ISSN: 0973-7510
E-ISSN: 2581-690X
Candiduria is a condition in which Candida species are found in the urine. Candiduria is commonly found in hospitalized patients, especially those with diabetes mellitus and those receiving medical care, especially using urine catheters. Although Candida is a normal part of the body’s flora, certain species can opportunistically cause urinary tract infections (UTIs). This study aimed to analyze the risk factors for Candida UTIs in patients hospitalized at Dr. Soetomo General Academic Hospital in Surabaya, Indonesia. This was an analytical, observational, and cross-sectional study included 52 patients hospitalized with Candida culture results, who underwent urine re-collection for confirmatory culture examination. The results showed a significant correlation between candiduria (p = 0.000); yeast, pseudohyphae, or hyphae found on Gram staining (p = 0.024); and previous antibiotic use (p = 0.027) with Candida UTIs. This suggests that candiduria, presence of yeast, pseudohyphae, or hyphae found on Gram staining, and previous antibiotic use are risk factors for Candida UTIs. In conclusion, these findings underscore the need for careful monitoring of antibiotic use and candiduria in hospitalized patients to prevent Candida UTIs.
Candiduria, Candida, Urinary Tract Infection, Diabetes, Medical Care
Candiduria, a condition in which Candida is found in the urine, commonly affects hospitalized patients.1,2 Candida is part of the normal flora of the human body and can act as an opportunistic pathogen causing urinary tract infections (UTIs); notably, the incidence of Candida UTIs has increased in recent years.1,3 Patients admitted to the intensive care unit with candiduria have a poor prognosis, with a mortality rate three times higher than that of patients without candiduria.1 The mortality rate of patients hospitalized in the intensive care unit was reported to be 48.8% in those with candiduria compared to 36.6% in those without candiduria (p < 0.001).4
Previous research at Dr. Soetomo General Academic Hospital in Surabaya showed that candiduria prevalence was 0.55% in hospitalized patients, with several influencing risk factors, including diabetes mellitus, antibiotic use, and intraurethral catheter use.5 In another study, candiduria was detected in 0.11-0.75% of outpatients, and was significantly increased in hospitalized patients between 3.49-10.63%. Other reports have shown that up to 90% of patients with Candida UTIs are hospitalized and use a urinary catheter.6 Further, a previous prospective study in patients with candiduria found that 2-4% of patients showed symptoms of urinary tract infections.7
Candida can cause UTIs either descending (hematogenous) or ascending from the perineum.8 Patients with Candida infection in the upper urinary tract are at risk for invasive candidiasis, which can increase mortality and prolong hospitalization.1,9 The increase in UTIs caused by Candida as an opportunistic infection in hospitalized patients can lead to significant health problems in the community, especially in immunocompromised patients admitted to the intensive care unit (ICU).10 The mortality rates of patients with candidemia admitted to the ICU were 61.8% and 31.3% of these patients had candiduria.2 A multicenter study of 1,765 patients in the ICU indicated that the hospitalized mortality rate was 48.8% in patients with candiduria compared to 36.6% in patients without candiduria (p < 0.001).4
Most patients with candiduria are asymptomatic, as it can indicate either contamination or colonization. Prolonged use of a urinary catheter can cause asymptomatic candiduria.8 Candiduria can also show symptoms of invasive infections, such as pyelonephritis and candidemia.4 Candiduria in symptomatic or asymptomatic patients should not be ignored or treated hastily, but requires evaluation by repeated urinalysis and urine culture.8,11 In this study, we discuss the significance of Candida as a causative agent of UTIs in hospitalized patients and the risk factors associated with Candida UTIs.
This study is an analytical observational study using cross-sectional analysis to analyze the risk factors of Candida UTIs in patients hospitalized at Dr. Soetomo General Academic Hospital in Surabaya, Indonesia. The study sample included inpatients who urine specimens were subjected to fungal culture at the Microbiology Laboratory of Dr. Soetomo General Academic Hospital, Surabaya, Indonesia, and yielded Candida-positive results; in total, 52 samples were collected from November 2023 to March 2024.
Inclusion criteria
The inclusion criteria were as follows: 1). Hospitalized patients older than 18 years with or without a urinary catheter and a Candida-positive urine culture result and had a repeat urine specimen collected; 2). Hospitalized patients aged more than 18 years who used urinary catheters with Candida-positive urine culture results and underwent catheter replacement after the fifth day of urinary catheter use; and 3). The urine culture results showed only Candida growth and no bacterial growth.
Exclusion criteria
The exclusion criteria were as follows: 1). A hospitalized patient older than 18 years with Candida-positive urine culture results did not undergo repeat urine specimen collection and urinary catheter replacement after the fifth day; and 2). Urine culture showed growth of both Candida and bacteria.
Urine specimen collection
In this study, urine specimen recollection was performed. Re-collection of urine specimens was carried out for patients who met the inclusion criteria. Before re-collection of urine specimen, informed consent was obtained from patients as research samples. In patients who used urinary catheters for less than 5 days and for patients who did not use urinary catheters, repeat specimen without changing the urinary catheter, but if the patient used a urinary catheter for more than 5 days, the urinary catheter was replaced with the permission from the clinician; after replacing the urinary catheter, the urine specimens was directly collected. Re-collected urine specimens (5-10 ml) were placed in a sterile container, labelled, and directly transported in a box to the microbiology laboratory.
Microscopic examination and fungal culture
Urine specimens were subjected to microscopic examination using Gram staining and fungal cultures. Urine specimens to be Gram-stained were not centrifuged; the urine sample was dispensed on a clean slide (up to 10 ml) using a sterile loop, and fixed. Gram staining was performed and the results were examined under a light microscope with a 10x objective lens and 10x ocular lens to evaluate polymorphonuclear (PMN) cells, epithelial cells, and the distribution of Candida. Then, a 100x objective lens and 10x ocular lens were used for oil immersion to observe the yeast, pseudohyphae, and hyphae.
The urine specimens were also examined for fungal cultures on Sabouraud Dextrose Agar (SDA) media. Urine specimens (up to 1 ml) were taken in a loop and semi-quantitatively streaked to count the number of colonies that grew. SDA media that were streaked, were incubated in an O2 incubator at 35-37 °C for 24-48 hours. SDA media with Candida growth were used for counting the number of colonies, identification, and antifungal susceptibility testing using the Vitex 2 system.
Statistical analysis
The results of the study are displayed as a table ( Table 1) containing percentage data, which were analyzed using a correlation test in the ‘EZR’ (Easy R) application. Statistical significance was set at p < 0.05 significant.
The total sample in this study comprised 52 inpatients, with 34 (65.38%) aged <60 years and 18 (34.62%) aged >60 years. The proportion of female patients was higher than that of male patients, with 42 (80.77%) female and 10 (19.23%) male patients. Of the patients with candiduria included in this study, 31 (59.62%) and 21 (40.38%) patients were treated in the high and low care rooms, respectively (Table 1).
Table (1):
Characteristics of the sample
| Variable | Total (n = 52) | ||
|---|---|---|---|
| n | % | ||
| Age | <60 years | 34 | 65.38% |
| >60 years | 18 | 34.62% | |
| Mean + SD | 53.25 + 13.32 | ||
| Gender | Woman | 42 | 80.77% |
| Man | 10 | 19.23% | |
| Treatment room | High care | 31 | 59.62% |
| Low care | 21 | 40.38% | |
| Risk factors | Diabetes Mellitus | 33 | 63.46% |
| Previous use of antibiotics | 51 | 98.08% | |
| Use of urinary catheters | 48 | 92.31% | |
| Gram Stain | Yeast/pseudohyphae/hyphae | 46 | 88.46% |
| No Yeast/pseudohyphae/hyphae | 6 | 11.54% | |
| Candida colony count | >105 CFU/ml | 41 | 7885% |
| <105 CFU/ml | 8 | 15.38% | |
| Candida culture results | Yes | 49 | 94.23% |
| No | 3 | 5.77% | |
| Candiduria | Symtomatic candiduria | 43 | 82.69% |
| Asymptomatic candiduria | 6 | 11.54% | |
Most patients included in this study, i.e., 48 (92.31%) patients used urinary catheters whereas 4 (7.69%) patients did not use urinary catheters. The average number of days of urinary catheter use in the study sample was 2.17 + 1.23 days. The risk factors found in this study were diabetes mellitus in 33 (63.46%) patients and previous antibiotic use in 51 (98.08%) patients.
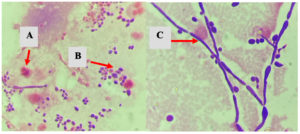
Gram staining of the urine samples in this study revealed yeast, pseudohyphae, or hyphae (Figure) in 46 (88.46%) samples. The Candida colony count in this study was ≥105 CFU/ml for 41 (78.85%) patients and less than 105 CFU/ml for 8 (15.38%) patients.
Figure. Microscopic examination of urine specimens with Gram stain (A: PMN cells; B: Yeast; C: Hypae)
In this study, re-collection of urine samples was performed, and 49 (94.23%) and 3 (5.77%) patients showed positive and negative Candida culture results, respectively. Further, 43 (82.69%) patients had positive Candida culture results and showed clinical symptoms or symptomatic candiduria, whereas 6 (11.54%) patients had positive Candida cultures but no clinical symptoms or asymptomatic candiduria.
Identification of Candida from the urine specimens in this study (Table 2) revealed Candida albicans in 20 (38.46%), Candida tropicalis in 19 (36.54%), Candida glabrata in 5 (9.62%), Candida parapsilosis in 4 (7.69%), and Candida krusei in 1 (1.92%) samples.
Table (2):
Antifungal susceptibility test of the Candida isolates
| Antifungal drugs | Candida isolate | |||||
|---|---|---|---|---|---|---|
| C. albicans No (%) | C. tropicalis No (%) | C. glabrata No (%) | C. parapsilosis No (%) | C. krusei No (%) | ||
| Fluconazole | S | 20 (100%) | 19 (100%) | – | 4 (100%) | – |
| R | 0 (0) | 0 (0) | – | 0 (0) | – | |
| Flucytosine | S | 20 (100%) | 19 (100%) | 5 (100%) | 4 (100%) | 1 (100%) |
| R | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Amphotericin B | S | 20 (100%) | 19 (100%) | 5 (100%) | 4 (100%) | 1 (100%) |
| R | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Voriconazole | S | 20 (100%) | 19 (100%) | 5 (100%) | 4 (100%) | 1 (100%) |
| R | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Caspofungin | S | 20 (100%) | 19 (100%) | 3 (60%) | 4 (100%) | 1 (100%) |
| I | 0 (0) | 0 (0) | 2 (40%) | 0 (0) | 0 (0) | |
| Micafungin | S | 20 (100%) | 19 (100%) | 5 (100%) | 4 (100%) | 1 (100%) |
| R | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
S: Sensitive, I: Intermediate, R: Resistant
The correlation analyses of several variables are presented in Table 3. Several variables showed a significant correlation with Candida UTIs, including candiduria (p = 0.000), Gram staining results (p = 0.024), and previous antibiotic use (p = 0.027). Age, sex, number of Candida colonies, diabetes mellitus, and use of urinary catheters were not significantly correlated with Candida UTIs (p > 0.05).
Table (3):
Correlation test of several variables with Candida UTIs
| Variable | Candida UTIs | P-value | ||
|---|---|---|---|---|
| Yes | No | |||
| Candiduria | Yes | 43 (82.69%) | 6 (11.53%) | 0.000* |
| No | 0 (0%) | 3 (5.76%) | ||
| Gram Stain | Yeast/Pseudohypae/Hypae | 40 (76.92%) | 6 (11.53%) | 0024* |
| No yeast found | 3 (5.76%) | 3 (5.76%) | ||
| Candida colony count | >105 CFU/ml | 35 (71.42%) | 6 (12.24%) | 0.248 |
| <105 CFU/ml | 8 (16.32%) | 0 (0) | ||
| Age | Mean + SD | 53.25 + 13.32 | 0.886 | |
| Gender | Female | 33 (63.46%) | 9 (17.30%) | 0.107 |
| Male | 10 (19.23%) | 0 (0%) | ||
| Diabetes mellitus | Yes | 27 (51.92%) | 6 (11.53%) | 0.826 |
| No | 16 (30.76%) | 3 (5.76%) | ||
| Previous antibiotic use | Yes | 43 (82.69%) | 8 (15.38%) | 0.027* |
| No | 0 (0) | 1 (1.92%) | ||
| Use of urinary catheters | Yes | 40 (76.92%) | 8 (15.38%) | 0.672 |
| No | 3 (5.76%) | 1 (1.92%) | ||
*The mean difference is significant at the p < 0.05 level
Candiduria is a condition characterized by the presence of Candida in the urine and is most common in hospitalized patients, with a prevalence of approximately 10-15%.2 Candida UTIs in hospitals are most commonly found in patients using urinary catheters and in those treated in intensive care units.2 Some risk factors for candiduria include age, female, diabetes mellitus, prolonged hospitalization, treatment in the intensive care unit, immunosuppressive therapy, use of broad-spectrum antibiotics, history of previous surgery (urological and non-urological), radiation therapy, genitourinary tuberculosis, neutropenia, and use of urinary tract instrumentation.12
In this study, most patients were younger than 60 years of age, and the average age was 53 years, with a higher proportion of women than of men (80.77% vs. 19.23%, respectively). However, the results of the correlation analysis of age and sex showed no statistically significant correlation between age (p = 0.886) and sex (p = 0.107) with the incidence of Candida UTIs. This may indicate that age and sex are risk factors for candiduria but not for Candida UTIs. In other studies, Candida was found to be more common at ages of less than 15 and >46 years. This is because the host defense is lower at extreme ages.13 Further, the presence of a shorter urethra and colonization of Candida species in the vulvo-vestibular region, which occurs mostly in women, increase the risk of ascending candiduria.14
Another study related to urinary catheter use and biofilm formation, conducted in Indonesia in 2017-2018, showed that the two risk factors influencing biofilm formation were female sex and duration of urinary catheter use (p < 0.05). Further, urinary catheter use for five or more days resulted in a higher biofilm mass than that with a shorter duration of catheter use.15 In this study, a second urine specimen could be immediately collected for patients who used a urinary catheter for up to 5 days, but a urinary catheter was used for more than the 5 days, the catheter was replaced before repeat collection of the urine specimen. This measure was applied to reduce the risk of biofilm formation. The prolonged use of a urinary catheter in the bladder can cause yeast and pseudohyphae to adhere to the urinary catheter, which can cause persistent candiduria and false-positive results; therefore, the replacement of a urinary catheter can provide resolution in some cases of candiduria.16 Urinary tract infections caused by Candida occur most often in the ascending regions related to urinary catheter use. A urinary catheter can be a portal for the entry of microorganisms into the urinary tract and can also be a place for microorganisms to colonize if used for a long time.12
Candiduria can be detected in outpatients at 0.11-0.75% and significantly higher in inpatients at 3.49-10.63%.6 In this study, candiduria was found in 59.62% of patients admitted to the intensive care unit (31 of 52 patients), and Candida UTIs accounted for 87% (27 of 31 patients). This shows that more than half of the patients with candiduria who had clinical symptoms of UTIs were admitted to the intensive care unit, which could increase the mortality rate of these patients if antifungal therapy was not administered. Candiduria is an indicator of invasive candidiasis, which can be serious, particularly in immunocompromised patients.17 In a prospective multicenter study, the incidence of candiduria was 22% in critically ill patients treated in the intensive care unit for >7 days. This may occur because patients with candiduria experience high antimicrobial exposure during treatment in the intensive care unit.14
Diabetes mellitus is a metabolic disorder that predisposes individuals to fungal infections, including Candida species, owing to its immunosuppressive effect in patients.18 In addition, diabetes mellitus is a predisposing factor for candiduria and Candida UTIs.19 In this study, 63.46% (33 of 52 patients) of patients with candiduria had diabetes mellitus while 84.37% (27 of 32 patients) of the patients Candida UTIs had diabetes mellitus. In this study, statistical analysis showed no correlation between diabetes mellitus and the incidence of Candida UTIs (p = 0.826); this could be attributed to the lack of data related to the duration and status of diabetes mellitus. Diabetes mellitus is a risk factor for asymptomatic candiduria; however, the occurrence of infection is also influenced by the duration of diabetes mellitus and glycemic control.
The presence of glucose in blood and the secretion of degrading enzymes can generally cause immunosuppression in patients, which can cause an imbalance between the host and yeast resulting in Candida transitioning from commensal to pathogenic and causing an infection.18 The presence of urogenital infections caused by Candida species is expected to become more widespread in patients with diabetes, especially in those with poor glycemic control. In patients with diabetes, disease duration and long-term poor glucose regulation alter the renal microvasculature and frequent polyuria/glycosuria, which may lead to more frequent urinary tract infections.17
The prevalence of candiduria in patients with a history of antibiotic use at Dr. Soetomo General Academic Hospital in Surabaya was 81.4%.5 In this study, patients with candiduria who previously used antibiotics constituted 98% (51 of 52 patients) of which 84.3% (43 of 51 patients) had UTIs due to Candida. The results of the correlation test showed a correlation between the previous use of antibiotics and incidence of UTIs caused by Candida (p = 0.027*). In a case-control study, the number of patients with candiduria increased 12-fold after the use of a urinary catheter, 6-fold after the use of broad-spectrum antibiotics, 6-fold in the presence of urinary tract abnormalities, and 2-fold in the presence of diabetes mellitus.20
Previous antibiotic use is a risk factor for UTIs due to Candida, and contributes to Candida colonization by suppressing normal bacterial flora, especially in the gastrointestinal tract, lower genital tract, and perineum. This can cause Candida overgrowth, allowing it to enter the urethra and colonize or infect the bladder.14 Further, antibiotics can interfere with phagocyte function and antibody formation, leading to impaired host defense mechanisms against Candida infection.21
The presence of candiduria triggers physicians to consider whether the patient has a urinary tract infection caused by Candida species or just bladder colonization or contamination22 A repeat urine specimen is used to confirm whether candiduria is caused by contamination, colonization, or infection. If the patient has a urinary catheter, urine specimen collection is repeated after urinary catheter replacement.8 Thus, a second urine specimen is obtained to verify the patient’s condition and to determine whether antifungal therapy or elimination of predisposing factors such as urinary catheter replacement is needed.
UTIs are divided into two parts (lower and upper tract infections), which can be caused by fungi or bacteria and can be symptomatic or asymptomatic.23 Most symptomatic Candida UTIs develop as ascending infections that start in the lower urinary tract with the same pathogenesis as that of bacterial UTIs. Patients with ascending infections may present with cystitis or pyelonephritis.22
Symptomatic candiduria has been observed in patients with cystitis, epididymoorchitis, prostatitis, pyelonephritis, and renal candidiasis.23 In contrast, in patients with asymptomatic candiduria, the presence of Candida in the urine may represent colonization and elimination of risk factors such as urinary catheter use is the recommended therapy.22
In a previous multicenter study, only 4% of patients with candiduria were asymptomatic, and in a smaller study, 14% of symptomatic patients had UTIs.24 In another prospective study, 2-4% of patients with candiduria had one of the symptoms of urinary tract infection.7 In this study, the prevalence of asymptomatic candiduria was 11.54% (6 of 52 patients), and that of symptomatic candiduria was 82.69% (43 of 52 patients); the statistical analysis results showed a significant correlation between candiduria and the incidence of UTIs because of Candida (p = 0.000*). The high prevalence of UTIs due to Candida in this study could be attributed to the inclusion of patients who were suspected by clinical doctors of having UTIs. Most of the study sample included patients who were admitted to the intensive care unit, used urinary catheters, had diabetes mellitus, and had previous antibiotic use, which are risk factors for Candida becoming a pathogen and causing an infection. This is supported by the results of previous studies, which reported an increase in the incidence of Candida infection in immunocompromised patients, especially those with diabetes mellitus.19 Further, the use of broad-spectrum antibiotics can decrease the number and function of commensal bacteria, which can increase the colonization of pathogenic microorganisms.18
In this study, the number of Candida colonies was determined to be ≥103 to ≥105 CFU/ml in patients with and without urinary catheter use; the statistical results showed no correlation between the number of colonies being >105 or <105 CFU/ml and the incidence of UTIs caused by Candida (p = 0.248). In this study, 16.3% (8 of 49 patients) of patients with Candida colony counts <105 CFU/ml were found to have UTIs due to Candida. Further, 14.6% (6 of 41 patients) had Candida colony counts >105 CFU/ml, but did not have Candida UTIs. This could be because these patients used a urinary catheter for more than two days; notably, using a urinary catheter is a risk factor for Candida colonization, which results in high colony counts, but lack of clinical symptoms in patients. This shows that the number of Candida colonies cannot be a reference for determining the presence of a UTIs due to Candida, but need a clinical data of the patients.
Urinary catheter can cause mechanical damage to the bladder and lead to the release and accumulation of fibrinogen in the bladder and the urinary catheter. A fibrinogen-coated urine catheter is a site for the attachment Candida albicans, resulting in a strong biofilm consisting of yeast, pseudohyphae, and hypha. Therefore, the attachment of fibrinogen and Candida albicans can cause bladder colonization and UTIs associated with urinary catheters.25
Another study showed that in patients without urinary catheters, Candida colony counts <104 CFU/ml in urine were correlated with the presence of renal infection. However, in patients with urinary catheters, there was no correlation between lower Candida colony counts and renal involvement, whereas colony counts ≥105 CFU/ml showed no evidence of UTIs caused by Candida.26 Another study also showed that the use of urinary catheters significantly led to bladder colonization by all Candida strains up to ≥105 CFU/ml, whereas with no urinary catheters, colonization by all Candida strains was significantly lower at ≤103 CFU/ml.25
Direct microscopic examination of urine specimens can be done using Gram staining. If yeast, pseudohyphae, or hyphae are found, the UTI may be caused by Candida.27 Urine specimens in this study were Gram-stained and observed directly microscopically. The results revealed yeast, pseudohyphae, or hyphae in 88.46% (46 of 52 patients) of candiduria cases, of which 86.9% (40 of 46 patients) were UTIs caused by Candida. The results of the correlation analysis showed a correlation between the discovery of yeast, pseudohyphae, or hyphae from Gram staining and the incidence of UTIs due to Candida (p = 0.024*). Thus, presence of yeast, pseudohyphae, or hyphae in Gram staining along with clinical symptoms can be an early sign of UTIs due to Candida.
A previous study at Dr. Soetomo Hospital identified nine different Candida species in patients with candiduria: Candida albicans (42.7%; n = 79), Candida tropicalis (31.4%; n = 58), Candida glabrata (10.8%; n = 20), Candida parapsilosis (10.3%; n = 19), Candida rugosa (2.2%; n = 4), Candida krusei (1.1%; n = 2), Candida lipolytica (0.5%; n = 1), Candida guilliermondii (0.5%; n = 1), and Candida lusitaniae (0.5%; n = 1).5 Candida albicans is the most commonly isolated yeast from urine, accounting for 50-70% of the isolates in various studies. Candida glabrata and Candida tropicalis are the next most common species found in urine cultures.24 In line with the results of this study, Candida albicans 20 (38.46%) was identified as the most common cause of candiduria, followed by Candida tropicalis in 19 (36.54%), Candida glabrata in 5 (9.62%), Candida parapsilosis in 4 (7.69%), and Candida krusei in 1 (1.92%). In the present study, Candida tropicalis was the most commonly isolated Candida species from patients with diabetes mellitus.
Candida albicans, which is a commensal microorganism on the skin and mucosal surface and can be an opportunistic pathogen of the genitourinary system, remains the most common cause of candidiasis.17 Non-albicans Candida is also emerging as a pathogen that can colonize mucosal surfaces.8
A limitation of this study was that it could not determine the pathogenesis of Candida UTIs, whether ascending or descending, because no fungal culture of blood specimens was performed to detect the presence of candidemia in patients with Candida UTIs. However, as the urine specimens were re-collected, so the pre-analytical process could be maintained according to the standards set for the study. Further, repeat specimens were collected from patients with candiduria to confirm whether candiduria was caused by colonization or a pathogen causing Candida UTIs. In this study, urinary catheters were also replaced if they had been used for more than five days; this was done to prevent Candida colonization in the urinary catheters.
A significant correlation was found between candiduria and previous antibiotic use with the incidence of Candida UTIs. In this study, Candida albicans was the predominant species in Candiduria and was found susceptible to all antifungals tested. C. tropicalis was predominantly isolated from patients with diabetes mellitus. These findings underscore the need for careful monitoring of antibiotic use and candiduria in hospitalized patients to prevent Candida UTIs.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Health Research Ethics Committee of Dr. Soetomo Hospital Surabaya, Indonesia, vide ethical fitness No. 0832/KEPK/XI/2023.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- He Z, Huo X, Lei D, Zhao H, Jia K, Wang F. Management of candiduria in hospitalized patients: a single-center study on the implementation of IDSA guidelines and factors affecting clinical decisions. Eur J Clin Microbiol Infect Dis. 2021;40(1):59-65.
Crossref - Sobel JD, Fisher JF, Kauffman CA, Newman CA. Candida Urinary Tract Infections-Epidemiology. Clin Infect Dis. 2011;52(suppl_6):S433-S436.
Crossref - Dias V. Candida species in the urinary tract: is it a fungal infection or not? Future Microbiol. 2020;15(2):81-83.
Crossref - Peman J, Ruiz-Gaitan A. Candidemia from urinary tract source: the challenge of candiduria. Hosp Pract. 2018;46(5):243-245.
Crossref - Anggraeni D, Endraswari PD, Rusli M, Kawilarang AP. Epidemiology and Risk Factors for Candiduria in Hospitalized Patients at Dr. Soetomo Hospital, Surabaya, Indonesia. Int J Res Publ. 2022;115(1).
Crossref - Gajdacs M, Doczi I, Abrok M, Lazar A, Burian K. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: results from a 10-year retrospective survey. Cent Eur J Urol. 2019;72(2):209-214.
Crossref - Malani AN, Kauffman CA. Candida urinary tract infections: treatment options. Expert Rev Anti Infect Ther. 2007;5(2):277-284.
Crossref - Alfouzan WA, Dhar R. Candiduria: Evidence-based approach to management, are we there yet? J Mycol Med. 2017;27(3):293-302.
Crossref - Fazeli A, Kordbacheh P, Nazari A, et al. Candiduria in Hospitalized Patients and Identification of Isolated Candida Species by Morphological and Molecular Methods in Ilam, Iran. Iran J Public Health. 2019;48(1):156-161.
Crossref - Eksi F, Hassan BA, Ugur BK, Yildiz H, Erinmez M, Ganidagli S. An epidemiologic analysis of Candida spp. urinary infections in intensive care unit. Rev Epidemiol e Control Infeccao. 2022;12(2):80-86.
Crossref - Kauffman CA, Fisher JF, Sobel JD, Newman CA. Candida Urinary Tract Infections-Diagnosis. Clin Infect Dis. 2011;52(suppl_6):S452-S456.
Crossref - Weinstein RA, Lundstrom T, Sobel J, Nosocomial Candiduria: A Review. Clin Infect Dis. 2001;32(11):1602-1607.
Crossref - Jain M, Dogra V, Mishra B, Thakur A, Loomba, Sood P, Bhargava A. Candiduria in catheterized intensive care unit patients/ : Emerging microbiological trends. Indian J Pathol Microbiol. 2011;54(3):552-555.
Crossref - Odabasi Z, Mert A. Candida urinary tract infections in adults. World J Urol. 2020;38(11):2699-2707.
Crossref - Gunardi WD, Karuniawati A, Umbas R, et al. Biofilm-Producing Bacteria and Risk Factors (Gender and Duration of Catheterization) Characterized as Catheter-Associated Biofilm Formation. Chaves Lopez C, ed. Int J Microbiol. 2021;2021:1-10.
Crossref - Fisher JF. Candiduria: When and how to treat it. Curr Infect Dis Rep. 2000;2(6):523-530.
Crossref - Talapko J, Mestrovic T, Skrlec I. Growing importance of urogenital candidiasis in individuals with diabetes: A narrative review. World J Diabetes. 2022;13(10):809-821.
Crossref - Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. Infections in Patients with Diabetes Mellitus. J Clin Med. 2019;8(1):76.
Crossref - Akinjogunla OJ, Divine-Anthony O, Ajayi AO, Etukudo IU, Etok IJ. Asymptomatic Candiduria among Type 1 and 2 Diabetes Mellitus Patients: Risk and Sociodemographic Factors, Prevalence, Virulence Markers and Antifungal Susceptibility. J Pure Appl Microbiol. 2020;14(2):1467-1478.
Crossref - Guler S, Ural O, Findik D, Arslan U. Risk factors for nosocomial candiduria. Saudi Med J. 2006;27(11):1706-1710.
- Bukhary ZA. Candiduria: a review of clinical significance and management. Saudi J Kidney Dis Transpl. 2008;19(3):350-360. http://www.ncbi.nlm.nih.gov/pubmed/18445893
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-e50.
Crossref - Behzadi P, Behzadi E, Ranjbar R. Urinary tract infections and Candida albicans. Cent Eur J Urol. 2015;68.
Crossref - Kauffman CA. Candiduria. Clin Infect Dis. 2005;41(Suppl_6):S371-S376.
Crossref - La Bella AA, Andersen MJ, Gervais NC, et al. The catheterized bladder environment promotes Efg1- and Als1-dependent Candida albicans infection. Sci Adv. 2023;9(9):eade7689.
Crossref - Wise GJ, Goldberg P, Kozinn PJ. Genitourinary Candidiasis: Diagnosis and Treatment. J Urol. 1976;116(6):778-780.
Crossref - Poloni JAT, Rotta LN. Urine Sediment Findings and the Immune Response to Pathologies in Fungal Urinary Tract Infections Caused by Candida spp. J Fungi. 2020;6(4):245.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.