among academics and professional circles.1 In response, a number of collaborations between medical and veterinary institutions have sprung up.12
The FAO and WHO, speaking on behalf of the FAO, WHO, and OIE, ultimately proposed a 50-point policy framework to address antibiotic resistance in animals.18 ‘Policy Option 49’ states that “antibiotic resistance in humans can be reduced significantly in animal husbandry”.18 ‘Annex 1’, under “Policy option 13”, suggests a number of basic principles for the use of antimicrobials in food animal production, such as a complete ban or restrictions on the use of particular antimicrobials that are considered crucial in human medicine, as specified by the WHO Expert Group on Public Health Importance of Antimicrobials.2 Ultimately, the issue of antibiotic resistance in zoonotic bacteria will only be taken seriously if the above-mentioned “general aspect” can be fully understood.4 Then, it becomes possible to go one step further, by identifying a number of examples of zoonotic bacteria, considered emerging threats for public health, which are resistant to a number of antimicrobials; this refers to colibacilli, such as Salmonellosis-causing Salmonella enterica from food.15
Regulatory measures and interventions
Considering the public threat posed by the presence of resistant Salmonella enterica strains detected in food, various severely stringent provisions have been adopted in specific countries to establish the microbiological criteria for these microorganisms in foods or in food-producing animals.7 For instance, in Poland, applicable regulations limiting the presence of resistant S. enterica follow Regulation of the Ministry of Agriculture and Rural Development of 2 August 2016, setting out rules for monitoring zoonoses and infectious diseases in animals.12 This is followed by regulation concerning repeated monitoring of foodstuffs of animal origin for the presence of Salmonella, extending the regulations by including a reference to minimum resistance thresholds for antibiotic agents for the control of infectious diseases caused by Salmonella enterica or less dangerous subtypes of these bacteria.15 Other regulations limiting parameter values are given by Commission Implementing Regulation (EU) No. 2018/333 regarding the establishment of the maximum limit for certain contaminants in foodstuffs.6 All the regulations mentioned above, including those discussed in the preceding paragraphs, do not establish the value of the epidemiological threshold, which should be unlike the relevant microbiological criterion but refers to the same provisions.2 Furthermore, microbiological criteria aim to guarantee food safety, protecting from bacterial infections or toxicosis.1 Additionally, bacteria that possess the possibility of transferring antibiotic resistance genes are also considered a potential health and environmental threat.10
The prevention of Salmonella spp. contamination in food is critical and important, requiring reduced levels of resistant strains throughout the food production chain 4. Food-producing animals and the environment should be constantly subjected to an accurate inspection, contributing to the control of potential sources of contamination.7 For instance, the application of antibiotic stewardship programs is also required in human patients to ensure the rational use of antibiotics in molecular and clinical medicine, proving that reduced use would lead to low pressure on bacterial strains for the development of resistance, relatively being the reason for achieving a change in the susceptibility patterns in terms of individual pharmacologic formulations.2 In addition, according to the concept of ‘One Health’ involving the monitoring of veterinary antibiotics used for therapy, treating food-producing animals in particular, it is estimated that their consumption constitutes 80% of the total use of antibiotics licensed in Europe.10
Due to the report of the European Medicines Agency (EMA), the overall EU sales of veterinary antibiotics have decreased by more than 20% since 2011.15 In such research pathways, ‘One Health’ is the basis for guidance issued by the World Health Organization (WHO), the UN Food and Agriculture Organization (FAO), and the European Centre for Disease Prevention and Control (ECDC) for the classification of veterinary antibiotics regarding the importance of antimicrobial resistance to human medicine.4 These guidelines were developed on the condition of introducing risk management measures to control the occurrence of MDR strains and to lead to reduced resistance, while other options for action can be taken such as not assigning pharmacological compositions to categorizations if a certain established target value of resistance to priority substances in humans is exceeded.7
Food safety regulations
Global food safety policies and norms have been initiated in most countries due to the widespread of antibiotic-resistant Salmonella.2 It has been well documented that aquatic animal products are one of the main origins of antimicrobial-resistant (AMR) bacteria.10 A new system comprising hazard analysis and critical control points has to be established.12 Food products can be exposed to a range of previous microbiological or viral pathogens on the farm.1 Processing chains of aquatic products are designed with different sources of pollutants such as disinfection by-products, chemical treatment products, pathogen contamination, and tropical effluents.4 Hygiene practices of a seafood establishment have been implemented to adopt domestic and regional guidelines.7 One of the major issues in controlling the spread of pathogens is the use of antibiotics and other chemical substances to control the growth of bacteria in aquacultured animals.2 Furthermore, the regulatory framework aims to set up and ease the security and quality of aquatic meals to address a regulatory gap throughout the food chain from the farm to the consumer.6
Comprehensive systemic literature analysis has been undertaken to review antibiotic susceptibility patterns based on individual sample origins.10 However, aquatic animal food products have been reviewed, classified, and discussed based on their main sample origins.2 We believe the review of antibiotic susceptibilities is directly related to each aquatic sample subset.4 Data on antibiotic susceptibility patterns in Salmonella isolated from Silurus triostegus, alcoholic beverages, ornamental fish aquariums, Botia strict pot, suckermouth armored catfish, frozen E. vermiculata, galangal mince, and substrate waste and red-sludge substrate ponds are available.15 In our opinion, available databases and the spread of antibiotic-resistant Salmonella or various habitats have been known, making it hard to trace the corresponding strain.10 Data related to the view of antibiotic susceptibility patterns in Salmonella can be projected regarding the scope and source of each review of food origins.6
Antibiotic stewardship programs
As a part of an initiative to reduce the phenomenon of antibiotic resistance, several countries have implemented antibiotic stewardship programs (ASPs) in human and veterinary medicine.7 The aim of the ASPs is to modify the antibiotic prescribing practices, establish surveillance systems, introduce a large number of public and professional educational or awareness campaigns to promote the use of antibiotics and limit resistance, and propose policies and strategies to change the behaviors of prescribers, industry, and the public.15 There are few studies on the impact of these programs in the specific case of Salmonella enterica.4 These studies show some limitations in reducing antibiotic resistance, particularly in veterinary medicine.10 This situation is due to many factors, including the addictive effect of antibiotics for animal weight gain and performance, the influence of the weather, poor management on farms, and consumers’ lack of knowledge about antibiotic resistance in the food of animal origin.7
To facilitate the process of setting up such programs in this sector, this study deals with issues related to the use of antibiotics and the distribution of antibiotic resistance in Salmonella isolated from food.6 In fact, the number of reported Salmonella enterica strains with a multiresistant phenotype is continuously increasing around the world.2 The pool of resistance observed is associated with reported patterns of antibiotic use in human and animal medicine.12 The strategy behind the diffusion of antibiotic resistance in Salmonella enterica strains isolated from food sources has developed rapidly over the last 15 years.18 This encouraging and promising interdisciplinary approach results from strict international control.1
Future perspectives and research directions
The excessive use of antibiotics exacerbates the emergence and spread of resistance, and developing novel antibiotics and alternative therapeutic strategies is a solution to address this issue.4 The development of novel agents based on alternative producing strategies is an attractive alternative in addressing foodborne infection.15 Bacteriophages are currently attracting attention because of their effective targeting of bacterial pathogens in animals and humans. 7 Phage-based biocontrol strategies are being pursued to prevent pathogenic bacterial dissemination through the consumption of contaminated food products, as suggested by specific phages targeted against Salmonella serovars that have caused acute systemic infection.2
Surveillance of the antimicrobial susceptibility and resistance of Salmonella enterica to specific antibiotics is the current recommended means of identifying the presence of salmonellosis in the general infectious population or in patients with confirmed non-typhoid salmonellosis.10 The antibiotic susceptibility patterns presented in this paper may aid global health stakeholders in comprehensively analyzing such epidemiological data.15 A total of 9 kinds of antibiotics showed less than 10% resistance, and as such, this data could be useful for the development of new methods acceptable for treating food, given that the natural reservoir of S. enterica is mostly food.4 A combination of bacteriophages specifically targeting serovars of S. enterica, traditional antimicrobial compounds, and removal of unhealthy animals is expected to decrease resistance.6 Certain antibiotics are expected to contribute towards making such decisions.2
Novel antibiotics and alternatives
New antimicrobial agents still used in humans can be attractive to prevent Salmonella contamination in food.12 This increasingly important field utilizes bioinformatics analysis to find broad-spectrum antimicrobial peptides in humans.2 Antimicrobial peptides have broad-acting activity against different cells and microorganisms.15 More recently, drugs have been used as potential repurposed agents to treat various diseases such as epilepsy and hypertension because of their antibacterial and antiviral properties. 10 While drug residues are closely monitored in many countries, there may be the possibility of developing new antibiotics for S. enterica.7 Drugs in this category may represent the newest group of broad-spectrum antimicrobials due to their broad tissue distribution, low bacterial resistance, and efficacy and potential for repositioning in the context of the use of antibiotics.4
The use of bacteriophages for the prophylaxis and treatment of infectious bacterial diseases has been considered an alternative tool in combating pathogens, such as in the reduction of S. Typhimurium contamination in or on fresh produce, ready-to-consume leafy greens, and cantaloupes.2 The use of antimicrobial drug cocktails may also be one of the promising approaches to reduce costs, overcome resistance, improve the effect of antibiotics, and ultimately reduce the problem of antibiotic resistance in foodborne zoonotic pathogens such as Salmonella as well.15 Dietary and non-dietary antioxidant and anti-inflammatory interventions are considered to be serious.6
Surveillance strategies
It is well known that antibiotic susceptibility patterns of Salmonella serovars might change within the production chain of food.7 Therefore, to improve monitoring strategies, big data analytics and genomic surveillance are increasingly being developed.4 This data is generally collected at the abattoir (after transport or a resting phase, for example) or during or after processing (cold smoking, marinating, or in situ with measurement and cleaning protocols performed in food processing at the retail level).2 At the same time, genetic profiles of strains are examined and subjected to a genomic or phenomic microarray.12 Many of these surveys have been conducted over several decades and are geographically limited and have excluded studies in which aquafarms, including surface water and whole aquatic populations (as opposed to clinically affected populations), are targeted to study clinically relevant antimicrobial resistance trends.15 The development of antimicrobial resistant phenotypes and the co-selection of genes resulting in a multidrug-resistant profile can take place at all stages of the production chain of food.10 The emergence of MDR serovars that are not initially treated with antimicrobials highlights the need for proactive surveillance.7
Thus, phylogenetic analysis and large-scale WGS allow for the examination of genetic relationships within and between human, animal, and environmental isolates at a relatively low cost 4. Heavy data processing of a huge amount of DNA data is now necessary.2 In addition, the support of (inter)national collaborative networks is a significant advantage in the fight against emerging international threats.12 Proactive and integrated surveillance within human, veterinary, food, and environmental fields can provide relevant information for assessing the ability to deal with emerging MDR trends in terms of capacity and performance.6,17 Rapid point-of-care tests such as single or multiplex nucleic acid tests and whole genome sequencing (WGS), where infrastructure allows, to guide appropriate therapy wherever indicated are suggested.10
This comprehensive review highlights the antibiotic susceptibility patterns of Salmonella enterica strains isolated from food sources, emphasizing the growing concern of antimicrobial resistance (AMR) in the global food chain. The findings underscore the variability in resistance profiles among different strains, influenced by geographic distribution, antibiotic usage in food production, and bacterial adaptation mechanisms. The increasing prevalence of multidrug-resistant Salmonella poses a significant threat to public health, complicating treatment options and raising concerns about food safety and infection control. The persistence of resistant strains in animal-derived food products, water systems, and processing environments highlights the urgent need for enhanced surveillance, responsible antibiotic stewardship, and strict regulatory policies to mitigate the spread of resistance. To combat this challenge, a multifaceted approach combining advanced molecular techniques, routine susceptibility testing, improved hygiene practices, and alternative antimicrobial strategies is essential. Strengthening global collaborations between public health authorities, food industries, and research institutions will play a crucial role in safeguarding food safety and preserving the efficacy of antibiotics against Salmonella enterica infections. Moving forward, continuous monitoring and research on emerging resistance trends, genetic mechanisms, and potential mitigation strategies will be essential in ensuring effective infection prevention, disease management, and sustainable food production practices.
ACKNOWLEDGMENTS
The author gratefully acknowledges technical and financial support provided by Jouf University, DSR, Sakaka, Saudi Arabia.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Havelaar AH, Kirk MD, Torgerson PR, et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12):e1001923.
Crossref - Foley SL, Lynne AM. Food animal-associated Salmonella challenges: Pathogenicity and antimicrobial resistance. J Anim Sci. 2008;86(E. Suppl.):E173-E187.
Crossref - Alcaine SD, Warnick LD, Wiedmann M. Antimicrobial resistance in nontyphoidal Salmonella. J Food Prot. 2007;70(3):780-790.
Crossref - Cabello FC. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ Microbiol. 2006;8(7):1137-1144.
Crossref - Davies R, Wales A. Antimicrobial resistance on farms: A review including biosecurity and the potential role of disinfectants in resistance selection. Compr Rev Food Sci Food Saf. 2019;18(3):753-774.
Crossref - Crim SM, Griffin PM, Tauxe R, et al. Preliminary incidence and trends of infection with pathogens transmitted commonly through food-Foodborne Diseases Active Surveillance Network, 10 US sites, 2006-2014. MMWR Morb Mortal Wkly Rep. 2015;64(18):495-499.
Crossref - Angulo FJ, Nargund VN, Chiller TC. Evidence of an Association Between Use of Anti-microbial Agents in Food Animals and Anti-microbial Resistance Among Bacteria Isolated from Humans and the Human Health Consequences of Such Resistance. Journal of Veterinary Medicine, Series B. 2004;51(8-9):374-379.
- Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5(5):607-625.
Crossref - Hendriksen RS, Seyfarth AM, Jensen AB, et al. Results of use of WHO Global Salm-Surv external quality assurance system for antimicrobial susceptibility testing of Salmonella isolates from 2000 to 2007. J Clin Microbiol. 2009;47(1):79-85.
Crossref - Heikal HSM, Abd El Naby WSH. Genetic improvement of litter size in four goat breeds in Egypt using polymorphism in bone morphogenetic protein 15 gene. Adv Anim Vet Sci. 2017;5(10):410-415.
Crossref - Li L, De Keuckelaere A, Uyttendaele M. Fate of Salmonella and Listeria monocytogenes in the presence of the human pathogen Escherichia coli O157 and indigenous microorganisms during storage on butterhead lettuce. Int J Food Microbiol. 2015;208(10):18-25.
Crossref - Hald T, Wingstrand A, Swanenburg M, von Altrock A, Thorberg BM. The occurrence and epidemiology of Salmonella in European pig slaughterhouses. Epidemiol Infect. 2003;131(3):1187-1203.
Crossref - Braden CR. Salmonella enterica serotype Enteritidis and eggs: A national epidemic in the United States. Clin Infect Dis. 2006;43(4):512-517.
Crossref - Mather AE, Reid SW, Maskell DJ, Parkhill J, Fookes MC, Harris SR, et al. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science. 2013;341(6153):1514-1517.
Crossref - Mather AE, Reid SWJ, Maskell DJ, et al. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science. 2013;341(6153):1514-1517.
Crossref - Doyle MP, Erickson MC. Summer meeting 2007-The problems with fresh produce: An overview. J Appl Microbiol. 2008;105(2):317-330.
Crossref - Hailu W, Alemayehu H, Wolde D, et al. Prevalence and antimicrobial susceptibility profile of Salmonella isolated from vegetable farms fertilized with animal manure in Addis Ababa Ethiopia. Sci Rep. 2024;14:19169.
Crossref - Heymann DL. Control of communicable diseases manual (19th ed.). Washington, DC: American Public Health Association. 2008.
- Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629 655.
Crossref - Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: A food production perspective. Int J Food Microbiol. 2007;117(3):237-257.
Crossref - Habib I, Mohamed MYI, Lakshmi GB, et al. High prevalence and genomic features of multidrug-resistant Salmonella enterica isolated from chilled broiler chicken on retail sale in the United Arab Emirates, Int J Food Microbiol. 2024;423:110828,
Crossref - Khan HA, Neyaz LA, Malak HA, et al. Diversity and antimicrobial susceptibility patterns of clinical and environmental Salmonella enterica serovars in Western Saudi Arabia. Folia Microbiol. 2024.
Crossref - Britto CD, John J, Verghese VP, Pollard AJ. A systematic review of antimicrobial resistance of typhoidal Salmonella in India. Indian J Med Res. 2019;149(2):151–163.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.