ISSN: 0973-7510
E-ISSN: 2581-690X
This study aimed to develop and evaluate a bioformulation combining Trichoderma asperellum MSU007 and the plant growth-promoting rhizobacterium (PGPR) Bacillus velezensis MSU01 to enhance rice growth. Coexistence assays demonstrated that T. asperellum MSU007 and the PGPR B. velezensis MSU01 could grow concurrently without mutual inhibition. A powdered bio-formulation was created that showed effective water solubility and moisture content stability across the three formulations. Viability assessments indicated that T. asperellum MSU007 and the PGPR maintained high survival rates over a 60 day storage period, with formulation 3 (T. asperellum MSU007 and B. velezensis MSU01 at a 3:1 ratio) consistently exhibiting the highest viability. The effect of the bioformulation on rice growth was significant. Formulation 3 was the most effective in enhancing rice seed germination and seedling growth. It achieved 100% germination by the fourth day and consistently promoted improvements in shoot and root lengths, as well as in the fresh and dry weights of shoots and roots. Formulations 1 and 2 also showed positive effects but were less effective than formulation 3. In summary, the Trichoderma-PGPR bioformulations, particularly formulation 3, significantly enhanced rice growth, offering a promising solution for improving crop yield.
PGPR, Powdered Bioformulation, Rice, Seed Germination, Trichoderma
Rice (Oryza sativa L.) is a staple food for more than half of the world’s population and serves as the primary source of carbohydrates.1 The growing global population and the increasing demand for food have increased the need for higher rice yields. Traditionally, to meet these demands, farmers rely heavily on chemical fertilizers to boost rice production. Although effective in the short term, the continuous use of chemical fertilizers poses significant risks, including escalating production costs, soil degradation, and adverse environmental impacts.2,3 These challenges underscore the need for sustainable agricultural practices that can maintain or enhance crop yields without compromising soil health.4
A promising approach to sustainable rice cultivation involves the use of beneficial microorganisms, such as Trichoderma spp. and plant growth-promoting rhizobacteria (PGPR). Trichoderma fungi enhance plant growth, nutrient uptake, and resistance to pathogens by colonizing roots and producing enzymes and metabolites that inhibit harmful pathogens.5,6 They also improve nutrient availability by decomposing organic matter.7 As plant growth-promoting fungi (PGPF), Trichoderma spp. stimulate growth, enhance crop quality, and increase productivity through favorable rhizosphere interactions and secondary metabolite production.8 For example, T. harzianum SQR-T037 produces harzianolide, which promotes tomato seedling growth, whereas T. virens and T. atroviride produce indole-3-acetic acid (IAA), which is crucial for root development. Studies have shown Trichoderma spp. enhance nutrient uptake and increase root surface area, aiding mineral competition.9,10 The potential use of Trichoderma metabolites as biofertilizers offers a sustainable alternative to synthetic fertilizers. In Northeast China, the native strain T. asperellum TaspHu1 was identified as an effective biocontrol agent and plant growth promoter, significantly improving tomato seedling growth and resistance to Alternaria alternata.11
PGPR, such as Bacillus velezensis, colonizes plant roots and promotes growth through various mechanisms.12 These mechanisms include nitrogen fixation, where atmospheric nitrogen is converted into a usable form; phosphate solubilization, which increases the availability of phosphate; and the production of growth-stimulating hormones such as auxins, cytokinins, and gibberellins.13 By enhancing nutrient uptake and promoting plant growth, PGPR contribute to improved crop yields and reduced reliance on chemical fertilizers. Wang et al.14 investigated the plant growth-promoting effects of three Bacillus strains isolated from the cucumber rhizosphere: B. velezensis SX13, B. paralicheniformis SX21, and B. tequilensis SX31. These strains can dissolve phosphorus and produce auxin (IAA, except for B. tequilensis SX31). When inoculated into the cucumber plant rhizosphere, these strains improved root structure, photosynthetic parameters, growth rate, and biomass accumulation compared to control plants. B. velezensis SX13 showed the most significant effects, promoting nutrient absorption and transport, enhancing photosynthesis, and increasing the activities of key carbon and nitrogen metabolism enzymes. This leads to higher levels of sugars, proteins, and amino acids in the leaves, boosting the overall biomass, element accumulation, and fruit quality and yield. The benefits of B. velezensis SX13 were dose dependent, highlighting the need for optimal application rates.
Modern agriculture faces significant challenges, particularly the need to sustainably and environmentally feed the growing global population. The use of microorganisms, including fungi such as Trichoderma spp. and bacteria such as Bacillus spp., has been extensively studied for their positive effects on crops. Combining these microorganisms offers even greater potential for enhancing plant growth and biocontrol capabilities through diverse mechanisms. Co-inoculation of Trichoderma with different PGPRs has shown important synergistic effects, promoting plant growth, increasing nutrient uptake, and boosting crop yield.15 For instance, under greenhouse conditions, applying a cell suspension of T. atroviride with Bacillus sp. and Pseudomonas sp. increased banana plant biomass by 37%, surpassing the individual effects of each microorganism.16 Trichoderma combined with rhizobia, such as Rhizobium and Bradyrhizobium species, increases plant biomass, nutrient uptake, and crop yield in peanuts and is associated with higher chlorophyll, carbohydrate, and foliar protein content.17 Triple inoculations, such as those in faba beans with T. harzianum, Rhizobium leguminosarum biovar viciae, and B. subtilis, further amplified the synergistic effects on plant biomass, and N and P uptake.18 In addition, using different carriers such as oil cakes and maize granules to formulate Trichoderma–Bacillus/Pseudomonas co-inoculants significantly enhanced their synergistic effects, as observed in soybeans under controlled greenhouse conditions.19
The combination of Trichoderma with PGPR has the potential to create a synergistic effect, offering a holistic approach for enhancing rice growth and yield. This study aimed to develop a formulation that integrates T. asperellum MSU007 with the PGPR strain B. velezensis MSU01 and to evaluate its effectiveness in promoting rice growth. By configuring these microorganisms at different ratios (1:1, 2:1, and 3:1), we sought to determine the optimal formulation for enhancing rice seed germination, seedling development, and overall plant health. This study focused on assessing the viability and survival rates of microorganisms in the formulated products over time, their effect on rice seed germination, and their effectiveness in promoting the growth of rice plants. The ultimate goal was to develop a sustainable, cost-effective alternative to chemical fertilizers that can support high rice yields while preserving soil health and contributing to environmentally friendly agricultural practices.
Testing the coexistence of Trichoderma asperellum MSU007 and PGPR
T. asperellum MSU007 is a biocontrol agent for plant diseases. B. velezensis MSU01, which has been tested for its plant growth-promoting properties, was obtained from the Microbiology Laboratory, Department of Biology, Faculty of Science, Mahasarakham University. To test the coexistence of T. asperellum MSU007 and B. velezensis MSU01, T. asperellum MSU007 was grown on potato dextrose agar (PDA) and incubated at 28 °C for 5–7 days, whereas B. velezensis MSU01 was grown on nutrient agar (NA) using the cross-streak method and incubated under the same conditions. After incubation, a 0.8 cm diameter mycelium plug of T. asperellum MSU007 was cut and transferred to the center of a new PDA plate. B. velezensis MSU01 was streaked in two 2 cm long streaks on opposite sides, 2.5 cm away from the fungus. Incubation was at 28 °C for another 5 to 7 days, and the growth of both microorganisms was observed on the agar plate.
Development of a powdered bioformulation for T. asperellum MSU007 combined with PGPR
Preparation of microbial inoculum
To prepare the B. velezensis MSU01 inoculum, the cells were grown in nutrient broth (NB) and incubated at 28 ± 2 °C with shaking at 150 rpm for 48 h. The culture was then centrifuged at 6,000 rpm for 15 min to precipitate the cells. The cell pellet was washed with 0.85% NaCl and a cell suspension was prepared in 0.85% NaCl. The cell density was adjusted to 108 CFU/mL by measuring the absorbance at 600 nm using a spectrophotometer with an OD value of 0.2. The spore suspension of T. asperellum MSU007 was prepared by growing the fungus on PDA medium and incubating at 28 °C for 7 days. The spores were scraped off and dissolved in 0.85% NaCl. Spore density was counted using a hemocytometer and adjusted to 108 spores/mL.
Preparation of powdered bioformulation
To prepare 1,000 g of the basic formulation, 975 g of talcum powder, 15 g of calcium carbonate, and 10 g of carboxymethyl cellulose (CMC) were mixed thoroughly and autoclaved at 121 °C for 30 min. The mixture was allowed to sit overnight and then autoclaved again. Under sterile conditions, 400 mL of the prepared PGPR cell suspension was mixed, incubated overnight, and stored in a zip bag at room temperature.
To prepare the T. asperellum MSU007 basic formulation, 500 mL of the prepared T. asperellum MSU007 spore suspension was mixed with 995 g of sterilized talcum powder, and 5 g of carboxymethyl cellulose (CMC) added, mixed thoroughly, and incubated overnight. The mixture was stored in a zip bag at room temperature.
Three formulas of T. asperellum MSU007 inoculated powder with PGPR were prepared as follows:
Formulation 1: T. asperellum MSU007 and PGPR in a 1:1 ratio.
Formulation 2: T. asperellum MSU007 and PGPR in a 2:1 ratio.
Formulation 3: T. asperellum MSU007 and PGPR in a 3:1 ratio.
The components were mixed thoroughly under sterile conditions and then dried at 50 °C, 24 h before testing.
Evaluation of the bioformulation for T. asperellum MSU007 in combination with PGPR
To determine the water solubility of the formulation, 1 g of the formulation was dissolved in 99 mL of distilled water using a magnetic stirrer set at 200 rpm. The time required for each formulation to completely dissolve in water was recorded.
To measure the moisture content of the formula, the formula was placed into an aluminum foil cup and baked at 105 °C for 5 h, allowed to cool to room temperature and then weighed. Moisture content was calculated using the following formula20:
moisture content (%) = [(W2 – W1) – (W3 – W1) / (W2 – W1)] x 100
Where,
weight W1 = weight of aluminum foil cup (g)
W2 = weight of bioproduct before baking in aluminum foil cup (g)
W3 = weight of aluminum foil cup + bioproduct after baking (g)
Assessment of the viability of microorganisms in the bioformulation for Trichoderma asperellum MSU007 in combination with PGPR
The dilution plate count method was used to assess the survival of T. asperellum MSU007 in the formula after storage at room temperature for 1, 30, or 60 days. Powdered samples from each formula were used to perform serially diluted by selecting dilution factors of 10-4 and 10-5 with 0.1 mL each. Diluted powders were spread onto rose bengal agar medium, incubated at 28 °C for 48–72 h, and T. asperellum MSU007 colonies counted to determine viability.
The viability of PGPR in the formula after storage under the same conditions and time intervals was assessed. Serial dilutions of the powder bioformulations were prepared at dilution factors of 10-4 and 10-5 and plated onto NA medium. After incubation at 28 °C for 24–48 h, PGPR colonies were counted and viability was calculated based on colony counts.
Evaluation of the efficiency of Trichoderma asperellum MSU007 bioformulation combined with PGPR in enhancing rice growth
Assessment of the impact of the powdered bioformulation on seed germination
To evaluate the impact of the bioformulation on seed germination, glutinous rice seeds (variety Kiaw-ngu) were procured from Ban Khok Si, Khlong Kham Subdistrict, Yang Talat District, Kalasin Province, Thailand. The seeds were surface-sterilized by immersing them in a 5% Clorox solution for 5 min, followed by rinsing twice with distilled water (dH2O); each rinse lasted 1 min. The seeds were air-dried before being subjected to the following four treatments:
Treatment 1 The seeds were soaked in Formula 1 for 24 h
Treatment 2 The seeds were soaked in Formula 2 for 24 h
Treatment 3 The seeds were soaked in Formula 3 for 24 h
Treatment 4 The seeds were soaked in distilled water (dH2O) for 24 h
These methods were designed to determine the efficacy of different formulations in promoting seed germination.
Testing the effect of the powdered bioformulation on rice seedling growth
To assess the effect of the bioformulation on the growth of rice seedlings, a randomized complete block design (RCBD) experiment was conducted using the following four treatments:
Treatment 1: Potting soil with Formula 1 was mixed at a ratio of 10 g formula per 200 g soil.
Treatment 2: Potting soil with Formula 2 was mixed at a ratio of 10 g formula per 200 g soil.
Treatment 3: Potting soil with Formula 3 was mixed at a ratio of 10 g formula per 200 g soil.
Treatment 4: The control method involved planting without mixing any formula with the soil.
Sterilized rice seeds were planted in pots, with 20 seeds per pot. Each treatment was replicated thrice. The rice seedlings were cultivated for 21 days, during which their growth parameters were recorded. The measurements included plant height (from the base to the tip of the longest shoot), root length (from the base of the plant to the tip of the longest root), and fresh weight (both plants and roots were weighed to determine their fresh weight). The results of these measurements were used to evaluate the effectiveness of different formulas in promoting rice seedling growth.
Testing the coexistence of Trichoderma asperellum MSU007 and PGPR
In a coexistence assay involving T. asperellum MSU007 and the PGPR strain B. velezensis MSU01 conducted using a co-culture method, both isolates were capable of concurrent growth without mutual inhibition. T. asperellum MSU007 and B. velezensis MSU01 grew together without inhibiting each other, indicating their potential for combined use to enhance plant growth. Studies have shown that the combination of Trichoderma and Bacillus strains can enhance plant growth and increase resistance to pathogens. For example, co-inoculation with T. harzianum and B. subtilis significantly improves the growth of tomato plants and protects them against Fusarium oxysporum.21 These microorganisms can synergistically suppress plant pathogens and induce systemic resistance in plants.22
Development of a powdered bioformulation for T. asperellum MSU007 combined with PGPR
A bioformulation was developed by combining T. asperellum MSU007 with powdered PGPR. Each formulation was a white powder and demonstrated effective resolution. The development of a powdered bioformulation combining T. asperellum MSU007 with PGPR represents a significant advancement in agricultural biotechnology. This formulation offers a practical and efficient means of delivering beneficial microorganisms to crops, enhancing plant growth and health. The bioformulation was a white powder, indicating a successful and uniform mixture of T. asperellum MSU007 and PGPR. The powdered form is advantageous because of its ease of application, storage, and transport. The formulation demonstrated effective resolution, indicating that the microorganisms remained viable and effective in powder form. This is crucial for ensuring the efficacy of bioformulations in promoting plant growth and health when applied to crops. Powdered bioformulations offer several benefits over liquid formulations, including stability, longer shelf life, and ease of handling and application. Powdered formulations of beneficial microbes maintain their viability and efficacy for extended periods.23 Lui et al.24 investigated the synergistic effects of co-cultivating T. atroviride SG3403 and B. subtilis22 on biocontrol and plant promotion with a focus on wheat head blight. The study found that T. atroviride SG3403 was compatible with B. subtilis22, and their co-culture produced antagonistic compounds that inhibited Fusarium graminearum growth and enhanced pathogen-related protein activities. A seed-coating agent made from the co-culture significantly protected against F. graminearum by inducing host plant defense genes. The dry, powdered bioseed coating agent proved to be an effective bioavailable formulation, suggesting its potential as a new microbial bio-fungicide.
Evaluation of the powdered bioformulation for Trichoderma asperellum MSU007 in combination with PGPR
The evaluation of the bioformulations containing T. asperellum MSU007 and PGPR focused on two key parameters: water solubility and moisture content. These parameters are critical for ensuring the efficacy, stability, and ease of application of the bioformulations.
Water solubility
The solubility of a bioformulation is essential for its effective application, as it ensures that beneficial microorganisms are evenly distributed when applied in water. The dissolution times for the three bioformulations were 25.97 s, 25.40 s, and 23.63 s, respectively, showing no significant differences (Table 1). This consistency in solubility is crucial for practical applications in agricultural settings as it ensures that the active ingredients are evenly dispersed when mixed with water, facilitating uniform application and enhancing the effectiveness.23 The observation that all formulations eventually precipitated was common in the powdered formulations. However, the initial solubility ensures that microorganisms are available for immediate application.
Table (1):
Water solubility and moisture content of Trichoderma asperellum MSU007 bioformulation combined with PGPR
Bio-formulation |
Water solubility (s) |
Moisture content (%) (Mean ± SD) |
|---|---|---|
1. |
25.97 ± 3.21ns |
3.47 ± 2.20ns |
2. |
25.40 ± 3.57 |
2.13 ± 1.67 |
3. |
23.63 ± 0.85 |
2.07 ± 0.81 |
ns = not significant (p > 0.05, LSD test)
Measurement of moisture content
The moisture content of a bioformulation affects its shelf life, stability, and ease of handling. The moisture contents were 3.47%, 2.13%, and 2.07% for bioformulations 1, 2, and 3, respectively, with no statistically significant differences (Table 1). Low moisture content is beneficial for the stability and longevity of a formulation, and a low moisture content in bioformulations is crucial for preventing microbial degradation and extending shelf life. Moisture levels below 5% are generally considered favorable for maintaining the viability of microorganisms in powdered formulations.25 This method aligns with the standard practices for preparing stable microbial formulations.26 These results indicated that the bioformulations of T. asperellum MSU007 combined with PGPR were consistent in solubility and had low moisture content, making them suitable for agricultural use.
Assess the viability of microorganisms in the powdered bioformulation for T. asperellum MSU007 in combination with PGPR
Viability Assessment of T. asperellum MSU007 in bioformulation with PGPR
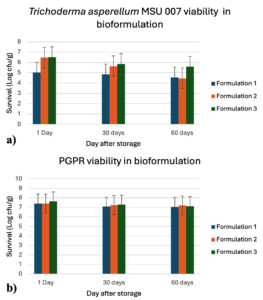
The viability of T. asperellum MSU007 in combination with the PGPR was assessed using three different bioformulations. This study found no statistically significant differences in the survival rates of T. asperellum MSU007 among the three bioformulations. Survival rates were monitored on days 1, 30, and 60 after storage, and the results are summarized in Figure 1a. After 1 day of storage, the survival rates of T. asperellum MSU007 were 5.02 ± 0.82, 6.47 ± 0.04, and 6.52 ± 0.09 log cfu/g for bioformulations 1, 2, and 3, respectively. After 30 days of storage, the survival rates decreased to 4.84 ± 0.12, 5.63 ± 0.03, and 5.85 ± 0.04 log cfu/g for bioformulations 1, 2, and 3, respectively. After 60 day of storage, the survival rates further declined to 4.55 ± 0.31, 4.46 ± 0.33, and 5.59 ± 0.03 log cfu/g for bioformulations 1, 2, and 3, respectively. The results indicated that while all three bioformulations maintained the viability of T. asperellum MSU007 over the 60 day storage period, formulation 3 consistently exhibited the highest survival rates.
Viability of PGPR in different bioformulations
This study assessed the viability of PGPR in three different bioformulations over a storage period of 60 days. The results are summarized in Figure 1b. After 1 day of storage, the survival rates of PGPR were 7.39 ± 0.09, 7.38 ± 0.04, and 7.62 ± 0.03 log cfu/g for bioformulations 1, 2, and 3, respectively. After 30 days of storage, the survival rates decreased to 7.07 ± 0.13, 7.23 ± 0.05, and 7.28 ± 0.02 log cfu/g for bioformulations 1, 2, and 3, respectively. After 60 days of storage, the survival rates further declined to 4 7.05 ± 0.07, 7.19 ± 0.08, and 7.11 ± 0.14 log cfu/g for bioformulations 1, 2, and 3, respectively. The results indicated that all three formulations effectively maintained PGPR viability over a 60 day storage period. Formulation 3 showed the highest initial viability and remained relatively stable over time.
Figure 1. Viability of microorganism in different formulations;
a) Trichoderma asperellum MSU 007 viability; b) PGPR viability
These results indicate that all three bioformulations were capable of maintaining the viability of both T. asperellum MSU007 and PGPR over a 60 day storage period. However, bioformulation 3 exhibited superior performance, particularly in maintaining the viability of T. asperellum MSU007. Microbial bioformulations have significant benefits, including sustainability, plant probiotic effects, and extended viability, making them a promising technology for future agriculture. To ensure the survival and effectiveness of microbial strains, various carrier materials are used to provide necessary nutrients and support. Selecting the right carrier material is crucial because it greatly influences the shelf life of microbial cells and the overall quality of the bioinoculants. Different carriers have distinct advantages and disadvantages, and their effectiveness can vary depending on the crops in which they are tested, each offering unique benefits.27 We utilized talcum powder and carboxymethyl cellulose (CMC) as carriers, similar to the work of Basheer et al.28 and Joshi et al.,29 who used talc and CMC as carriers to prepare bioformulations of Bacillus sp., Pseudomonas putida, and P. jessenii MP1. This bioformulation enhances seed germination and plant growth, stabilize microbial survival, and increase soil nutrient status in crops such as cowpeas, lady fingers, cucumbers, lettuce, and chickpeas.30 The use of talc as a carrier to prepare bioformulations of P. fluorescens enhanced plant growth and nutrient status, reducing the disease index in rice. Kumar et al.31 studied the shelf-life of T. viride in talc-based formulations. The formulation was prepared by adding three different volumes of T. viride biomass (30, 40, and 50 mL/g of talc). The initial mean cfu of T. viride for these volumes were 227.2 × 109, 256.00 × 109, and 291.03 × 109 cfu/g, respectively. Over 120 days of storage, the cfu decreased to 70.33 × 109, 80.67 × 109, and 96.67 × 109 cfu/g, corresponding to viability reductions of 69.04%, 68.48%, and 66.78%, respectively. Despite this decline, sufficient spores remained viable after 120 days, indicating the potential for longer storage.
Evaluation of the efficiency of T. asperellum MSU007 bioformulation combined with PGPR in enhancing rice growth
Assessment of the impact of the powdered bioformulation on rice seed germination
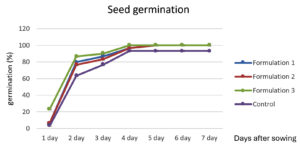
The effect of the bioformulation on rice seed germination was assessed over a 7-day period. The results are summarized in Figure 2. Bioformulations 1 and 2 showed similar trends in rice seed germination, with a gradual increase from 6.67% on the first day to 100% by the day 5, maintaining this rate until day 7. Bioformulation 3 exhibited the highest initial germination rate of 23.33% on the first day, reaching 100% germination by day 4 and maintaining this rate for 7 days. The control (dH2O) showed the lowest initial germination rate of 3.34%, with a slower increase in germination percentage than the other bioformulations (Figure 2). By day 5, germination reached 93.33% and remained at this level for 7 days. In summary, bioformulation 3 was the most effective in enhancing rice seed germination, achieving 100% germination by day 4, while bioformulations 1 and 2 reached 100% germination by day 5. The control was the least effective and did not achieve 100% germination within 7 days. These results align with those of previous studies, demonstrating the effectiveness of Trichoderma spp. and PGPR in promoting seed germination and plant growth. Harman et al.5 reported that Trichoderma spp. can enhance seed germination and seedling vigor owing to their ability to produce growth-promoting metabolites and control plant pathogens. Similarly, Vessey32 highlighted the role of PGPR in improving seed germination and plant growth through various mechanisms, including nutrient solubilization, production of growth hormones, and suppression of plant diseases. In this context, the higher initial germination rate observed with bioformulation 3 may be attributed to a more effective synergy between T. asperellum MSU007 and the specific PGPR used, leading to a more rapid enhancement of seed germination conditions. This indicates that the formulation and combination of microbial inoculants play crucial roles in the effectiveness of bioformulations.
Testing the effect of the powdered bioformulation on rice seedling growth
The effects of the bioformulation on rice seedling growth were evaluated by measuring various growth parameters, as summarized in Table 2. The results indicated that bioformulation 3 was the most effective in promoting rice seedling growth, including shoot length, root length, shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight.
Table (2):
Plant growth promotion of the powdered bioformulation on rice seedling growth
| Formulation | Rice seedling growth | |||||
|---|---|---|---|---|---|---|
| Shoot length (cm) (Mean ± SD) | Root length (cm) (Mean ± SD) | Shoot fresh weight (g) (Mean ± SD) | Root fresh weight (g) (Mean ± SD) | Shoot dry weight (g) (Mean ± SD) | Root dry weight (g) (Mean ± SD) | |
| 1. | 26.94 ± 1.10ab | 12.41 ± 0.63ab | 0.77 ± 0.03b | 0.84 ± 0.05a | 0.39 ± 0.02b | 0.33 ± 0.032ns |
| 2. | 28.73 ± 2.41a | 12.66 ± 0.92ab | 0.78 ± 0.03b | 0.89 ± 0.03a | 0.42 ± 0.03ab | 0.36 ± 0.02 |
| 3. | 30.02 ± 2.97a | 14.23 ± 0.98a | 0.88 ± 0.03a | 0.95 ± 0.05a | 0.47 ± 0.05a | 0.40 ± 0.02 |
| Control (dH2O) | 23.40 ± 0.82b | 9.56 ± 3.12b | 0.66 ± 0.03c | 0.71 ± 0.08b | 0.36 ± 0.03b | 0.31 ± 0.01 |
The means followed by the same letter were not significantly different according to least significant difference test (LSD), P < 0.05
ns = not significant (p > 0.05, LSD test)
Shoot length
Bioformulation 3 had the longest shoot length (30.02 cm), which was significantly greater than that of the control (23.40 cm). Bioformulations 1 and 2 also promoted shoot length (26.94 cm and 28.73 cm, respectively), compared to the control. Previous research supports these findings, demonstrating that Trichoderma spp. can enhance shoot growth by producing phytohormones and facilitating nutrient uptake.5,22
Root length
Bioformulation 3 exhibited the greatest root length (14.23 cm), followed by bioformulations 2 (12.66 cm) and 1 (12.41 cm), with the control exhibiting the shortest root length (9.56 cm). Trichoderma and PGPR promote root development by producing indole-3-acetic acid (IAA) and other growth-promoting substances.21
Shoot fresh weight
Bioformulation 3 had the highest shoot fresh weight (0.88 g), which was significantly higher than that of the control (0.66 g). Bioformulations 1 and 2 also increased shoot fresh weight compared to that of the control. Enhanced nutrient uptake and stress tolerance conferred by Trichoderma spp. and PGPR likely contributed to the increased shoot biomass.5
Root fresh weight
All bioformulations significantly increased the root fresh weight compared to the control. Bioformulation 3 had the highest fresh root weight (0.95 g), followed by bioformulations 2 (0.89 g) and 1 (0.84 g). Improved root length and increased root biomass were consistent with the beneficial effects of the bioformulations on plant growth.33
Shoot dry weight
Bioformulation 3 resulted in the highest shoot dry weight (0.47 g), followed by bioformulations 2 (0.42 g) and 1 (0.39 g). The control group had the lowest shoot dry weight (0.36 g). These findings are consistent with those of previous studies showing that Trichoderma spp. can enhance plant dry matter accumulation by improving nutrient-use efficiency and pathogen suppression.7
Root dry weight
Although root dry weight differences were not significant across formulations, bioformulation 3 showed the highest value (0.40 g), whereas the control had the lowest value (0.31 g). This suggests that, even without significant differences, the trend toward increased root biomass with bioformulations indicates potential long-term benefits for plant growth and health.5
We also investigated the impact of the bioformulations on plant growth and yield, specifically focusing on rice seedlings. Shivangi et al.34 examined the effects of seed biopriming with various bioagents, including PGPR-1, a rhizobial biofertilizer (Rhizobium strain B1), and the biological control agent T. viride, on growth, yield, and disease incidence in French bean cv. Contenders during the 2017 and 2018 kharif seasons. Similarly, our results showed that seed biopriming with PGPR-1 and Rhizobium strain B1 significantly improved various plant growth parameters and yield metrics compared to carbendazim seed treatment and untreated control. Bioformulation 3 was the most effective in promoting rice seedling growth, demonstrating the greatest improvements in shoot and root lengths, as well as fresh and dry weights of shoots and roots. Bioformulations 1 and 2 also showed positive effects compared with the control but to a lesser extent than bioformulation 3. These results highlight the potential of T. asperellum MSU007 combined with PGPR to significantly enhance plant growth and development.
The development and evaluation of a bioformulation combining T. asperellum MSU007 and the PGPR, B. velezensis MSU01, demonstrated significant potential for enhancing rice growth. Coexistence assays confirmed that T. asperellum MSU007 and B. velezensis MSU01 can grow together without mutual inhibition, forming the basis for a stable and effective bioformulation. The powdered bioformulation exhibited effective water solubility and stable moisture content, indicating its practicality for agricultural use. Viability assessments over a 60 day storage period indicated that both T. asperellum MSU007 and the PGPR maintained high survival rates, with bioformulation 3 (T. asperellum MSU007 and B. velezensis MSU01 in a 3:1 ratio) showing the highest viability. This bioformulation proved to be the most effective in enhancing rice seed germination and seedling growth, achieving 100% germination by the fourth day and significantly improving shoot and root lengths, as well as the fresh and dry weights of shoots and roots. Bioformulations 1 and 2 also improved rice growth parameters but were less effective than bioformulation 3. Overall, the Trichoderma-PGPR bioformulation, especially bioformulation 3, is a promising solution for enhancing rice growth and potentially improving crop yields. This study provides a solid foundation for further research and potential commercialization of bioformulations to support sustainable agriculture.
ACKNOWLEDGMENTS
The authors are thankful to Mahasarakham University for the financial support and research equipments. The authors are also grateful to Dr. Adrian Roderick Plant for English language editing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This research project was financially supported by the Mahasarakham University, Thailand, with grant No. 6802038/2568.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Sen S, Chakraborty R, Kalita P. Rice – not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci Technol. 2020;97:265-285.
Crossref - Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418(6898):671-677.
Crossref - Zhang W, Dou Z, He P, et al. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc Natl Acad Sci USA. 2013;110(21):8375-8380.
Crossref - Pretty J. Agricultural sustainability: concepts, principles and evidence. Phil Trans R Soc B. 2008;363(1491):447-465.
Crossref - Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2(1):43-56.
Crossref - Benitez T, Rincon AM, Limon MC, Codon AC. Biocontrol mechanisms of Trichoderma strains. Int Microbiol. 2004;7(4):249-260.
- Lorito M, Woo SL, Harman GE, Monte E. Translational research on Trichoderma: From ‘Omics to the field. Annu Rev Phytopathol. 2010;48:395-417.
Crossref - Zin NA, Badaluddin NA. Biological functions of Trichoderma spp. for agriculture applications. Ann Agric Sci. 2020;65(2):168-178.
Crossref - Yedidia I, Srivastva AK, Kapulnik Y, Chet I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil. 2001;235(2):235-242.
Crossref - Vinale F, Nigro M, Sivasithamparam K, et al. Harzianic acid: a novel siderophore from Trichoderma harzianum. FEMS Microbiol Lett. 2013;347(2):123-129.
Crossref - Yu Z, Wang Z, Zhang Y, Wang Y, Liu Z. Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil. Microbiol Res. 2021;242:126596.
Crossref - Kloepper JW, Ryu CM, Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathol. 2002;94(11):1259-1266.
Crossref - Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica (Cairo). 2012:963401.
Crossref - Wang J, Qu F, Liang J, Mingfei Y, Hu X. Bacillus velezensis SX13 promoted cucumber growth and production by accelerating the absorption of nutrients and increasing plant photosynthetic metabolism. Sci Hortic. 2022;301:111151.
Crossref - Poveda J, Eugui D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas):Development of microbial synergistic bio-inoculants in sustainable agriculture. Biological Control. 2022;176:105100.
Crossref - Chaves NP, Pocasangre LE, Elango F, Rosales FE, Sikora R. Combining endophytic fungi and bacteria for the biocontrol of Radopholus similis (Cobb) Thorne and for effects on plant growth. Sci Hortic. 2009;122(3):472-478.
Crossref - Neelipally RTKR, Anoruo AO , Nelson S. Effect of co-inoculation of Bradyrhizobium and Trichoderma on growth, development, and yield of Arachis hypogaea L. (Peanut). Agron. 2020;10(9):1415.
Crossref - Firdu Z, Alemu T, Assefa F. The synergistic effects of Trichoderma harzianum AAUT14 and Bacillus subtilis AAUB95 on faba bean (Vicia faba L.) growth performance and control of chocolate spot compared to chemical fungicides under greenhouse conditions. Arch Phytopathol Plant Prot. 2021;55(2):1-14.
Crossref - Muthukumar A, Eswaran A, Sangeetha G. Induction of systemic resistance by mixtures of fungal and endophytic bacterial isolates against Pythium aphanidermatum. Acta Physiol Plant. 2011;33:1933-1944.
Crossref - Sawatphanit N, Sutthisa W, Kumlung T. Bioformulation developmentof Bacillus velezensis strain N1 to control rice bacterial leaf blight. Trends Sci. 2022;19(21):6315.
Crossref - Vinale F, Sivasithamparam K, Ghisalberti EL, et al. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol Mol Plant Pathol. 2008;72(1-3):80-86.
Crossref - Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48:21-43.
Crossref - John RP, Tyagi RD, Prevost D, Brar SK, Pouleur S, Surampalli RY. Bio-encapsulation of microbial cells for targeted agricultural applications. Biotech Advances. 2010;28(4):496-507.
Crossref - Liu H, Li T, Y Li, Wang X, Chen J. Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the biocontrol of wheat head blight. J Fungi. 2022;8(12):1250.
Crossref - Arora NK, Tewari S, Singh S, Lal N, Maheshwari DK. PGPR for protection of plant health under saline conditions. In: Maheshwari D (eds) Bacteria in Agrobiology: Stress Management Springer (Berlin). 2011:239-258.
Crossref - Young CC, Rekha PD, Lai WA, Arun AB. Encapsulation of plant growth promoting bacteria in alginate beads enriched with humic acid. Biotechnol Bioeng. 2006;95(1):76-83.
Crossref - Khan A, Singh AV, Gautam SS, et al. Microbial bioformulation: a microbial assisted biostimulating fertilization technique for sustainable agriculture. Front Plant Sci. 2023;14:1270039.
Crossref - Basheer J, Ravi A, Mathew J, Krishnankutty RE. Assessment of plant-probiotic performance of novel endophytic Bacillus sp. in talc-based formulation. Probiotics Antimicrob Proteins. 2019;11(1):256-263.
Crossref - Joshi D, Chandra R, Chandra SD, Kumar S, Goel R. Impacts of bioinoculants Pseudomonas jesenii MP1 and Rhodococcus qingshengii S10107 on chickpea (Cicer arietinum L.) yield and soil nitrogen status. Pedosphere. 2019;29(3):388-399.
Crossref - Saravanakumar D, Harish S, Loganathan M, et al. Rhizobacterial bioformulation for the effective management of Macrophomina root rot in mungbean. Arc Phytopathol Plant Protect. 2007;40(5):323-337.
Crossref - Kumar S, Kumar R, Om H. Shelf-life of Trichoderma viride in talc and charcoal based formulations. Indian J Agril Sci. 2013;83(5):566-569.
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255(2):571-586.
Crossref - Compant S, Duffy B, Nowak J, Clement C, Barka EA. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol. 2005;71(9):4951-4959.
Crossref - Shivangi N, Bharat NK, Manish K. Effect of seed biopriming with indigenous PGPR, Rhizobia and Trichoderma sp. on growth, seed yield and incidence of diseases in french bean (Phaseolus vulgaris L.). Legume Research. 2021;44(5):593-601.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.