ISSN: 0973-7510
E-ISSN: 2581-690X
Mint cultivation requires heavy use of chemical pesticides to manage pathogens and insect pests. To develop a biological alternative to these pesticides, we investigated the potential of plant growth-promoting rhizobacteria (PGPR) to stimulate the natural defense mechanisms of mint, potentially inducing systemic acquired resistance. In the present study, we utilized a bacterial consortium comprising four PGPR strains, Sphingobacterium suaeda, Pseudomonas sp., Bacillus cereus and Bacillus pumilus, to promote mint growth and activate natural defense mechanisms. The defense mechanisms of mint were analyzed by measuring phenylalanine ammonia lyase (PAL) activity at both the root and aerial levels, a key enzyme in phenolic and lignin metabolism, along with the levels of phenolic compounds and lignin. The results showed that the PGPR consortium substantially boosted mint growth, leading to a 28% increase in the number of leaves, a 25% increase in shoot height, a 34% improvement in aerial dry biomass, and an 80% increase in root biomass. Similarly, the PGPR consortium stimulated PAL activity and increased the levels of phenolic compounds and lignin in both the roots and shoots. The stimulation of these remote defense mechanisms at the shoot level evokes a systemic defense reaction known as the expression of systemic acquired resistance in plants. These results present promising opportunities for improving the bioprotection of mint against pathogens.
Green Mint, PGPR, Natural Defenses, Bioprotection
Spearmint is known for its refreshing scent and is used in cooking, traditional medicine, and the food, pharmaceutical, cosmetic, and perfume industries worldwide. Morocco is the main producer of mint, an essential ingredient in tea. However, like other plants, mint is exposed to risks posed by pathogens, including fungi, oomycetes, bacteria, and viruses. These pathogens attack mint in various ways, jeopardizing its health and development, and leading to significant crop losses and deterioration in the quality of mint-derived products.1 Current agricultural practices to combat plant diseases rely primarily on the use of chemical pesticides. However, this intensive use has resulted in potential environmental damage, affecting soils, groundwater, targeted and non-targeted organisms, and human health.2 To control pests, farmers use synthetic pesticides such as bitertanol and tebuconazole for fungal diseases. Farmers mainly use cypermethrin, cyfluthrin, and deltamethrin.3 In Morocco, no synthetic pesticides were approved for use on mint crops before 2013. Approximately 54% of food poisoning cases are caused by the use of agricultural pesticides [FAO/WHO, 2004]. Death is a consequence of this poisoning. According to information from the World Health Organization, between 1 and 5 million cases of pesticide poisoning occur annually, and the affected individuals include mostly vulnerable people such as the elderly, children, infants, and fetuses. Factors that increase sensitivity to pesticides include malnutrition and dehydration [FAO/WHO 2004]. Therefore, it is essential to develop more environmentally friendly agriculture that respects the environment, farmers, and consumers, while reducing dependence on chemical inputs. Therefore, the scientific community is now investigating alternative strategies. In the 20th century, it was shown that such plants can defend themselves from pathogens by infecting crops with other non-virulent strains. This approach, known as induced systemic resistance (ISR), enhances the ability of plants to resist diseases.4 Various biotic elicitors such as polysaccharides, lipids, (glyco) peptides, and (glyco) proteins have been reported to induce the vegetation immune system, which is resistant to various types of biotic stressors. These molecules act as pathogen-associated molecular patterns (PAMPs) or microbe-associated molecular patterns (MAMPs) and are recognized by pattern recognition receptors (PRRs) to initiate protection reactions termed PAMP-triggered immunity (PTI).5 The perception of an elicitor triggers a series of intracellular events, including changes in receptor conformation or the activation of various effectors such as G proteins, lipases, phosphatases, protein kinases, and ion channels. This initiates downstream cellular responses, such as phosphorylation, dephosphorylation, ion transport through the plasma membrane, and the release of signaling molecules, such as calcium ions (Ca2+), nitric oxide (NO), reactive oxygen species (ROS), and secondary messengers, such as inositol 1,4,5-trisphosphate (IP3), cyclic adenosine monophosphate (cAMP), and cyclic guanosine monophosphate (cGMP).6
Plant growth-enhancing rhizobacteria constitute a diverse assembly of microorganisms associated with plant roots capable of mitigating the severity or occurrence of diseases. Several PGPR strains can be applied to plant roots and used as ISR elicitors.7 PGPR reduce disease in crops and make plants more resistant to pathogens by inducing physiological modifications,7 protein synthesis, peptides, and chemicals that contribute to plant defense strategies.8
Plant-protective responses include the production of the phenylalanine ammonia lyase (PAL) enzyme and an increase in phenolic content.7 PAL is an enzyme that stimulates the conversion of L-phenylalanine to trans-cinnamic acid, which is an intermediary in the synthesis of phenolics. These phenolic compounds are antimicrobial agents that are deposited beyond the infection sites. In addition, lignin is deposited at infection sites and acts as an antimicrobial agent.9
To develop a biological alternative that respects the environment and human health, which is becoming increasingly urgent, the present study aimed to explore the potential of a consortium of four compatible species of plant growth-enhancing rhizobacteria to stimulate the immune system of mint, causing the stimulation of acquired plant defense against pests: Sphingobacterium suaeda T47, Pseudomonas sp. DN 13-01, Bacillus cereus 263AG5, and Bacillus pumilus X22. This bacterial consortium has already been shown to promote the growth of tomato, bean, and corn plants,10 enhance biological protective responses, and improve the resistance of date palm.11 The induction of mint biological defense by the PGPR consortium was analyzed through the activity of PAL, a key enzyme in phenylpropanoid metabolism, and the content of lignin and phenolic compounds.
Bacterial strains
In this research, four strains were utilized: the Sphingobacterium suaeda strain “B17”, Pseudomonas sp. strain “BR2”, Bacillus pumilus strain “BV2”, and Bacillus cereus strain “BV3”. These strains were originally sourced from phosphate washing sludge storage basins situated at the Merah Lahrach mining site in Khouribga (32°44’51.4 “N 6°50’56.5 “W). They were selected based on their interesting PGP traits (phosphorus solubilization, phytohormone production, potassium solubilization, nitrogen fixation, and siderophore production).10 The bacteria have been shown to exhibit biocompatibility with each other during consortium formation.10
Preparation of inoculum
Each strain underwent individual culturing in 250 mL of liquid LB medium for 48 hours at 28 °C with agitation set at 120 rpm. Subsequently, each strain was centrifuged separately at 10,000 rpm for 15 minutes at 4 °C. The resulting pellet was washed thrice with sterile distilled water before dilution in a sterile physiological saline solution (0.9% NaCl). The optical density of each bacterial suspension was measured separately and adjusted to 0.4 at 600 nm by adding physiological water. This adjustment ensured a standardized bacterial concentration, typically equivalent to approximately 108 colony-forming units/mL (CFU/mL). Subsequently, the four bacterial strains were mixed to form a consortium, which was used for plant inoculation. The interactions between the four bacterial strains in the consortium BC were assessed. The analysis examined the potential antagonistic effects, and the findings indicated the absence of significant growth inhibition among the strains.
Experimental design and plant growth
The research utilized the “Mentha spicata” variety, commonly known as green mint, propagated from cuttings, ensuring genetic uniformity with the parent plant. This study evaluated the plant growth and development of green mint. The experiments were conducted in a greenhouse equipped with side windows for ventilation. In total, 120 pots were used in this study, with each pot containing three spearmint plants. Of these, 60 pots were treated with the bacterial consortium and the remaining 60 pots served as the control group. The plants were grown in plastic pots measuring 16 cm in height and 19 cm in diameter and were disinfected with bleach before being filled with soil. In preparation for transplanting the green mint into pots, the cuttings were initially planted in black bags suitable for this purpose. Once the cuttings sprouted, they were transplanted to pots filled with a composite of 25% compost and 75% soil.
To apply the bacterial consortium to mint plants at the root level, 10 mL of the prepared bacterial mixture was added to 100 mL of tap water. This method allows for the effective delivery of beneficial bacteria directly to the plant root system, promoting a relationship between the microorganisms and mint plants. Weekly application ensured consistent and sustained exposure to the consortium, enhancing its potential to positively influence the development and health of the mint plants. Therefore, the choice of weekly application for three weeks aligns with the objective of maximizing the benefits of PGPR-mediated ISR and plant growth enhancement.
Plant sampling and studied parameters
Three samples were collected from both treated and untreated plants at 24-hour and 48-hour intervals following each inoculation (Figure 1). Three inoculations were performed: the second inoculation was performed 7 days after the first inoculation, and the third inoculation was performed 14 days after the second inoculation. The collected samples of the plant shoots (aerial part: stems and leaves) and roots were kept in conditions of -20 °C in a refrigerator for biochemical analyses (PAL enzyme activity, content of lignin and phenolic compounds).
Phenylalanine ammonia lyase (PAL) activity
PAL activity was analyzed as described by Liu et al.12 The protocol began with an extraction step, during which 100 mg of shoot and root samples were ground in 2 mL of a buffer solution (containing 100 mM boric acid, pH 6.1 mM EDTA, and 1% PVPP) and the homogenate was centrifuged at 10,000 × g for 30 min. Next, an assay was performed by taking 200 µL of enzyme extract and adding 1 mL of 100 mM boric acid buffer and a further 200 µL of 20 mM L-phenylalanine. The mixture was incubated at 30 °C for 60 minutes, then the reaction was stopped by adding 100 µL of 6 N HCl. Finally, the absorbance was read at 290 nm with a molar extinction coefficient of 16.890 L/mol/cm.12
Phenolic compound content
The protocol, as described by Ribיreau-Gayon and Stonestreet13 with modifications, involved the following steps. Leaves and roots (100 mg) were ground and combined with 1 mL of 50% ethanol. The resulting extracts were transferred to Eppendorf tubes and stored in a refrigerator until use. Subsequently, 0.25 mL of chloroform was added to 3 ml of the extract, followed by vortexing and centrifugation at 1000 rpm for 5 min to obtain a supernatant and pellet. A mixture of 0.5 ml of water extract and 0.5 mL of Na2CO3 (20%) was prepared in tubes, followed by the addition of Folin-Ciocalteu reagent (0.5 mL) after a 3-minute wait period. The tubes were then shaken and incubated at 40 °C for 30 minutes before measuring the absorbance at 760 nm. The phenol assay was performed using the method based on the Folin-Ciocalteu method of Ribיreau-Gayon Stonestreet.13 To prepare the mixture, 0.5 mL of extract was added to test tubes, followed by the addition of 0.5 mL of 20% Na2CO3. After mixing, the solution was left to stand for 3 min before adding the Folin-Ciocalteu reagent (0.5 mL). The tubes were then mixed and placed at a temperature of 40 °C for 30 minutes. Absorbance was measured at 760 nm. The amount of phenolic compounds was calculated using gallic acid as the standard curve and the results were expressed in milligrams of plant material.
Lignin content
To measure the quantity of lignin, the roots were first mixed with 2 mL of 99.5% (v/v) ethanol and centrifuged at 7000 rpm for 20 min. The resulting pellets were dried overnight at room temperature. Next, 10 mg of the dried residue was placed in a screw-top tube, and 0.5 mL of 2 M HCl and 0.1 mL of thioglycolic acid were added. The samples were heated to 100 °C for 8 hrs and left on ice. The sample was then centrifuged at 7000 rpm for 20 min at 4 °C. The resulting pellet was washed with distilled water and resuspended in 5 mL 1 M NaOH. The solution was gently stirred at 20 °C for 18 hours and then centrifuged at 7000 g for 20 minutes. The supernatant was collected in a test tube, and 1 mL of concentrated HCl was added. The lignin-thioglycolic acid complex was allowed to precipitate at 4 °C for 4 hours and then centrifuged at 7000 rpm for 20 minutes. The resulting pellet was dissolved in 1 mL of 1 M NaOH and the absorbance was measured at 280 nm using a NaOH blank. This protocol has been described previously by Bruce and West.14
Plant growth
Once the experimental period was completed, the stimulatory effect of rhizobacteria on plant biomass was assessed. This involved separating the roots and shoots of the plants and measuring various parameters, including plant height, fresh weight of both parts, and leaf count. To determine dry weight, samples of the root and shoot parts were placed in an oven at 75 °C until a stabilized weight was achieved, typically taking two days.
Statistical analysis
The data regarding the comparison of plant growth, PAL activity, phenolic substance accumulation, and lignin content in the roots and shoots of green mint between treated and control plants were subjected to analysis of variance (ANOVA). The objective was to determine significant differences between means. For this analysis, a multiple comparison test with a confidence level of 95% was conducted using the Tukey method in Minitab software. Results were indicated as mean ± standard error (SE) from three separate experiments. Statistical significance was set at p < 0.05.
Effect of inoculation with the PGPR consortium on plant growth
The results presented in Table demonstrate the effects of inoculating the growth-enhancing rhizobacterial consortium on various plant developmental parameters. The findings indicated that the application of the bacterial consortium (consisting of Sphingobacterium suaeda, Pseudomonas sp., B. pumilus, and B. cereus) significantly influenced (p < 0.05) the development of mint plants compared with the control group. Specifically, in the shoots, plants treated with the inoculum exhibited a significant increase of approximately 28.07% in leaf number, 25.24% in leaf length, 34.12% in fresh weight, and 32.81% in dry weight compared to non-inoculated plants. Similarly, in the roots, inoculated plants demonstrated significant improvements, with approximately 42.95% longer length, 66.67% higher fresh weight, and 80.31% greater dry weight than non-inoculated plants.
Table:
Effect of inoculation with the bacterial consortium on the vegetative growth parameters of green mint plants. For the same parameter, values with different letters are significantly different (p < 0.05)
| Samples | Treatment | Leaves number | Length (cm) | FW (g) | DW (g) |
|---|---|---|---|---|---|
| Shoot | Control | 22.8 + 2.16b | 22.5 + 2.07b | 4.85 + 1.20b | 0.96 + 0.17b |
| + PGPR Consortium | 29.2 + 2.27a | 28.33 +2.94a | 6.51 + 1.87a | 1.27 + 0.23a | |
| Root | Control | – | 22.16 + 8.75b | 1.32 + 0.86b | 0.35 + 0.22b |
| PGPR Consortium | – | 31.5 + 6.83a | 2.20 + 0.84 | 0.63 + 0.20a |
FW: Fresh weight, DW: Dry weight
PGPR induction of natural defense in green mint
PAL activity
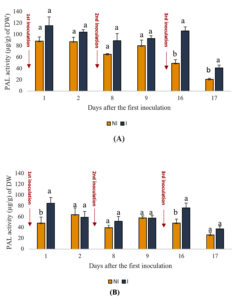
The activity of PAL, an enzyme involved in the biosynthesis of secondary metabolic compounds crucial for plant defense mechanisms, was assessed in the shoots and roots of plants at various time intervals under inoculated and uninoculated conditions. Figure 2 illustrates PAL activity dynamics. The results indicated a significant (p < 0.05) increase in PAL activity in both shoots and roots of mint plants following PGPR application. Specifically, in the shoot, PAL content ranged from 41 to 115 µg/g in inoculated plants compared to 20 to 88 µg/g in uninoculated plants across the observed time points. Similarly, in the root, PAL content varied between 37 and 84 µg/g for inoculated plants and between 26 and 63 µg/g for uninoculated plants. These findings suggest a potential positive influence of PGPR inoculation on PAL activity, thereby enhancing plant defense responses.
Figure 2. Effects of PGPR on PAL activity in spearmint aerial shoots (A) and roots (B). The letters (a, b) indicate significant differences between treatments (Control (NI), Inoculated (I)) in each point time using the Tukey test. Values indicate the mean (±SD) of n = 3. Scores with the same letter are not significantly different at p = 0.05
Phenol content
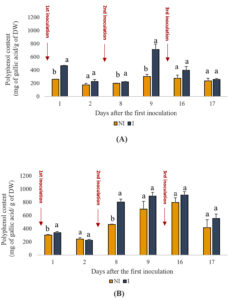
Phenolic content is a crucial indicator of plant health and antioxidant potential. The results presented in Figure 3 illustrate the effect of inoculating the PGPR consortium on phenolic content. Notably, inoculated plants consistently exhibited higher phenolic contents than uninoculated plants throughout the experimental period. For instance, on day one, the phenolic content in the inoculated shoots was approximately 78% higher than that in the uninoculated shoots, suggesting a substantial effect of PGPR inoculation on phenolic synthesis. Similar trends were observed in root phenolic content, with inoculated plants showing percentage increases ranging from 12% to 116% compared with uninoculated plants across different time points. These findings underscore the significant (p < 0.05) influence of PGPR treatment on enhancing phenolic content in mint plants, which is indicative of improved antioxidant capacity and stress resilience.
Figure 3. Effects of PGPR on the phenolic content of spearmint aerial shoots (A) and roots (B). The letters (a, b) indicate significant differences between treatments (Control (NI), Inoculated (I)) in each point time using the Tukey test. Values indicate the mean (±SD) of n = 3. Scores with the same letter are not significantly different at p = 0.05
Lignin content
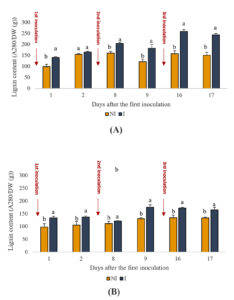
Lignin, a key component of plant cell walls, plays a crucial role in defense mechanisms against environmental stress. The data illustrate the lignin content in both the shoot and root tissues of plants over time (Figure 4), with and without inoculation with PGPR. Across all observed time points, the lignin content in both shoot and root tissues of inoculated plants consistently surpassed that of the uninoculated plants (p < 0.05). For instance, on day 9, the lignin content in the shoots of the inoculated plants was 182 units, which was significantly higher than that of the uninoculated plants (122 units). Similarly, in the roots, the lignin content in inoculated plants on day 16 was 172 units compared to 134 units in uninoculated plants. This increase suggests a significant enhancement in the structural integrity and stress resilience of the plants owing to PGPR inoculation.
Figure 4. Effect of PGPR on the lignin content of the aerial parts of spearmint plants shoots (A) and roots (B). The letters (a, b) indicate significant differences between treatments (Control (NI), Inoculated (I)) in each point time using the Tukey test. Values indicate the mean (±SE) of n = 3. Scores with the same letter are not significantly different at p = 0.05
Implementing sustainable agricultural practices involves reducing dependency on chemical inputs, including fertilizers and pesticides. PGPR have shown promising potential as alternatives to traditional chemical inputs and offer environmentally friendly solutions to improve soil health, plant growth, and crop productivity. As demonstrated in a previous study by Benbrik et al.,10 selected bacterial strains are known for their ability to improve plant growth and development in various crops, including maize and tomato. Therefore, the objectives of this study embarked on a novel path, aiming to assess biocontrol through induced systemic resistance and to stimulate mint growth, using the selected bacterial consortium, for the first time.
Application of the bacterial consortium significantly enhanced the developmental parameters of mint plants compared with the control. This effect could be due to the capacity of the bacteria to solubilize potassium and phosphorus, in addition to possessing various plant growth-promoting properties such as siderophore synthesis, indole-3-acetic acid (IAA) production, hydrogen cyanide (HCN) production, and ammonium (NH4) fixation, which are associated with the growth and development of various plants.10 Furthermore, the four PGPR produced a range of enzymes, including alkaline phosphatases and acid, protease, pectinase, cellulase, amylase, and lipase enzymes, leading to the hydrolysis of different polymers in the soil and consequently enhancing nutrient acquisition by the plants.10
This study clearly demonstrated the ability of the selected bacterial consortium10 to increase PAL enzyme activity and lignin and polyphenol content in the roots and shoots of inoculated spearmint plants. PAL is an enzyme essential for the biosynthesis of secondary metabolic compounds, such as polyphenols, flavonoids, lignins, coumarins, and stilbenes. It plays a pivotal role in catalyzing the conversion of phenylalanine to cinnamic acid via the phenylpropanoid pathway. These secondary metabolites present in plants perform a variety of functions, notably by providing antioxidant and antimicrobial properties and strengthening the cell wall. These compounds serve a vital function as defense mechanisms by protecting plants from damage caused by free radicals, pathogens, and environmental stresses. Systemic resistance mediated by PGPR is frequently linked to the initiation of defense mechanisms, which involve the early and heightened expression of various defense enzymes such as PAL, glucanase, chitinase, and peroxidase. It also includes the accumulation of phenolics, phytoalexins, lignins, and other components.15
Our observations corroborate the results obtained by Del Rosario Cappellari et al.,16 who reported significantly higher PAL activity in Mentha piperita plants inoculated with bacterial strains (P. putida, P. fluorescens, and B. subtilis) than in untreated controls. In an investigation conducted by Kandan et al.,17 tomato plants treated with P. fluorescens exhibited heightened PAL and peroxidase activity, as well as increased levels of phenols and lignification. Other studies have suggested a potentially protective role of these metabolites under stressful conditions. Khanna et al.18 applied P. aeruginosa and B. gladioli to Solanum lycopersicum under Cd-stress conditions. Their work showed that PGPR are involved in polyphenol synthesis and stimulation of PAL enzyme activity, thereby increasing phenolic compound levels to cope with cadmium stress. A study by Asghari et al.,19 who treated Mentha pulegium L. with a group of growth-promoting bacteria, as well as the study of Chiappero et al.,20 who infected Mentha piperita with P. fluorescens and B. amyloliquefaciens, showed improved growth and greater increase of total phenolic content in inoculated mint plants compared to uninoculated plants, even under water stress conditions.
Cell wall modifications have been associated with PGPR, which protect plants from phytopathogens. The increased lignin content may play a role in pathogen inhibition and disease suppression. The Biocontrol agent B. pumilus has been observed to increase lignin deposition in the epidermal tissues of pearl millet; this protective reaction occurs more quickly in PGPR-primed plants infected with the pathogen Sclerospora graminicola than in unprimed plants treated with turmeric rhizomes containing Serratia nematodiphila and P. plecoglossicida and exhibits antifungal activity.21 Bakshi et al.22 observed that Brassica juncea L. plants inoculated with P. aeruginosa and B. gladioli showed increased PAL expression in response to chlorpyrifos toxicity. Martins et al.23 reported that common beans treated with Bacillus subtilis induced an increase in total soluble phenolics in the presence of the pathogen Curtobacterium flaccumfaciens. Similar results have been observed for P. putida inoculated onto bean roots infected with Fusarium solani.24 These studies highlight the role of PGPR in inducing PAL enzyme action and the production of phenolic compounds, and their essential role in stimulating natural defenses by activating the ISR defense of different plants. The increase in PAL activity after the third inoculation, rather than the first, likely reflects the cumulative response of the plants to repeated treatments. The induction of phenolic compounds and lignin after the first and second inoculations, but no significant increase in PAL activity, may reflect the plant’s initial rapid defense strategy. Phenolic compounds and lignin are critical for strengthening cell walls and providing immediate protection against stress and pathogens. Plants may utilize pre-existing metabolic pathways or reserves to produce these compounds quickly without requiring a marked immediate increase in PAL activity. PAL, an upstream enzyme, may show delayed activation because its role becomes more critical for sustained synthesis over time as the plant requires the ongoing production of phenolic precursors to maintain its defense response. However, further studies are needed to deepen our understanding of these mechanisms and explore the protective effects of these molecules in various environmental and stress contexts.
In this study, we had the opportunity to refine the application of the formulated consortium to enhance its effectiveness in promoting biocontrol through induced systemic resistance. This involved the assessment of PAL production, phenolic content, and lignin production. In addition, integrating diverse microbial strains within the consortium to complement each other’s functions and adaptability to varying environmental conditions can further enhance their performance. Despite the promising results obtained in this study, the employment of advanced techniques, such as precision agriculture and molecular analysis, can provide insights into the complex dynamics between the consortium and its environment, allowing for more targeted and effective biocontrol strategies. Ultimately, by capitalizing on these approaches, we can develop more robust and sustainable solutions for crop protection, ensuring enhanced agricultural productivity and environmental conservation.
The use of chemical controls to prevent plant diseases poses a significant problem in Morocco, with potential risks to the environment by affecting soil, groundwater, targeted and non-targeted organisms, and human health. Therefore, we explored PGPR as a promising biological solution to replace chemical pesticides. Four strains of PGPR (Sphingobacterium suaeda, Pseudomonas sp., B. pumilus, and B. cereus) were employed in our study, applied to spearmint plants to highlight and assess the positive effects of this consortium on biocontrol, systemic resistance induction, and phytostimulation.
These results unequivocally demonstrated the beneficial effects of inoculation on spearmint. Inoculated plants exhibited remarkable growth in terms of height and fresh and dry weights compared with non-inoculated plants. Furthermore, the activity of the PAL enzyme, as well as the concentrations of total phenols and lignin, significantly increased in treated plants compared to those in untreated plants. These observations underscore the positive impact of plant growth-promoting rhizobacteria inoculation on the development and resistance of spearmint.
In conclusion, these results show that the consortium of the four PGPR strains used in the present study has the capacity to promote the growth of spearmint and stimulate its natural defenses in a systemic manner. These results suggest that the nutritional status and immune system of mint can be induced by the PGPR consortium used, which would be at the origin of acquired systemic resistance, contributing to the development of an alternative bioprotection and bioproduction of mint in a pesticide-free environment.
ACKNOWLEDGMENTS
The authors are thankful to the CNRST-Morocco, the National Agency for Medicinal and Aromatic Plants (ANPMA) – Taounate (Morocco), and Cadi Ayyad University – Marrakech for supporting this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Hirissou F. La Resistance Des Plantes. Chambre d’Agriculture Dordogne. 2020. https://nouvelle-aquitaine.chambres-agriculture.fr/fileadmin/user_upload/FAL_commun/publications/Nouvelle-Aquitaine/24_Agronomie_ResistanceDesPlantes.pdf
- Tudi M, Ruan HD, Wang L, et al. Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health. 2021;18(3):1112.
Crossref - Edwards J, Bienvenu FE. Evaluation of selected fungicides to control mint rust on Scotch spearmint. Crop Protection. 2000;19(3):195-199.
Crossref - Nithya R, Sharma R, Rao VP, Gopalakrishnan S, Thakur RP. Biochemical characterisation of grain mould resistant and susceptible genotypes and PGPR-induced resistance in the host to Curvularia lunata and Fusarium proliferatum. Arch Phytopathol Plant Protection. 2013;46(8):980-989.
Crossref - Aitouguinane M, Alaoui-Talibi ZE, Rchid H, et al. Polysaccharides from Moroccan green and brown seaweed and their derivatives stimulate natural defenses in olive tree leaves. Appl Sci. 2022;12(17):8842.
Crossref - Mishra AK, Sharma K, Misra RS. Elicitor recognition, signal transduction and induced resistance in plants. J Plant Interact. 2011;7(2):95-120.
Crossref - Girish N, Umesha S. Effect of plant growth promoting rhizobacteria on bacterial canker of tomato. Archives of Phytopathology and Plant Protection. 2005;38(3):235-243.
Crossref - Meena M, Swapnil P, Divyanshu K, et al. PGPR mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J Basic Microbiol. 2020;60(10):828-861.
Crossref - Singh A, Sarma BK, Upadhyay RS, Singh HB. Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol Res. 2013;168(1):33-40.
Crossref - Benbrik B, Elabed A, Iraqui M, et al. A phosphocompost amendment enriched with PGPR consortium enhancing plants growth in deficient soil. Commun Soil Sci Plant Anal. 2021;52(11):1236-1247.
Crossref - Ziane SO, El-Alaoui TZ, Koraichi SI, Douira A, Amir S, Meddich A, El Modafar C. Synergistic effects of arbuscular mycorrhizal fungi associated to plant growth-promoting rhizobacteria in suppression of soil-borne Fusarium wilt of date palm. Biocatal Agric Biotechnol. 2023;51:102753.
Crossref - Liu H, Jiang W, Bi Y, Luo Y. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol Technol. 2005;35(3):263-269.
Crossref - Ribereau-Gayon P, Stonestreet E. Determination of anthocyanins in red wine. Bulletin de la Societe chimique de France. 1965;9:2649-2652
- Bruce RJ, West CA. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989;91(3):889-897.
Crossref - Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. The Plant Cell. 1995;7(7):1085-1097.
Crossref - del Rosario Cappellari L, Chiappero J, Santoro MV, Giordano W, Banchio E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci Hortic. 2017;220:193-198.
Crossref - Kandan A, Commare RR, Nandakumar R, Ramiah M, Raguchander T, Samiyappan R. Induction of phenylpropanoid metabolism by Pseudomonas fluorescens against tomato spotted wilt virus in tomato. Folia Microbiologica. 2002;47(2):121-129.
Crossref - Khanna K, Jamwal VL, Sharma A, et al. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere. 2019;230:628-639.
Crossref - Asghari B, Khademian R, Sedaghati B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Scientia Horticulturae. 2020;263:109132.
Crossref - Chiappero J, del Rosario Cappellari L, Alderete LGS, Palermo TB, Banchio E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind Crops Prod. 2019;139:111553.
Crossref - Jagtap RR, Mali GV, Waghmare SR, Nadaf HN, Nimbalkar MS, Sonawane KD. Impact of Plant Growth Promoting Rhizobacteria Serratia nematodiphila RGK and Pseudomonas plecoglossicida RGK on Secondary Metabolites of Turmeric Rhizome. Biocatal Agric Biotechno. 2023;47: 102622.
Crossref - Bakshi P, Sharma P, Chouhan R, et al. Interactive effect of 24-epibrassinolide and plant growth promoting rhizobacteria inoculation restores photosynthetic attributes in Brassica juncea L. under chlorpyrifos toxicity. Environ Poll. 2023;320:120760.
Crossref - Martins SJ, de Medeiros FHV, de Souza RM, de Resende MLV, Junior PMR. Biological control of bacterial wilt of common bean by plant growth-promoting rhizobacteria. Biological Control. 2013;66(1):65-71.
Crossref - Anderson AJ, and Guerra D. Responses of bean to root colonization with Pseudomonas putida in a hydroponic system. Phytopathology. 1985;75(9):992-99.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.