ISSN: 0973-7510
E-ISSN: 2581-690X
A sustainable and environmentally beneficial method is the synthesis of green nanoparticles using various plant components. Extracts from medicinal and herbal plants were used to synthesize nano silver. Aqueous extracts of Solenostemma argel, Citrullus colocynthis, Syzygium aromaticum, Foeniculum vulgare, Maerua crassifolia, and Elettaria cardamomum have been tested as antimicrobial and antifungal agents. In a lab-scale system, the effects of these plant extracts with or without silver nanoparticles (AgNPs) were tested and evaluated. The plant extracts used were Solenostemma argel (SA-AgNPs), Citrullus colocynthis (CC-AgNPs), Syzygium aromaticum (SAR-AgNPs), Foeniculum vulgare (FV-AgNPs), Maerua crassifolia (MC-AgNPs), and Elettaria cardamomum (EC-AgNPs). According to the findings of the antibacterial tests, SA-AgNPs was the most effective plant extract combined with AgNPs, demonstrating high antibacterial activity. Conversely, the extracts from Solenostemma argel resulted in the most significant inhibitions of Candida albicans fungus growth. The potential bioassay activity of these synthetic nanoparticles were tested alongside the identical plant extracts (without the silver nanoparticles). Solenostemma argel exhibited the maximum zone of inhibition towards Bacillus subtilis (35 mm) and Candida albicans (34 mm), indicating its strong antimicrobial properties. Antibacterial activity results indicate that plant extracts combined with AgNPs possess promising antimicrobial activities against a range of pathogenic microorganisms. This study is crucial to the progression of green chemistry and may result in the development of novel antibacterial and antifungal agents that capitalize on the natural properties of medicinal plants while eliminating the need for toxic chemicals. This study recommends using medicinal plant extracts enhanced with AgNPs to control human pathogenic microbes, including Gram-positive, Gram-negative bacteria and yeasts.
Green Approach, Silver Nanoparticles, Medicinal Plants, Antimicrobial Activity, Methanol Aqueous Extracts
The green biosynthesis of metal nanoparticles (NPs) from plant extracts has garnered important attention in modern medicine. Metallic nanoparticles have exclusive mechanical, chemical, magnetic, and electrical properties that keep them very interesting in the field of nanotechnology. The base for integration and control on both molecules and atoms is provided by nanoscale material fabrication.1 There have been reports of significant antimicrobial activity for silver nanoparticles.2 Silver nanoparticles are synthesized by a variety of techniques, including biological, heat evaporation, photochemical reduction, and electrochemical reduction. According to published reports, these techniques are highly costly and necessitate the application of hazardous chemicals that endanger both the human health and environment. The manufacture of silver nanoparticles from plant extract is gaining more attention these days due to their environmentally beneficial properties. Plant extraction serves as a reducing and capping agent to manufacture nanoparticles.3,4
Biogenic routes for the manufacture of metal and metal oxide nanoparticles (NPs) by means of aqueous plant extract and microorganisms have garnered significant consideration owing to their stability, cost-effectiveness, clinical adaptability, stability, and biocompatibility.5,6 Numerous investigations into the antimicrobial activity of silver nanoparticles (AgNPs) synthesized biogenically utilizing biological materials such as plant and herbal extract and microorganisms as reducing agents have been carried out recently.7,8 The first report on the synthesis of biogenic AgCl-NPs using the extract of the aerial part of Pulicaria vulgaris Gaertn. and their application as antibacterial, antifungal and antioxidant agents pharmaceutical plant was performed by Sharifi-Rad et al.9 Plant extract proteins, flavonoids, ketones, aldehydes, tannins, carboxylic acids, phenolic acids, and aldehydes oxidize Ag+ to Ag0 to generate AgNPs.10 Metal nanoparticle synthesis using plant and herbal extracts is one of the most convenient, cost-effective, and ecofriendly and harmless procedures for reducing the use of harmful chemicals. In recent years, various ecofriendly techniques for the fast synthesis of silver nanoparticles have been stated, utilizing aqueous extracts from many plant parts including leaves, bark, and roots. Medicinal herbs, also mentioned as medicinal plants, have long been recognized and used in traditional medical methods. For a number of reasons, comprising protection and defense against bacteria, fungi, insects, and several diseases, plants generate many of chemical compounds.11 Unlike synthetic antimicrobials, which can cause adverse effects, medicinal plants can be used therapeutically. Sharifi-Rad et al.12 reported the green synthesis of AgNPs by the L. royleana leaf extract with biopharmaceutical, catalytic applications and in vitro anti-arthritic activity. Also, a quick, extremely simple one-step green synthesis of the AgNPs by the root extract of A. tribuloides Delile, with specific biological activity that may be exploited for biomedical applications as a new drug combination.13 The anticancer, antimicrobial, anti-inflammatory, and antioxidant properties of Solenostemma argel are well-known.14 Citrullus colocynthis is used as antioxidant, anti-microbial, anti-bacterial and anti-hyperlipidemic activities.15 Syzygium aromaticum has analgesic, antioxidant, anticancer, antiseptic, anti-depressant, antispasmodic, anti-inflammatory, antiviral, antifungal, and antibacterial activities.16 Foeniculum vulgare exhibits antifungal, antibacterial, antioxidant, antithrombotic and hepatoprotective activities.17 Maerua crassifolia has analgesic, anti-inflammatory and antipyretic activities.18 It has been demonstrated that Elettaria cardamomum can treat cardiac, digestive and kidney problems as well as asthma, tooth and gum infections, cataracts, nausea, and diarrhea.19 This study focus on the green synthesis of silver nanoparticles with antimicrobial properties utilizing aqueous extracts from indigenous plants in Yanbu Province, one of the Saudi Arabian cities. Aqueous extracts of Solenostemma argel, Citrullus colocynthis, Syzygium aromaticum, Foeniculum vulgare, Maerua crassifolia, and Elettaria cardamomum were used in the current study to synthesize biogenic silver nanoparticles that were subsequently investigated as antimicrobial and antifungal agents. The primary goal of comparing extracts with and without silver nanoparticles is to determine if the plants themselves have strong antibacterial activity or antifungal activities whether the silver nanoparticles improve the plant extracts’ bioassay capabilities.
Plant materials
Variable plant parts (leaves, flowers and seeds) of the medicinal plants, such as Solenostemma argel SA (leaves), Citrullus colocynthis CC (seeds), Foeniculum vulgare FV (seeds), and Maerua crassifolia MC (seeds), were collected from the desert areas surrounding Yanbu city, Saudi Arabia. Other plants Syzygium aromaticum SAR (flowers), and Elettaria cardamomum EC (seeds) were purchased from the local Market, thoroughly cleaned with tap water and then distilled water to remove any debris or other unwanted materials. After being washed, the leaves were allowed to air dry at room temperature.
The plant species were identified using a traditional approach including dichotomous keys; published plant descriptions, illustrations and photographs; and comparison with properly identified herbarium specimens. Specimen samples are pressed in a plant press and dried on a plant dryer. Specimen’s vouchers were deposited in a local herbarium in a faculty of science at Yanbu, KSA, Taibah university with voucher numbers as follows: Solenostemma argel (Voucher No. SA0011), Citrullus colocynthis (Voucher No. CC0012), Foeniculum vulgare (Voucher No. CC0023), Maerua crassifolia (Voucher No. MC0012).
Plant crude extract and Extraction
A household blender was used to grind the dried plant components into a fine powder in order to prepare aqueous plant extracts. Each plant’s 100 grams of fresh leaves were weighed and ground in a blend mixer with 100 milliliters distilled water. After that homogenates were placed into the glass flasks, mixed thoroughly till all the contents were mixed adequately and then the flasks were stored at 4 °C for 2 days. The plant extracts were first filtered through cotton textile mesh before being utilized on the Whatman No. 1 paper. After that, the plant material homogenates were spun down and supernatants were then stored at 4 °C until needed (Figure 1).
Preparation of AgNO3 Solution
4.245 grams of silver nitrate (Sigma-Aldrich, Switzerland) were dissolved in 250 mL of distilled water in 100 mL of deionized water (DW). After that, a 1 mM solution was made by adding 1 mL of the prepared AgNO3 solution to 1000 mL of DW.20
AgNPs green nanoparticles synthesis
Plant’s extract were added to 0.10 M AgNo3 at a volume ratio of 1:2 at room temperature, and the mixture was stirred for 30 minutes to synthesize AgNPs using various plant materials (SA-AgNPs, CC-AgNPs, SAR-AgNPs, FV-AgNPs, MC-AgNPs and EC-AgNPs). By using the 0.01 M D-sorbitol technique recommended by Devatha et al.21 and Bhardwaj & Singh,22 the stability of the nanoparticles was enhanced. The reduction of Ag1+ is indicated by the color’s disappearance. After filtering, washing with ethanol, and vacuum-drying at 50 °C, the resulting solid was used right away.23-27
Transmission Electron Microscopy (TEM)
The size and morphology of the bio-synthesized silver nanoparticles (Ag NPS) were examined by transmission electron microscopy (TEM). The TEM analysis was performed on a Hitachi® 7700 transmission electron microscope, operated at an accelerating voltage of 100 kV. Additionally, images were recorded using a Jeol® JEM-ARM 200F NEOARM, operating at 200 kV. A drop of the AgNPS suspension was placed on a carbon-coated copper grid and allowed to dry at room temperature before imaging.
Silver nanoparticles: characterization and analysis
A steady color shift was obtained, and any precipitate was removed by centrifuging the solution for 10 mins. at 5000 rpm. After that, supernatant was centrifuged at 12,000 rpm for 30 mins. Any remaining plant extract was eliminated by washing the pellet with deionized DW. Prior to using the final pellet for characterization, it was suspended in DW.
Spectrophotometric analysis
A UV-visible spectrophotometer (Shimadzu) was used to perform spectral scanning (200-800 nm) in order to detect the absorption maxima of the synthesized AgNPs.25
Fourier Transform Infrared Spectrometer (FT-IR)
The AgNPs and plant extract solution was assessed using an FT-IR spectrometer (Shimadzu) with a 500-4000 cm-1 region and an 8 cm-1 resolution. KBr (300 mg) was added to the sample containing the synthesized AgNPs (1 mg) to form a hydraulic pellet, which was subsequently compressed and subjected to FTIR spectroscopic analysis. Peaks were observed for the synthesized particles’ functional groups.28
Evaluation of the antimicrobial activity of the various plant extracts
The antibacterial activity of plant material extracts was investigated. 100% concentrated plant extracts were utilized. Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus and Bacillus subtilis bacteria and Candida albicans fungus were employed in this experiment. In order to inject 50 µl of extract, tiny holes were made in each plate after the bacterial suspension had been spread out on the nutrient agar medium. The impact of 50 µl of each 100% concentrated extract on the used bacterial and fungal strains was examined. The bacterial plates were incubated overnight at 37 °C, whereas the fungal plates were cultured for three days at 28 °C. The plates were later examined and photographed.
Antimicrobial activity assays
The antimicrobial activity of biogenic AgNPs was evaluated using the agar-well diffusion method in compliance with the National Committee for Clinical Laboratory Standards (NCCLS).29 Media containing Muller Hinton agar and Potato Dextrose agar media were transferred into petri dishes and allowed it to solidify completely. The agar wells were created using a cork borer that had a diameter of 6.0 mm.
100 µg of each sample were added to the agar wells and the samples were then incubated for 4 hours to facilitate the diffusion of the test samples. Individual inoculations of Klebsiella pneumoniae (ATCC 13883), Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 8739), Bacillus subtilis (ATCC 6633) and Candida albicans (ATCC 10221) strain cultures were applied using 0.1 ml of each culture. Furthermore, Gentamycin was used as a positive control for antibacterial activity, whereas Fluconazole was used as an antifungal for C. albicans. 14 × 106 colony-forming units per milliliter of the inoculation cultures were subsequently disseminated onto agar plates. The plates were then incubated in an incubator set at 37 °C for a maximum of 24 hours. According to the recommendations established by the Clinical and Laboratory Standards Institute (CLSI), the experiments were conducted in triplicate, and the mean diameter (in mm) of the inhibition zones was used to measure the inhibitory activity.30
Silver nitrate contains silver ions (Ag+), which are reduced by the plant extract to form silver nanoparticles. The unique qualities of silver, including its good conductivity, catalytic activity, and chemical stability, make silver nitrate an effective reducing agent. Herbal extracts reduced the aqueous silver ions in solution, leading to the formation of silver hydrosol. Each plant has a different time frame for color change.31 The visual change in color after 48 hours as can be seen from Figure 2 indicates that the reduction of silver ions (Ag+) to elemental silver (Ag0) has occurred, resulting in formation of nano-silver. The plant extracts’ color change upon exposure to the aqueous silver ions confirmed the creation of silver nanoparticles. The darker coloration is a common indicator of nanoparticle synthesis, as AgNPs have distinctive surface plasmon resonance properties, which cause these visible changes.32 The variation in colors among the samples suggests differences in the rate or extent of nanoparticle formation, likely inclined by the chemical composition of each extract. The third row shows the silver nanoparticle suspensions formed before drying. These are the results after silver ions have been reduced but are still in solution or suspension. Each beaker shows a slightly different color and appearance, possibly indicating differences in nanoparticle size, shape, or concentration. The appearances of yellowish-brown color suggest the formation of silver nanoparticles.33 The bottom row shows the dried silver nanoparticles (AgNPs) obtained from each plant extract. Overall, this sequence illustrates the synthesis of silver nanoparticles using plant-based green synthesis, showing the plant extract, the solution after reduction, and the dried nanoparticles.
Figure 2. Preparation of biogenic AgNPs, and color changes during the bio-reduction of AgNO3 into AgNPs
Statistical analysis
The experiments were designed as completely randomized design. Data were subjected to one way ANOVA analysis. Mean values were compared according to Duncan Multiple Range test at p = 0.05. Data were analyzed using SPSS (20) package.
Spectrophotometric analysis
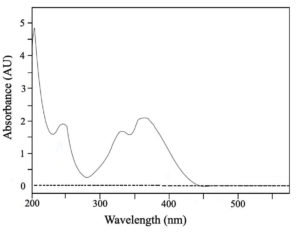
Figure 3 displays the AgNPs’ UV-vis spectral patterns generated from the aqueous extract of Solenostemma argel. The NPs appeared to be polydisperse based on the existence of a wide-ranging absorption band at a wavelength 380 nm. The absorption band of the NPs showed a nearly symmetrical or identical form, as determined by surface plasmon resonance (SPR). According to Samimi et al.,34 these findings indicated that spherical NPs were formed with little to no aggregation. In order to prevent a high absorbance value, the sample was analyzed after being diluted with a fixed volume of deionized water to confirm the bioreduction of Ag+ to Ag0.35 Distilled water served as a blank. For example, larger nanoparticles show light dispersion and have peaks that broaden and migrate towards longer wavelengths, whereas small nanoparticles have peaks about 400 nm and mostly absorb light. It has been noted that the optical characteristics may also alter when particle aggregation causes the conduction electrons close to the particle’s surface to delocalize.36 The expansion of the peak indicated the polydispersed nature of the particles.28
Figure 3. Graph with absorption spectrum from UV-Vis spectrophotometer of the synthesized silver nanoparticles of Solenostemma argel extract
FTIR spectrum of silver nanoparticles
Figure 4 displays the FTIR spectrum analysis of AgNO3 NPs formed by means of Solenostemma argel aqueous extract. Phytocomponents in plant extracts contain functional groups that reduce silver ions to AgNP.37-39 According to the chemical analysis of Solenostemma argel, phenolic compounds and flavonoids are responsible for their antioxidants.40 The main phenolic acid in the methanolic extract was gallic acid (71.70%), followed by syringic acid, p-coumaric acid, caffeic acid, gallic acid, and ferulic acid.41 In this context, the bioformation of silver nanoparticles is caused by other phenols containing hydroxyl groups (-OH) found in Solenostemma argel. There were noticeable absorption peaks in the 800-3750 cm-1 range of the FTIR spectra. The methyl and alcohol groups were identified by fingerprint peaks at 1400 cm-1 and 11700 cm-1, respectively. The bioreduction of silver ions to AgNPs by these alcohol groups contained in plant extract.42,43 Figure 4A displays the FTIR spectra of the Solenostemma argel aqueous extract, while Figure 4B displays the AgNPs’ spectra. In the bands at 3429.67 cm-1, the amine/phenol functional groups’ N-H stretching vibrations are visible. AgNPs have been observed to bind with hydroxyl and carboxyl groups based on the bands seen at 2999.09 and 2892.07 cm-1. AgNPs may have bound to the C=O band, as indicated by the band at 1754.67cm-1. Consequently, the synthesized AgNO3 are stabilized by the observed functional groups through their capping and reducing actions.44
Morphological analysis of AgNPs by TEM
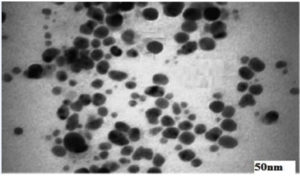
The TEM micrograph (Figure 5) revealed that the synthesized AgNPs were predominantly spherical shapes with a narrow size distribution. The particles appeared well-dispersed, with sizes ranging between 10 nm to 50 nm. This observation confirms the uniformity and nanoscale nature of the synthesized particles.
Antimicrobial activity of the plants extract
Agar-well diffusion was used to assess the antimicrobial activity of the plant extract. Table 1 displays the inhibition zones’ diameter, which was used to measure the antibacterial activity.
Table (1):
Different plant extracts’ antimicrobial efficacy against bacterial isolates is measured through determining the zone of inhibition (ZOI) diameter (mm)
| Plant | Inhibition zone diameter | |||
|---|---|---|---|---|
| Bacillus subtilis | Staphylococcus aureus | Escherichia coli | Klebsiella pneumoniae | |
| Solenostemma argel (SA) | 22.3 ± 0.34d | 18.4 ± 0. 22b | 19.4 ± 0.32c | 17.7 ± 0.09b |
| Citrullus colocynthis (CC) | 21.7 ± 0.21c | 17.4 ± 0.42b | 16.7 ± 0.12b | 16.5 ± 0.19a |
| Syzygium aromaticum (SAR) | 17.6 ± 0.25a | 16.3 ± 0.12a | 15.9 ± 0.17a | 16.1 ± 0.11a |
| Foeniculum vulgare (FA) | 21.9 ± 0.24cd | 21.5 ± 0.19d | 16.8 ± 0.22b | 17.9 ± 0.12b |
| Maerua crassifolia (MC) | 21.6 ± 0.17c | 19.9 ± 0.12c | 16.2 ± 0.21a | 17.1 ± 0.23b |
| Elettaria cardamomum (EC) | 19.8 ± 0.32b | 19.9 ± 0.31c | 16.1 ± 0.11a | 17.9 ± 0.12b |
| Gentamycin (Antibiotics) | 21.8 ± 0.11c | 15.7 ± 0.27a | 16.2 ± 0.13a | 18.5 ± 0.12c |
Means followed by the same letter within each spices are not significantly different according to Duncan Multiple range test at p < 0.05. Each treatment consisted of three replicates and each sample contained three Petri dishes. Values are the means ± standard error
Antimicrobial activity of the biogenic silver nanoparticles
The biogenic AgNPs’ antibacterial activity was evaluated using the agar-well diffusion technique. The antibacterial activity of various plant and herbal extracts combined with silver nanoparticles (AgNPs) was evaluated against several bacterial isolates. The diameter of the inhibitory zones was measured in millimeters (mm) in order to assess the antimicrobial activity as displayed in Figure 6 and Table 2 below.
Table (2):
Antimicrobial activity of various plants extracts AgNPs against bacterial species; the diameter (mm) of inhibition zone have been measured
| Plant | Inhibition zone diameter | ||||
|---|---|---|---|---|---|
| B. subtilis | S. aureus | E. coli | K. pneumoniae | C. albicans | |
| Solenostemma argel | 35 ± 0.2d | 26 ± 0.1c | 25 ± 0.1d | 24 ± 0.2b | 34 ± 0.2c |
| Citrullus colocynthis | 31 ± 0.1b | 25 ± 0.21c | 22 ± 0.2c | 21 ± 0.1a | 28 ± 0.1b |
| Syzygium aromaticum | 27 ± 0.1a | 22 ± 0.22b | 18 ± 0.2a | 20 ± 0.2a | 32 ± 0.2c |
| Foeniculum vulgare | 33 ± 0.1c | 32 ± 0.2e | 20 ± 0.1b | 26 ± 0.1c | 28 ± 0.1b |
| Maerua crassifolia | 32 ± 0.15c | 30 ± 0.11d | 20 ± 0.18b | 24 ± 0.1b | 25 ± 0.1a |
| Elettaria cardamomum | 35 ± 0.12d | 33 ± 0.2e | 22 ± 0.1c | 25 ± 0.2c | 29 ± 0.1b |
| Gentamycin (Antibiotics) | 30 ± 0.1b | 17 ± 0.3a | 20 ± 0.2b | 25 ± 0.1c | 25 ± 0.3a |
Means followed by the same letter within each spices are not significantly different according to Duncan Multiple range test at p < 0.05. Each treatment consisted of three replicates and each sample contained three Petri dishes. Values are the means ± standard error.
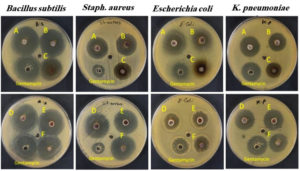
Figure 6. The antibiotic Gentamycin was employed as a control for the antibacterial activity of AgNPs synthesized utilizing the A: Solenostemma argel, B: Citrullus colocynthis, C: Syzygium aromaticum, D: Foeniculum vulgare, E: Maerua crassifolia, F: Elettaria cardamomum.
Tests were conducted to evaluate the antibacterial activity of the biogenic AgNPs synthesized using variable plant extracts to that of Gentamycin. According to the results illustrated in Figure 6, S. argel, C. colocynthis, F. vulgare, M. crassifolia and E. cardamomum have higher antibacterial activity against B. subtilis, with superior activity of S. argel and E. cardamomum. The findings demonstrated that most biogenic AgNPs showed higher activity against S. aureus than Gentamycin. Despite being a commonly used antibiotic, gentamicin showed less efficacy against S. aureus (17 mm), which raises significant questions regarding the possibility of plant-derived extracts as alternatives or adjuncts for traditional antibiotics, especially in light of the growing antibiotic resistance.45 Furthermore, the results demonstrated that, in contrast to the S. aromaticum extract, which demonstrated less antibacterial activity, S. argel, C. colocynthis, and E. cardamomum demonstrated higher antibacterial activity than Gentamycin against E. coli. Meanwhile, F. vulgare and M. crassifolia demonstrated similar antibacterial activity to Gentamycin. F. vulgare presented strong inhibition against S. aureus (32 mm) and only F. vulgare exhibited greater antibacterial action against K. pneumoniae (26 mm) than Gentamycin. Further supporting its use in traditional medicine for treating bacterial infections.46 The reason is that silver exhibits greater microbial activity and effectiveness when proteinaceous material and inorganic binding proteins are present. In vivo, silver binds to inorganic structures utilizing standard molecular biology procedures. Silver nitrate is reduced to silver nanoparticles by photosynthesis in green leaves, which also provide additional H+ ions. It is hypothesized that the organic matrix’s silver binding proteins, which provide amino acid moieties that serve as nucleation sites, provided the molecular basis for the production of these silver crystals.47,48 The overall results indicate the antibacterial activities of all the synthesized biogenic AgNPs.
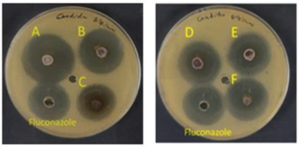
The antifungal efficacy of several plant extracts against Candida albicans was assessed both with and without silver nanoparticles (AgNPs). The following Table 3 provides an overview of the findings. The results (Table 3 and Figure 7) of the biogenic AgNPs’ test against the fungal isolate C. albicans demonstrated that S. argel, S. aromaticum, and E. cardamiomum had higher antifungal activity. F. vulgare and C. colocynthis showed moderate activity. But the least effective antifungal activity was displayed by biogenic AgNPs synthesized using M. crassifolia. The results reveal that adding silver nanoparticles (AgNPs) to all tested plant extracts showed an increase in their antifungal activity. Solenostemma argel (SA) showed the maximum activity, increasing from 21.4 mm to 22.3 mm, indicating a potential synergistic effect of AgNPs on antifungal efficacy. This improvement is consistent with studies by Rai et al. and Majeed et al.49,50 that found that natural compounds’ antifungal effectiveness increased when coupled with AgNPs. The inclusion of AgNPs appeared to marginally boost the activity of all extracts, indicating their potential in overcoming antifungal resistance. AgNPs have been demonstrated to improve antifungal properties in a variety of plant extracts, with similar results reported.51,52 This is most likely due to their capacity to adversely impact fungal cell membranes and walls and obstruct metabolic activities.53 Furthermore, the overall results indicate that integrating AgNPs with herbal antifungal agents is a potential approach for developing more effective treatments against fungal infections, even though the differences were statistically significant for some extracts. This study backs up the prospective use of nanotechnology in therapeutics, which is becoming more popular when combined with traditional medicine.54
Table (3):
Antifungal activity without and with AgNPs. against C. albicans
| Plant | Inhibition zone diameter | |
|---|---|---|
| Antifungal activity C. albicans | Antifungal activity AgNPs. against C. albicans | |
| Solenostemma argel (SA) | 21.4 ± 0.22d* | 22.3 ± 0.34c |
| Citrullus colocynthis (CC) | 20.4 ± 0.42c | 21.7 ± 0.21b |
| Syzygium aromaticum (SAR) | 18.7 ± 0.31a | 19.1 ± 0.32a |
| Foeniculum vulgare (FV) | 18.9 ± 0.19ab | 19.5 ± 0.24a |
| Maerua crassifolia (MC) | 19.1 ± 0.12ab | 19.3 ± 0.17a |
| Elettaria cardamomum (EC) | 19.3 ± 0.12ab | 19.6 ± 0.25a |
| Fluconazole (Antifungal) | 18.5 ± 0.31a | 19.0 ± 0.32a |
Means followed by the same letter within each spices are not significantly different according to Duncan Multiple range test at p < 0.05. Each treatment consisted of three replicates and each sample contained three Petri dishes. Values are the means ± standard error.
Figure 7. Antifungal activity of AgNPs against C. Albicans utilizing the A: Solenostemma argel, B: Citrullus colocynthis, C: Syzygium aromaticum, D: Foeniculum vulgare, E: Maerua crassifolia, F: Elettaria cardamomum; Fluconazole (Antifungal)
Phytochemicals’ natural reducing and capping properties have drawn much interest in the biosynthesis of silver nanoparticles (AgNPs) from plant extracts and their antibacterial applications. The preferential role and mechanisms of functional phytochemicals from various plants in the synthesis of AgNPs, and their catalytic and antibacterial capabilities are still mostly unexplored.55 Phytochemicals’ varied effects on the production of AgNPs vary greatly depending on the plant species. More research should be done to quantitatively analyze the changes in the specific functional phytochemicals depending on various plant tissues or species to elucidate the preferential role (e.g., reduction, immobilization, dispersion, etc.) of phytochemicals for AgNPs biosynthesis.56,57 Furthermore, the large-scale production and application of biosynthetic AgNPs with the consideration of improving environmental and economic benefits could be facilitated by optimizing the processes of plant extract preparation and AgNPs synthesis by analyzing the influencing factors underlying the predominant phytochemicals.
Solenostemma argel, Syzygium aromaticum, Foeniculum vulgare, and Elettaria cardamomum are beneficial medicinal plants. They can be effectively used for the biogenic synthesis of AgNPs, which can be employed as antifungal and antibacterial drugs. AgNPs can dramatically boost the antibacterial effectiveness of widely recognized antibiotic drugs when they interact synergistically. This presents a novel and promising option in the fight against a variety of highly contagious, recently developed bacteria that are multidrug resistant. A deeper comprehension of each phytochemical, their concentrations, and their interactions will open the door to the synthesis of biogenic NPs with specific shapes. Further research is required to test their ability to overcome growth and to use them to overcome seed dormancy and stimulating seedling growth of crop plants.
ACKNOWLEDGMENTS
The author thanks the Department of Biology, Taibah University, for providing the research facilities. The author is highly thankful to Prof. Rashad Kebeish (Faculty of Science, Taibah University) for his assistance, insightful discussion, and manuscript revision.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Asif M, Yasmin R, Asif R, Ambreen A, Mustafa M, Umbreen S. Green synthesis of silver nanoparticles (AgNPs), structural characterization, and their antibacterial potential. Dose-Response. 2022;20(1):15593258221088709.
Crossref - Gong P, Li H, He X, et al. Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. Nanotechnology. 2009;20(2):285604.
Crossref - Verma A, Mehata MS. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J Radiat Res Appl Sci. 2016;9(1):109-115.
Crossref - Velusamy P, Das J, Pachaiappan R, Vaseeharan B, Pandian K. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Ind Crops Prod. 2015;66:103-109.
Crossref - Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17-28.
Crossref - Ahmed S, Ikram S. Biosynthesis of gold nanoparticles: a green approach. J Photochem Photobiol B. 2016;161:141-153.
Crossref - Remya VR, Abitha VK, Rajput PS, Rane AV, Dutta A. Silver nanoparticles green synthesis: a mini review. Chem Int. 2017;3(2):165-171.
- Rafique M, Sadaf I, Rafique MS, Tahir MB. A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol. 2017;45(7):1272-1291.
Crossref - Sharifi-Rad M, Pohl P. Synthesis of Biogenic Silver Nanoparticles (AgCl-NPs) Using a Pulicaria vulgaris Gaertn. Aerial Part Extract and Their Application as Antibacterial, Antifungal and Antioxidant Agents, Nanomaterials. 2020;10(4):638.
Crossref - Vanlalveni C, Lallianrawna S, Biswas A, Selvaraj M, Changmai B, Rokhum SL. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021;11(5):2804-2837.
Crossref - Winslow LC, Kroll DJ. Herbs as medicines. Arch Intern Med. 1998;158(20):2192-2199.
Crossref - Sharifi-Rad M, Elshafie HS, Pohl P. Green synthesis of silver nanoparticles (AgNPs) by Lallemantia royleana leaf Extract: Their Bio-Pharmaceutical and catalytic properties. J Photochem Photobiol A Chem. 2024;448:115318.
Crossref - Sharifi-Rad M, Pohl P, Epifano F, Alvarez-Suarez JM. Green Synthesis of Silver Nanoparticles Using Astragalus tribuloides Delile. Root Extract: Characterization, Antioxidant, Antibacterial, and Anti-Inflammatory Activities. Nanomaterials. 2020;10(12):2383.
Crossref - Elsanhoty RM, Soliman MS, Khidr YA, et al. Pharmacological activities and characterization of phenolic and flavonoid compounds in Solenostemma argel extract. Molecules. 2022;27(23):8118.
Crossref - Batiha GES, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10(2):202.
Crossref - Pavithra M, Sridhar KR, Keshavachandra K. Diverse Medicinal Attributes of Indigenous Flora of Southwest India. Biodiversity, Conservation and Sustainability in Asia: Springer, Cham. 2022.
Crossref - Rather MA, Dar BA, Sofi SN, Bhat BA, Qurishi MA. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab J Chem. 2016;9(2):S1574-S1583.
Crossref - Akuodor GC, Essien AD, Akpan JL, et al. Analgesic, anti-inflammatory and antipyretic activities of methanolic leaf extract of Maerua crassifolia. J Coast Life Med. 2016;4(3):225-230.
Crossref - Ashokkumar K, Murugan M, Dhanya MK, Warkentin TD. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.)] Maton-A critical review. J Ethnopharmacol. 2020;246:112244.
Crossref - Alnuaimi MT, Hamdan NT, Abdalraheem E, Aljanabi ZZ. Biodegradation of malathion pesticide by silver bio-nanoparticles of Bacillus licheniformis extracts. Research on Crops. 2019;20(spl):79-84.
Crossref - Devatha CP, Thalla AK, Katte SY. Green synthesis of iron nanoparticles using different leaf extracts for treatment of domestic waste water. J Clean Prod. 2016;139:1425-1435.
Crossref - Bhardwaj A, Ritika, Singh AK. Murraya Koenigii plant extract mediated green synthesis of metallic nanoparticles and their applications: A Review. Plant Nano Biology. 2024;8:100076.
Crossref - Kołodziejczak-Radzimska A, Jesionowski T. Zinc oxide-from synthesis to application: a review. Materials, 2014;7(4):2833-2881.
Crossref - Wang T, Jin X, Chen Z, Megharaj M, Naidu R. Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ. 2014;466:210-213.
Crossref - Majumdar S, Trujillo-Reyes J, Hernandez-Viezcas JA, White JC, Peralta-Videa JR, Gardea-Torresdeyc JL. Cerium biomagnification in a terrestrial food chain: influence of particle size and growth stage. Environ Sci Technol. 2016;50(13):6782-6792.
Crossref - Prieto-Méndez J, Aquino-Torres E, Pérez-Ríos SR, Trejo-González N, Prieto-García, F. Seed germination of beans (Phaseolus vulgaris) with nano-particles of iron. Bulgarian Journal of Agricultural Science, 2019; 25(2):283-287.
- Pariona N, Martinez AI, Hernandez-Flores H, Clark-Tapia R. Effect of magnetite nanoparticles on the germination and early growth of Quercus macdougallii. Sci Total Environ. 2017;575:869-875.
Crossref - Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Applied Nanoscience. 2016;6:399-408.
Crossref - Cockerill FR, Wikler MA, Bush, Dudley MN, Eliopoulos GM, Hardy DJ. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing: twenty-second informational supplement. 2011.
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition. CLSI document M07-A9. Clinical and Laboratory Standards Institute, PA: NCCLS, USA, 2012.
- Savithramma N, Rao ML, Rukmini K, Devi PS. Antimicrobial activity of silver nanoparticles synthesized by using medicinal plants. 2011:1394-1402.
- Hammed V, Bankole AA, Akinrotimi O, Ayanleye O. Silver nanoparticles (AGNPs): A review on properties and behavior of silver at the nanoscale level. International Journal of Science and Research Archive. 2024;12(2):1267-1272.
- Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275(2):496-502.
Crossref - Samimi S, Maghsoudnia N, Eftekhari RB, Dorkoosh F. Lipid-based nanoparticles for drug delivery systems. Characterization and Biology of Nanomaterials for Drug Delivery. 2019;47-76.
Crossref - Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 2008;65(1):150-153.
Crossref - Yu SJ, Yin YG, Liu JF. Silver nanoparticles in the environment. Environmental Science: Processes & Impacts. 2013;15(1):78-92.
Crossref - Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep. 2017;7(1):15867.
Crossref - Shumi G, Demissie TB, Eswaramoorthy R, Bogale RF, Kenasa G, Desalegn T. Biosynthesis of silver nanoparticles functionalized with histidine and phenylalanine amino acids for potential antioxidant and antibacterial activities. ACS Omega. 2023;8(27):24371-24386.
Crossref - Ozcelik B, Kara A. Evaluation of biological activities of silver nanoparticles (AgNPs) synthesized by green nanotechnology from birch (Betula spp.) branches extract. Turk J Anal Chem. 2023;5(2):151-161.
Crossref - Abdel-Sattar E, El-Shiekh RA. A Comprehensive Review on Solenostemma argel (Del.) Hayne, An Egyptian Medicinal Plant. Bulletin of Faculty of Pharmacy Cairo University. 2024;62(1):3.
- Osman HM, Shayoub MH, Babiker EM, Mounzer ME. The effect of ethanolic leaves extract of Solenostemma argel on blood electrolytes and biochemical constituents of albino rats. Sudan J Sci. 2014;(SJS):6(1):48-52.
- Raja K, Saravanakumar A, Vijayakumar R. Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;97:490-494.
Crossref - Sre PRR, Reka M, Poovazhagi R, Kumar MA, Murugesan K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;135:1137-1144.
Crossref - Bakri MKB, Rahman MR, Khui PLN, Jayamani E, Khan A. Use of sustainable polymers to make green composites. Advances in Sustainable Polymer Composites. 2021:109-129.
Crossref - Vashist H, Jindal A. Antimicrobial activities of medicinal plants-Review. Int J Res Pharm Biomed Sci. 2012;3(1):222-230.
- Gani HM, Hoq MO, Tamanna T. Pharmacological and phytochemical analysis of Foeniculum vulgare Mill: A review. International Journal of Unani and Integrative Medicine. 2019;3(2):13-8.
- Arya V. Living Systems: eco-friendly nanofactories. Dig J Nanomater Biostruct (DJNB). 2010:5(1):9-21.
- Prabhu N, Raj DT, Yamuna GK, Ayisha SS, Puspha J, Innocent D. Synthesis of silver phyto nanoparticles and their antibacterial efficacy. Dig J Nanomater Biostruct (DJNB). 2010;5(1):185-189.
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76-83.
Crossref - Majeed M, Hakeem KR, Ul Rehman R. Synergistic effect of plant extract coupled silver nanoparticles in various therapeutic applications-present insights and bottlenecks. Chemosphere. 2022;288(Pt 2):132527.
Crossref - Machado AL, Wady A, Zamperini CA, Zucolotto V, Vergani CE. Antifungal activity of silver nanoparticles against candida albicans. J Dent Res. 2011;90.
- Begum T, Follett PA, Mahmud J, et al. Silver nanoparticles-essential oils combined treatments to enhance the antibacterial and antifungal properties against foodborne pathogens and spoilage microorganisms. Microb Pathog. 2022;164:105411.
Crossref - Wen H, Shi H, Jiang N, Qiu J, Lin F, Kou Y. Antifungal mechanisms of silver nanoparticles on mycotoxin producing rice false smut fungus. iscience. 2023;26(1).
Crossref - Hussain MA, Ahmed D, Anwar A, et al. Combination therapy of clinically approved antifungal drugs is enhanced by conjugation with silver nanoparticles. Int Microbiol. 2019;22(2):239-246.
Crossref - Liu L, Yu C, Ahmad S, Ri C, Tang J. Preferential role of distinct phytochemicals in biosynthesis and antibacterial activity of silver nanoparticles. J Environ Manage. 2023;344:118546.
Crossref - Afreen A, Ahmed R, Mehboob S, et al. Phytochemical-assisted biosynthesis of silver nanoparticles from Ajuga bracteosa for biomedical applications. Mater Res Express. 2020;7(7):075404.
Crossref - Mallikarjuna K, Sushma NJ, Narasimha G, Manoj L, Raju BDP. Phytochemical fabrication and characterization of silver nanoparticles by using Pepper leaf broth. Arab J Chem. 2014;7(6):1099-1103.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.