Bacteria that are important for nutrition and health predominantly live in a healthy gut. Gut dysbiosis results from alterations in gut homeostasis. Contemporary probiotics are used to treat gastrointestinal (GI) problems. Probiotic-producing genera include Lactobacillus, Clostridium, Bifidobacterium, and Streptococcus, which account for many probiotic strains currently in use. Recent improvements in culturomics, using new methods combined with gnotobiotic animal models, offer a solid foundation for the development of innovative host-specific probiotic treatments. The GI tract begins from the mouth and ends at the anus, and it controls food consumption and digestion. Along with aiding food digestion, the GI tract acts as an immune system and a physical barrier against potentially hazardous germs, foreign objects, and antigens. The principal location of nutritional absorption is the gut, which includes the stomach and the small and large intestines. Contemporary probiotics contain well-characterized live microbes that can manipulate the gut and provide health benefits. Based on the available literature, the normal gut microbiota can be restored to preserve gut integrity and host health. Changes or dysfunctions in the microbiome can lead to various illnesses, such as inflammatory bowel disease, obesity, and autoimmune disorders. Prebiotics, probiotics, and fecal microbiota transplantation are only a few of the treatment strategies discussed in this article, along with their advantages, drawbacks, and potential future research areas. Furthermore, it highlights the current studies linking the gut microbiota to COVID-19 and their potential implications for disease treatment and prevention. A topic on the future of microbiome research and how it will enhance general wellness is presented in the article’s conclusion.
COVID-19, Gut-Brain Axis, Gut-Skin Axis, Oral Microbiota, Therapeutic Interventions
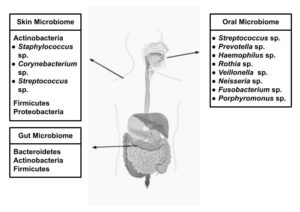
The human body harbors trillions of microbes, collectively with their genetic material, called the “microbiome”. The total number of protozoa, archaea, eukaryotes, viruses, and predominantly bacteria in and on the body is ten times that in human cells. Most of these microbes are beneficial; however, the human body does not function well in their absence. Figure 1 shows the different types of human microbiota and the key microbial species involved. Humans are hybrids of primates and microbes that co-evolve with the bacteria, bacteriophages, viruses, yeasts, and fungi that live in the gut. The gut microbiome plays an essential role in maintaining human health, which contains nearly 10-100 trillion microbial cells and primarily comprises various bacterial commensals and opportunistic bacteria in a homeostatic ratio.1 The gut microbiome is a key immunological defense system, continuously safeguarding the impact against opportunistic bacteria.2 The establishment of “war and peace” at the gut mucosal surface and a constant state of “tug of war” inside the human body results from the coexistence of microbial consortia inside the gut.
Figure 1. Illustration showing the different types of human microbiota and their associated key microbial species
The human body contains different types of microorganisms depending on the ecology in which they reside, such as oily areas on the scalp and back, dry areas on the forearms, and wet areas on the mouth and nose. The gut is the largest and most significant habitat. It is the most complex and diverse and plays a significant role in fighting infections, stimulating and suppressing the immune system, and signaling cells. Any change in the microbiome brought on by aging, nutrition, pharmaceuticals, ethnicity, geography, and way of life can lead to diseases, medications, and health system dysfunction.3 They even regulate our metabolism. If it is not operating properly for whatever reason as a result of what we eat or the antibiotics we take, it may result in several diseases, such as colon cancer, colitis, obesity, and diabetes. The bacteria in the gastrointestinal system are referred to as the gut microbiome and exist mutually, benefiting health and providing a safe environment. Every individual is born with a certain type of microbiome. Microorganisms such as bacteria, fungi, parasites, and viruses live all over the body’s surface as part of an ecosystem. Similar to fingerprints, an individual’s microbiome is unique. It changes over time as people are exposed to new people, environments, and diets. The largest numbers of microbes live in the small and large intestines, and they play a major role in body functions, supporting human health by boosting the immune system and aiding in food digestion; thus, they are referred to as supporting organs.
Autism, anxiety, obesity, autoimmune diseases, and even cancer have all been related to disturbances in the gut flora.4 A diversified microbiome is necessary for good health; the more diverse the microbiome, the more likely the person is to be in good health. A shift in gut microflora is one of the hallmarks of aging. Compared to the microbiome of young and healthy people, the gut microbiome of older people is often less diverse.
Oral Microbiome
The oral microbiome serves as a gateway to the gut. It is an ecosystem of microorganisms, primarily bacteria, found in the mouth. The microbial community in the oral cavity comprises commensal, symbiotic, and pathogenic microorganisms. Papenfort and Bassler investigated the synthesis of diverse, complex natural products, including macrolides and polyketides, which are diffusible quorum-sensing compounds exhibiting significant antibacterial and immunomodulatory properties by facilitating communication between microbes and their hosts.5 The oral microbiota resides in saliva, on gum tissue and teeth surfaces, and within biofilms. These microorganisms play a significant role in maintaining oral homeostasis, protecting the oral cavity, and preventing disease development. The major genera with the largest representation in the oral cavity include Streptococcus, Prevotella, Haemophilus, Rothia, Veillonella, Neisseria, Fusobacterium, and Porphyromonas.6 Endogenic and exogenic factors such as nutrition, smoking, alcohol, antibiotics, and pregnancy can all affect the oral microbiota.7 Thus, the physiological change can disrupt the microbial balance in the oral cavity by promoting hazardous bacteria and decreasing healthy bacteria. This causes various oral infectious disorders such as dental caries and periodontitis.
According to previous studies, the oral microbiome is crucial for preserving oral health and preventing diseases, including dental caries, periodontal disease, and oral cancer.8 S. salivarius, for instance, has been shown to prevent the growth of harmful bacteria in the oral cavity, while other bacterial species, such as S. mutans, are significantly associated with dental caries.9
The skin microbiome
It is now known that the trillions of microorganisms in our gut are crucial to our health. Various microbiomes have recently received increasing attention. The skin microbiome is composed primarily of bacteria but also includes a variety of archaea, fungi, and viruses. Without these microorganisms, the skin cannot act as the first line of defense against diseases. For instance, the skin is the home to beneficial microorganisms like Cutibacterium acnes, which break down the greasy substances produced by the sebaceous glands to produce an acidic environment that is difficult for pathogens to colonize.10 Staphylococcus epidermidis and other commensal bacteria generate antimicrobial chemicals that kill potential invaders.11 Recent developments in the study of microbial genomes explain that there are various strains of this bacterium, and not all cause acne. Antibiotics are frequently used for treatment of acne, but they attack all Cutibaterium strains, including commensal strains.
Another crucial function played by S. epidermidis is the production of substances that strengthen the tight connections between skin cells and safeguard the skin’s structural integrity.12 These bacteria strengthen our immune systems while also bolstering physical barriers. S. epidermidis, for instance, interacts with regulatory T cells shortly after birth, effectively teaching the immune system to tolerate commensal skin microorganisms, while remaining vigilant against intruders. This delicate, well-balanced environment is vital to human health, but occasionally loses equilibrium.
Another inflammatory condition linked to changes in the normal skin microbiota is eczema. Staphylococcus aureus skin infections are frequently associated with this condition. These microorganisms can break down tight connections between epidermal cells, allowing them to enter and inflame the dermis. According to a recent study, specific commensal Staphylococcus strains release an antibiotic peptide that inhibits the growth of harmful Staphylococcus aureus.13 Recent studies have demonstrated the potential of probiotics against pathogens.14
The gut microbiome
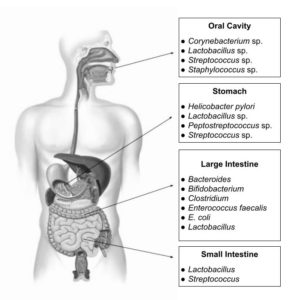
The large community of microbes inhabiting the GI tract is essential for many facets of human health, including digestion, metabolism, immunological response, and mental health. Figure 2 shows the distribution of different microorganisms throughout the GI tract. The gut is home to trillions of microorganisms, and it shows that any alterations in the gut microbiome can lead to changes in immune function, leading to the development of various diseases, including cancer, allergies, and autoimmune diseases.15 The composition of the gut microbiome is different in people with obesity and metabolic diseases, and these differences can affect how the body uses food and energy.16
Several studies have also suggested that treatments that concentrate on the gut microbiome, like probiotics or fecal microbiota transplantation, may be useful in enhancing metabolic health outcomes.17 Gut dysbiosis results from any alteration to the healthy gut homeostasis. Contemporary probiotics are used to treat GI problems. These genera include Lactobacillus, Clostridium, Bifidobacterium, and Streptococcus, which account for most probiotic strains currently in use. Recent developments in culturomics have made it possible to produce unique, host-specific probiotic treatments by combining modern methods with gnotobiotic animal models.18 The most recent advancements in the development of microbe-based medications, which are being utilized to treat a wide range of illnesses, have been highlighted in this review article.
Studies suggest that the gut microbiome may contribute to neurological and mental health issues.19 The changes in the gut microbiota are linked to changes in brain structure and behavior. Therapies that focus on the gut microbiome may be useful in treating neurological diseases such as Parkinson’s disease, Alzheimer’s disease, and depression.20
The gut-skin axis
The gut-skin axis also plays a major role in healthy well-being. Psoriasis is a chronic inflammatory skin disorder affecting millions of people worldwide. Although the exact origin of psoriasis remains unknown, recent findings indicate that the gut microbiota may significantly influence the pathophysiology of the condition.21 An important field of study investigating the link between gut microbiota and skin health is the gut-skin axis, which refers to the bidirectional communication between the gut and the skin. Celoria and co-workers reported that gut dysbiosis and leaky gut significantly contribute to the development of psoriasis, possibly by influencing the immune system and inducing inflammation.22 Therapeutic approaches that target the gut microbiota, such as probiotics, prebiotics, and dietary changes, may provide viable psoriasis therapy alternatives.23
Microbes and disease connection
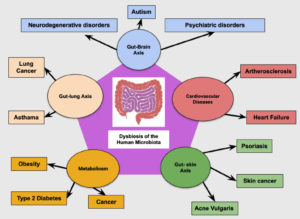
An imbalance in gut flora is known as gut dysbiosis. When gut dysbiosis occurs, beneficial bacteria are lost, potentially harmful bacteria rapidly multiply in the gut, or there may be fewer bacteria in the gut (Figure 3).
Cancer
Cancer is a complex disease resulting from a combination of hereditary and environmental factors. According to recent findings, the human microbiome may contribute to the development and spread of cancer.24 Many malignancies, including colorectal, liver, gastric, and pancreatic cancers, are linked to gut dysbiosis. The ability of the gut microbiome to affect the immune system, control inflammation, and produce metabolites that can either promote or inhibit tumor growth is considered to be the reason for its effect on cancer.25 Moreover, while some gut bacteria produce anti-cancer chemicals, others can produce carcinogenic substances. Hence, the composition of the gut microbiome can substantially affect cancer prevention and treatment.
The synthesis of metabolites such as short-chain fatty acids (SCFAs), which can have anti-inflammatory and antitumor effects, is one way the gut microbiota may contribute to cancer.26 According to studies, certain bacterial species, such as Fusobacterium nucleatum, are linked to a higher risk of colorectal cancer.27,28 Further studies are required to completely comprehend the mechanisms underlying the complicated and multifaceted interaction between cancer and the gut. However, the gut microbiome plays a significant role in the emergence of cancer and may be a useful target for cancer prevention and treatment.
Cardiovascular diseases
The human microbiome has been linked to cardiovascular disease in several ways. Certain bacteria in the gut microbiome can produce compounds such as trimethylamine N-oxide (TMAO), which is associated with an elevated risk of cardiovascular disease. TMAO is thought to promote atherosclerosis or plaque build-up in the arteries by enhancing cholesterol deposition and promoting inflammation.29
The gut microbiota impacts the immune system, which may further affect the development of atherosclerosis and other cardiovascular disorders.30 Chronic inflammation, a major factor in the emergence of atherosclerosis, has been linked to dysbiosis in the gut microbiota.31 According to recent studies, the oral microbiome may also influence cardiovascular disease. Studies have found that certain bacteria in the oral microbiome, such as Porphyromonas gingivalis, may promote inflammation and contribute to the development of atherosclerosis.32 One study provides evidence for a potential link between periodontitis and colorectal cancer, as well as a potential mechanism through which the oral microbiome may influence cancer development and progression.33 Researchers suggest that periodontitis may contribute to cardiovascular disease through chronic inflammation and the promotion of atherosclerosis.34
These findings suggest that the human microbiome plays a significant role in developing CVDs. Altering microbiome composition may be a promising therapeutic approach for preventing or treating cardiac illnesses.
Respiratory tract infection
Prevotella and Bifidobacterium are abundant among tuberculosis (TB) patients.35 Functional investigations have revealed decreased production of vitamins and amino acids and enhanced butyrate and propionate metabolism in TB individuals. Therefore, these results indicate a possible role of microbial dysbiosis in the pathogenesis of TB by improving the anti-inflammatory milieu in the host. Through the aid of next-generation sequencing technologies, researchers made progress in understanding the gut microbiome’s (GM) role in the immune system development and homeostasis, host nutrition, and metabolic disorders.36 The suggested “gut-lung axis” has been linked to the alteration of GM composition and function in the development of lung illness.37 Marsland et al.38 and Budden et al.39 predicted the cross-talk between the respiratory and gut mucosal surfaces, which permits the bidirectional flow of microbial products, metabolites, and cytokines; this is something we are only now starting to comprehend. Lung diseases, such as cystic fibrosis and asthma, have been linked to GM. In the TB patient population increased number of Bacteroides has been reported.40 Studies have shown an antagonistic relationship between Bacteroides and Prevotella in GM,41 and it has been observed that Prevotella species are colonizing better in the gut when consuming plant-derived carbohydrates.
Inflammatory bowel disease
The development and progression of inflammatory bowel disease (IBD) are significantly influenced by the gut microbiome. Studies have found that people with IBD have an imbalanced gut microbiota, leading to Crohn’s disease and colitis.42 This imbalance can cause intestinal lining damage and inflammation, resulting in symptoms such as diarrhea, weight loss, and stomach pain.
Porras and co-workers used metagenomics, proteomics, and functional assays of bacterial species associated with IBD capable of degrading extracellular matrix components (ECM). Researchers have discovered that several bacterial species are associated with IBD and can destroy the ECM.43 The findings suggest that targeting these specific gut commensals and their enzymatic activities could be a potential therapeutic strategy for treating IBD by preserving the integrity of the ECM and reducing inflammation. However, further studies are required to understand the complex relationship between the host immune system and the gut bacteria involved in the pathogenesis of IBD.
Chronic kidney disease
Chronic kidney disease (CKD) is a degenerative illness characterized by a steady decline in kidney function. According to recent research, the pathophysiology and development of CKD may be significantly influenced by the gut microbiota. Patients with CKD have a unique gut microbiome profile compared to healthy people, with less microbial diversity and alterations in the abundance of bacterial taxa.44 These alterations in the gut microbiome have been associated with oxidative stress, systemic inflammation, and other elements that accelerate the development of CKD. Patients with CKD have elevated serum levels of various components such as creatinine and urea because the kidneys cannot filter them out, and many other end-metabolites are also high in their blood. Therefore, when the urea concentration increases, gut bacterial strains can metabolize urea and produce harmful uremic toxins. These uremic toxins cause inflammation and alter gut integrity. In addition, the high levels of urea and uremic toxins produced by these harmful bacterial strains also lead to a decrease in beneficial bacteria or bacterial strains that may have an anti-inflammatory effect on the gut.45 Moreover, probiotics and prebiotics, the two therapies that modify the gut microbiome, may be therapeutic in CKD.46
Obesity
There is compelling evidence, based on studies examining the connection between healthy gut microbiota and obesity, that changes in the composition and function of the gut microbiota may lead to obesity and other related metabolic diseases.47,48 It has been demonstrated that obese individuals have a distinct gut microbiota from lean ones, with more Firmicutes and fewer Bacteroidetes.49 Many of which are involved in the release of short-chain fatty acids and the control of gut hormone release; this change in microbiota composition may affect energy balance and glucose metabolism.50
Moreover, obesity-related diseases, such as type 2 diabetes and non-alcoholic fatty liver disease, have been linked to inflammation and immune system dysregulation.51 These processes may be influenced by the gut microbiota owing to their effects on immune system activity and gut barrier function. Despite various obstacles, there is considerable interest in the therapeutic potential of alterations in the gut microbiota in the management of obesity and associated disorders. Promising outcomes have been reported in several prospective studies investigating probiotics, prebiotics, and fecal microbiota transplantation as prospective treatments.52 However, further research is needed to determine the efficacy and safety of these interventions and to establish optimal strategies for developing personalized microbiota-based therapeutics.
Gut-brain connection in neurological diseases
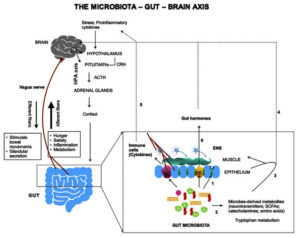
The gut microbiome plays a crucial role in maintaining the overall health of the host by regulating various physiological processes such as digestion, metabolism, and immune function. Recent studies have also shown that alterations in the gut microbiome may contribute to the pathogenesis of neurological diseases such as autism spectrum disorder (ASD), Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis.52-54 Dysbiosis is responsible for neurological diseases as it refers to the imbalance of the gut microbial community, which produces pro-inflammatory molecules that can cause inflammation and damage the central nervous system (CNS) (Figure 4). According to a study published in Cell Reports, changes in myelination of the prefrontal cortex, which is essential for healthy brain function, are linked to altered gut microbiomes in mice. Changes in the gut microbiome are also associated with alterations in behavior and cognition.55 Transplanting a normal microbiome into altered mice partially reverses these changes. While this study focused on the potential connection between the gut microbiome and brain function, further investigation is necessary to completely comprehend the intricate relationships between the gut microbiome and human brain function.
Figure 4. Microbiota and the gut-brain axis. The gut-brain axis forms a bidirectional network involving three major pathways-the neural pathway (vagus nerve, enteric nervous system), the immune pathway (cytokines), and the endocrine pathway80
The gut-Covid connection
The human gut is one of the largest organs in the body and regulates the immune response. It also helps protect against different infections like SARS-CoV-2 (or COVID-19). In the context of COVID-19, researchers have discovered that patients infected with the disease have a gut microbiome distinct from those not affected by the disease. Patients with COVID-19 lack beneficial microorganisms that contribute to strong immune responses. During COVID-19, gastrointestinal symptoms such as diarrhea, nausea, and vomiting are common.56 The respiratory and gastrointestinal tracts are additional targets for SARS-CoV-2 infection owing to their high levels of angiotensin-converting enzyme-2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) expression. Numerous human illnesses, including ulcerative colitis, Crohn’s disease, diabetes, obesity, cancer, and a few viral infections, have strong immunomodulatory effects on microbiota.
Whether the changes in the gut microbiota seen in COVID-19 patients result from or are the cause of the illness remains a matter of debate. However, there is evidence that healthy gut microbiota might boost the immune system and possibly ward off illnesses such as COVID-19. Several studies have revealed that those with diverse and healthy gut microbiota typically respond to infections better and have a stronger immune system.57 In contrast, changes in the gut microbiome, such as those brought on by antibiotic usage or a poor diet, can weaken the immune system and make a person more vulnerable to infections.
An in-depth analysis of the connection between the microbiota and COVID-19 has revealed an intricate interaction between the microbiota, host genetics, and environmental factors that influence the severity of the illness. Gang and coworkers revealed modifications in the bacterial diversity, density, and composition of COVID-19 patients’ microbiotas.58 These modifications are thought to play a role in the dysregulation of the immune response, intensifying inflammation, and damaging tissue. Therefore, the authors emphasize the importance of additional investigations into the connection between the microbiota and COVID-19 and the potential for microbiome-focused therapeutic approaches to enhance COVID-19 patient outcomes.
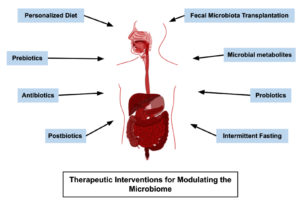
Therapeutic interventions for a healthy human microbiome
In the context of the human microbiome, the term “therapeutic interventions” refers to the application of various techniques to alter or manage the microbial population within the body to improve health or treat diseases. Some common treatment techniques are illustrated in Figure 5. The following sections will discuss a closer look at these therapeutic interventions for promoting a healthy human microbiome.
Probiotics
In the early 20th century, Nobel winner Metchnikoff proposed probiotics (meaning “for life”), hypothesizing that consuming yogurt’s helpful bacteria could postpone senility and enhance health. The demand for probiotics as nutritional supplements has steadily increased over the past few years owing to efforts to enhance lifestyle and food quality.59 These foods contain live bacteria and are recommended for maintaining a balanced population of bacteria in the intestines. Several yeast and bacterial strains are considered to be probiotics. Fermentation produces probiotic foods, such as curd and kimchi, which contain vitamin B, folic acid, and other minerals. Probiotic foods have a wide range of health advantages; however, there are some limitations to consider. One disadvantage is that not all probiotic strains have been extensively investigated, making it difficult to choose optimal strains for specific medical conditions.60 Probiotic results may vary depending on a person’s microbiome and general health. Another problem is that some probiotic foods may not contain sufficient live and active cultures to improve health.
Prebiotics
Prebiotics promote the growth of beneficial gut microbes. Prebiotic meals are typically made of complex carbohydrates or fibers, which are usually indigestible by human cells but can feed on some types of intestinal bacteria. The potential roles of numerous prebiotic compounds have been previously investigated. The three most commonly used prebiotics are fructose, galacto-oligosaccharides and trans-galacto-oligosaccharides. The fermentation of prebiotics by gut microbiota results in the production of short-chain fatty acids (SCFA), including lactic acid, butyric acid, and propionic acid. For instance, propionate affects bone marrow-derived dendritic cells, macrophages, and T helper 2 in the airways.61 The pH of the colon is lowered by SCFAs.62 Other prebiotic fermentation products, such as xylo-oligosaccharides, inulin, lactulose, and galacto-oligosaccharides, can boost the body’s natural defenses against harmful microbes.63 Prebiotics, in short, are foods that support the growth of good bacteria in our gut, assisting in maintaining gut health. Bananas are among the best prebiotic foods because they contain inulin fiber, which provides food for beneficial bacteria in the stomach. Rice, potatoes, sago, and casava are excellent prebiotics. Some people may experience gastrointestinal discomfort due to various prebiotic fibers, including bloating. This could be due to the fermentation of prebiotics by gut bacteria, which produce gas as a byproduct. However, these symptoms are usually mild and temporary, and can be improved by gradually increasing prebiotic-rich foods and drinking plenty of water.
Regular exercise
Several aspects of the human body including the microbiome benefit from regular exercise. Researchers have discovered that exercise increases the diversity of beneficial bacteria in the stomach in animal and human trials.64 Multiple studies have highlighted how exercise and food can improve gut health.65 Additionally, studies have shown that regardless of diet, exercise has the power to alter the makeup and functionality of gut bacteria. High-intensity aerobic exercise and longer workouts were found to have the biggest effects on gut bacteria diversity and function in relation to general health.66 They also observed that slim people are more likely to benefit from exercise than people who are overweight or obese.
Reduce stress
Consider the “butterflies” sensation that you get when excited or frustrated. Gut health experts frequently mention the “gut-brain connection” and refer to the gut as “the second brain.” According to research, anxiety and depression are affected by the gut and vice versa; they can raise the risk of IBS, and people with IBS are more likely to suffer from these mental health issues.67 Many aspects of health, including gut health, depend on effective stress management. Psychological stressors, even those that are only momentary, have been demonstrated in animal studies to change bacteria in the intestines.68 Psychological and environmental stressors, such as excessive heat, cold, noise, and others, harm a person’s gut health. Sleep deprivation, circadian clock disruption, meditation, deep-breathing exercises, and progressive muscle relaxation are techniques used to reduce stress. Stress can be reduced by exercising regularly, getting enough sleep, and maintaining healthy eating habits. Prebiotics boost immunity by increasing the number of defense microorganisms in the body. Studies in animals and humans have shown that prebiotics can decrease the number of harmful Lactobacilli and Bifidobacteria.69
Limit alcohol intake
Excessive alcohol consumption may also negatively affect the microbiome. Repeated alcohol consumption is associated with gastritis and intestinal inflammation. Heartburn, prolonged discomfort, ulcers, and bacterial infections can result from inflammation. This indicates an unhealthy gut. According to previous research, this type of inflammation alters the microbiota, including how well it functions, and therefore can throw it off balance.70
Take antibiotics only when necessary
Antibiotic resistance can develop, even though antibiotics are commonly used to treat bacterial diseases. Misuse or overuse of antibiotics harms the immune system and gut microbiome.71 Some studies indicate that the gut still lacks several helpful bacterial species even six months after use.72 The Centers for Disease Control and Prevention estimates that about 30% of antibiotic prescriptions in the USA are unnecessary.73 Therefore, the organization urges patients to speak with their doctor before using antibiotics or considering other therapies.
Sleep enough
Sufficient, good-quality sleep improves happiness, cognitive function, and digestive health. According to a 2014 study, irregular sleep habits and disrupted sleep can have harmful effects on gut flora, thereby increasing the risk of inflammatory disorders.74
Fecal microbiota transplantation (FMT)
The digestive system is home to countless bacteria that are either harmless or beneficial; however, diseases that require antibiotic therapy can kill many healthy bacteria in the colon, allowing the harmful bacterium Clostridium difficile to thrive. The toxins secreted by C. difficile can damage the colonic lining, resulting in colitis. C. difficile can cause potentially fatal diseases such as toxic megacolon, in which the colon becomes dangerously enlarged. Antibiotics are typically used to treat colitis. However, recurring infections occur; therefore, when antibiotic treatment fails, fecal microbial transplantation (FMT) is an alternative therapy. This represents a novel treatment option for patients with recurrent infections. The FMT technique uses beneficial bacteria from a donor to restore the beneficial bacteria in the colon.75
Intermittent fasting (IF)
A nutritional strategy is known as intermittent fasting (IF) cycles between periods of fasting and eating. This method of eating focuses on eating rather than the food itself. It has been demonstrated that IF may have several possible health advantages, including weight loss, increased insulin sensitivity, decreased inflammation, and enhanced cognitive performance. The gut microbiome has been demonstrated to improve with IF by boosting microbial diversity and changing the composition to be more helpful, with an abundance of good bacteria and a depletion of potentially dangerous ones.76,77 The gut microbiota changes its composition and function due to the stress response of IF. Moreover, IF increases the amount of short-chain fatty acids, which have anti-inflammatory characteristics and support gut health, produced in the gut.78 Future research may investigate the ideal timing and duration of fasting intervals and the effects of various fasting routines (such as alternate-day fasting and time-restricted meals) on the gut flora and overall health.
The human microbiome, which includes skin, stomach, and oral microbiomes, is essential for preserving human health. Inflammatory bowel illness, diabetes, and obesity have been linked to dysbiosis in microbial populations. Recent studies have shown a connection between COVID-19 and the gut microbiome, with changes in the microbiota influencing disease severity. Certain microbiome-related disorders have shown promise in treatment with therapeutic methods such as fecal microbiota transplantation. A diverse and balanced gut microbiota can be achieved by eating a balanced diet, exercising frequently, and reducing stress. Prebiotics and probiotics can be used as supplements to promote the growth of beneficial microorganisms. Although the human microbiome is well understood, much remains to be discovered. Further research is needed to fully comprehend the precise mechanisms by which the microbiome influences human health and to create innovative therapeutic approaches for disorders associated with the microbiome. Given the current knowledge gaps, evidence that can be applied to clinical practice is required. Ideally, this will come from randomized controlled studies that evaluate changes in the gut microbiota composition and health outcomes using consistent prebiotics, probiotics, or fecal microbiota transplantation matrices.
The human microbiome, encompassing diverse microbial communities across the gut, skin, and oral environments, plays an indispensable role in maintaining human health. This review highlights the profound influence of the microbiome on immunity, metabolism, and disease management, emphasizing its involvement in conditions such as obesity, cancer, cardiovascular disease, and neurological disorders. Emerging evidence underscores the potential of microbiome-targeted interventions, such as probiotics, prebiotics, and faecal microbiota transplantation, in therapeutic strategies. While current findings are promising, significant gaps in knowledge remain, necessitating future research to unravel the intricate mechanisms governing host-microbiome interactions and to develop evidence-based clinical applications.
Advancements in metagenomics, artificial intelligence, and personalized medicine hold the potential to revolutionize healthcare by enabling microbiome-based therapies tailored to individuals. These therapies could prevent and treat diseases, paving the way for precision health care. This paper advocates the transformative potential of the evolving field of microbiome research, emphasizing its capacity to advance personalized medicine and significantly enhance human health outcomes through innovative, microbiome-focused therapeutic strategies.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MS conceptualized and designed the study. MD, SK and K performed literature review. MD, SK, K and MS wrote the manuscript. K and MS reviewed and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
Not applicable.
ETHICS STATEMENT
Not applicable.
- Kulkarni AS, Kumbhare SV, Dhotre Dhiraj P, Shouche YS. Mining the core gut microbiome from a sample Indian population. Indian J Microbiol. 2019;59(1):90-95.
Crossref - Price LB, Hungate BA, Koch BJ, Davis GS, Liu CM. Colonizing opportunistic pathogens (Cops): The beasts in all of us. PLoS Pathog. 2017;13(8):e1006369.
Crossref - Das S, Khanna C, Singh S, Nandi S, Verma R. Impact of human microbiome on health. In: Sharma SG, Sharma NR, Sharma M, eds. Microbial Diversity, Interventions and Scope. Singapore: Springer Singapore; 2020:349-373.
Crossref - Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013.
Crossref - Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016;14(9):576-588.
Crossref - Zhao H, Chu M, Huang Z, et al. Variations in oral microbiota associated with oral cancer. Sci Rep. 2017;7(1):11773.
Crossref - Giannella L, Grelloni C, Quintili D, et al. Microbiome changes in pregnancy disorders. Antioxidants. 2023;12(2):463.
Crossref - Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883-893.
Crossref - Kilian M, Chapple ILC, Hannig M, et al. The oral microbiome – an update for oral healthcare professionals. Br Dent J. 2016;221(10):657-666.
Crossref - Skowron K, Bauza-Kaszewska J, Kraszewska Z, et al. Human skin microbiome: impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms. 2021;9(3):543.
Crossref - Nurxat N, Wang L, Wang Q, et al. Commensal Staphylococcus epidermidis Defends against Staphylococcus aureus through SaeRS Two-Component System. ACS Omega. 2023;8(20):17712-17718.
Crossref - Zheng Y, Hunt RL, Villaruz AE, et al. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe. 2022;30(3):301-313.e9.
Crossref - Newstead LL, Varjonen K, Nuttall T, Paterson GK. Staphylococcal-produced bacteriocins and antimicrobial peptides: their potential as alternative treatments for Staphylococcus aureus infections. Antibiotics. 2020;9(2):40.
Crossref - Piewngam P, Zheng Y, Nguyen TH, et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018;562(7728):532-537.
Crossref - Cani PD, De Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765.
Crossref - Xu Z, Jiang W, Huang W, Lin Y, Chan FKL, Ng SC. Gut microbiota in patients with obesity and metabolic disorders – a systematic review. Genes Nutr. 2022;17(1):2.
Crossref - Quaranta G, Guarnaccia A, Fancello G, et al. Fecal microbiota transplantation and other gut microbiota manipulation strategies. Microorganisms. 2022;10(12):2424.
Crossref - Kumar R, Sood U, Gupta V, Singh M, Scaria J, Lal R. Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. Indian J Microbiol. 2020;60(1):12-25.
Crossref - Kandpal M, Indari O, Baral B, et al. Dysbiosis of gut microbiota from the perspective of the gut-brain axis: role in the provocation of neurological disorders. Metabolites. 2022;12(11):1064.
Crossref - Zhou Y, Wang Y, Quan M, Zhao H, Jia J. Gut microbiota changes and their correlation with cognitive and neuropsychiatric symptoms in alzheimer’s disease. J Alzheimers Dis. 2021;81(2):583-595.
Crossref - Buhas MC, Gavrilas LI, Candrea R, et al. Gut microbiota in psoriasis. Nutrients. 2022;14(14):2970.
Crossref - Celoria V, Rosset F, Pala V, et al. The skin microbiome and its role in psoriasis: a review. Psoriasis (Auckl). 2023;13:71-78.
Crossref - Thye AYK, Bah YR, Law JWF, et al. Gut-skin axis: unravelling the connection between the gut microbiome and psoriasis. Biomedicines. 2022;10(5):1037.
Crossref - Long Y, Tang L, Zhou Y, Zhao S, Zhu H. Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. BMC Med. 2023;21(1):66.
Crossref - Garrett WS. Cancer and the microbiota. Science. 2015;348(6230):80-86.
Crossref - Vivarelli S, Salemi R, Candido S, et al. Gut microbiota and cancer: from pathogenesis to therapy. Cancers. 2019;11(1):38.
Crossref - Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22(2):557.
Crossref - Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570-580.
Crossref - Tang WHW, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124(10):4204-4211.
Crossref - Rahman MM, Islam F, Harun-Or-Rashid M, et al. The gut microbiota (Microbiome) in cardiovascular disease and its therapeutic regulation. Front Cell Infect Microbiol. 2022;12:903570.
Crossref - Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183-1196.
Crossref - Li Y, Zhu M, Liu Y, et al. The oral microbiota and cardiometabolic health: A comprehensive review and emerging insights. Front Immunol. 2022;13:1010368.
Crossref - Mu W, Jia Y, Chen X, Li H, Wang Z, Cheng B. Intracellular Porphyromonas gingivalis promotes the proliferation of colorectal cancer cells via the mapk/erk signaling pathway. Front Cell Infect Microbiol. 2020;10:584798.
Crossref - Leng Y, Hu Q, Ling Q, et al. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front Cardiovasc Med. 2023;10:1114927.
Crossref - Maji A, Misra R, Dhakan DB, et al. Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environ Microbiol. 2018;20(1):402-419.
Crossref - Cassotta M, Forbes-Hernandez TY, Calderon Iglesias R, et al. Links between nutrition, infectious diseases, and microbiota: emerging technologies and opportunities for human-focused research. Nutrients. 2020;12(6):1827.
Crossref - Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6.
Crossref - Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Annals ATS. 2015;12(Suppl 2):S150-S156.
Crossref - Budden KF, Gellatly SL, Wood DLA, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55-63.
Crossref - Eshetie S, van Soolingen D. The respiratory microbiota: new insights into pulmonary tuberculosis. BMC Infect Dis. 2019;19(1):92.
Crossref - Ley RE. Prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol. 2016;13(2):69-70.
Crossref - Shan Y, Lee M, Chang EB. The gut microbiome and inflammatory bowel diseases. Annu Rev Med. 2022;73(1):455-468.
Crossref - Porras AM, Zhou H, Shi Q, et al. Inflammatory bowel disease-associated gut commensals degrade components of the extracellular matrix. mBio. 2022;13(6):e02201-22.
Crossref - Khiabani SA, Asgharzadeh M, Samadi Kafil H. Chronic kidney disease and gut microbiota. Heliyon. 2023;9(8):e18991.
Crossref - Plata C, Cruz C, Cervantes LG, Ramirez V. The gut microbiota and its relationship with chronic kidney disease. Int Urol Nephrol. 2019;51(12):2209-2226.
Crossref - Pantazi AC, Kassim MAK, Nori W, et al. Clinical perspectives of gut microbiota in patients with chronic kidney disease and end-stage kidney disease: where do we stand? Biomedicines. 2023;11(9):2480.
Crossref - Dao MC, Clיment K. Gut microbiota and obesity: Concepts relevant to clinical care. Eur J Intern Med. 2018;48:18-24.
Crossref - Liu BN, Liu XT, Liang ZH, Wang JH. Gut microbiota in obesity. WJG. 2021;27(25):3837-3850.
Crossref - Geng J, Ni Q, Sun W, Li L, Feng X. The links between gut microbiota and obesity and obesity related diseases. Biomed Pharmacother. 2022;147:112678.
Crossref - Saez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci. 2016;17(6):928.
Crossref - Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31-55.
Crossref - Ullah H, Arbab S, Tian Y, et al. The gut microbiota-brain axis in neurological disorder. Front Neurosci. 2023;17:1225875.
Crossref - Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: How gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9.
Crossref - Doroszkiewicz J, Groblewska M, Mroczko B. The role of gut microbiota and gut-brain interplay in selected diseases of the central nervous system. Int J Mol Sci. 2021;22(18):10028.
Crossref - Hoban AE, Stilling RM, Ryan FJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6(4):e774-e774.
Crossref - Zhang J, Garrett S, Sun J. Gastrointestinal symptoms, pathophysiology, and treatment in COVID-19. Genes Dis. 2021;8(4):385-400.
Crossref - Rocchi G, Giovanetti M, Benedetti F, et al. Gut microbiota and covid-19: potential implications for disease severity. Pathogens. 2022;11(9):1050.
Crossref - Gang J, Wang H, Xue X, Zhang S. Microbiota and COVID-19: Long-term and complex influencing factors. Front Microbiol. 2022;13:963488.
Crossref - Kumar R, Sood U, Gupta V, Singh M, Scaria J, Lal R. Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. Indian J Microbiol. 2020;60(1):12-25.
Crossref - Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (Isapp) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491-502.
Crossref - Nastasi C, Candela M, Bonefeld CM, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5(1):16148.
Crossref - Xiong RG, Zhou DD, Wu SX, et al. Health benefits and side effects of short-chain fatty acids. Foods. 2022;11(18):2863.
Crossref - You S, Ma Y, Yan B, et al. The promotion mechanism of prebiotics for probiotics: A review. Front Nutr. 2022;9:1000517.
Crossref - Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017:1-8.
Crossref - Rojas-Valverde D, Bonilla DA, Gomez-Miranda LM, Calleja-Nunez JJ, Arias N, Martinez-Guardado I. Examining the interaction between exercise, gut microbiota, and neurodegeneration: future research directions. Biomedicines. 2023;11(8):2267.
Crossref - Bonomini-Gnutzmann R, Plaza-Diaz J, Jorquera-Aguilera C, Rodriguez-Rodriguez A, Rodriguez-Rodriguez F. Effect of intensity and duration of exercise on gut microbiota in humans: a systematic review. Int J Environ Res Public Health. 2022;19(15):9518.
Crossref - Staudacher HM, Black CJ, Teasdale SB, Mikocka-Walus A, Keefer L. Irritable bowel syndrome and mental health comorbidity – approach to multidisciplinary management. Nat Rev Gastroenterol Hepatol. 2023;20(9):582-596.
Crossref - Galley JD, Nelson MC, Yu Z, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14(1):189.
Crossref - Klatt NR, Canary LA, Sun X, et al. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest. 2013;123(2):903-907.
Crossref - Bishehsari F, Magno E, Swanson G, et al. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38(2):163-171.
- Dudek-Wicher RK, Junka A, Bartoszewicz M. The influence of antibiotics and dietary components on gut microbiota. Prz Gastroenterol. 2018;13(2):85-92.
Crossref - Palleja A, Mikkelsen KH, Forslund SK, et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3(11):1255-1265.
Crossref - CDC. Centres for Disease Control and Prevention. Measuring outpatient antibiotic prescribing. https://www.cdc.gov/antibiotic-use/data/outpatient-prescribing/index.html. Published November 14, 2023. Accessed May 9, 2024
- Voigt RM, Forsyth CB, Green SJ, et al. Circadian disorganization alters intestinal microbiota. PLoS ONE. 2014;9(5):e97500.
Crossref - Fischer M, Sipe B, Cheng YW, et al. Fecal microbiota transplant in severe and severe-complicated Clostridium difficile?: A promising treatment approach. Gut Microbes. 2017;8(3):289-302.
Crossref - Khan MN, Khan SI, Rana MI, Ayyaz A, Khan MY, Imran M. Intermittent fasting positively modulates human gut microbial diversity and ameliorates blood lipid profile. Front Microbiol. 2022;13:922727.
Crossref - Saglam D, Colak GA, Sahin E, Ekren BY, Sezerman U, Bas M. Effects of Ramadan intermittent fasting on gut microbiome: is the diet key? Front Microbiol. 2023;14:1203205.
Crossref - Ahsan M. The effect of intermittent fasting on the gut microbiome. News-Medical. https://www.news-medical.net/health/The-Effect-of-Intermittent-Fasting-on-the-Gut-Microbiome.aspx. Published February 7, 2023. Accessed May 9, 2024
- Upadhyaya S. Potential health benefits of probiotics, prebiotics and synbiotics: A review. J Food Microbiol. 2023;7(4):151.
Crossref - Verma H, Phian S, Lakra P, et al. Human Gut Microbiota and Mental Health: Advancements and Challenges in Microbe-Based Therapeutic Interventions. Indian J Microbiol. 2020;60:405-419.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.