ISSN: 0973-7510
E-ISSN: 2581-690X
This study aimed to examine the antioxidant and antimicrobial activities and the phytochemical composition of three Parmeliaceae lichens from the western Himalayas. Three lichen species (Everniastrum cirrhatum (Fr.) Hale, Parmotrema reticulatum (Taylor) M. Choisy, and Usnea orientalis Motyka) were analyzed for antimicrobial, antioxidant assay, and chemical analyses using Gas chromatography-mass spectrometry (GC-MS). The chemical constituents were identified, and the percentage of components obtained was calculated. Antimicrobial activity was tested with the agar well diffusion method, and the total antioxidant capacity was measured using the phosphomolybdenum method (Total Antioxidant Capacity – TAC) and the hydrogen peroxide radical scavenging test. GC-MS analysis of methanol extracts from the lichens revealed 57 compounds in all three species. Methanol extracts from these lichens demonstrated strong antimicrobial activity (inhibition zones: 8.6 ± 0.09 to 28.2 ± 0.23 mm) with the highest activity against Salmonella typhi. They also exhibited low minimum inhibitory concentrations (MICs) of 0.125 mg against many microbes. The antioxidant capacity of methanol extracts ranged from 1.256 to 1.991 mg/g ascorbic acid equivalent. Interestingly, the hexane extract of E. cirrhatum showed the highest hydrogen peroxide scavenging activity at 91.13%. This study highlights the antioxidant and antimicrobial properties of these Parmeliaceae lichens, attributed to their diverse secondary metabolites. These findings suggest promising prospects for the development of novel antimicrobial and antioxidant agents from lichen extracts, warranting further exploration in pharmacological and biomedical research.
Biological Activity, Gas Chromatography-mass Spectroscopy, Antioxidants, Antimicrobial
Lichens are composite organisms comprising of two microorganisms, a fungus and an algae, along with various non obligate viruses, bacteria, and fungi forming a microecosystem. Some fungi grow on the outer surface of lichen called lichenicolous fungi, while others live inside the lichen, known as endolichenic fungi. Both the main lichen and these secondary fungi produce special chemicals that have medicinal uses. Worldwide, there are about 20,000 types of lichens known, and India alone has more than 3,000 different species. This shows how diverse lichens are in India and around the world.1 Lichens thrive in diverse and harsh environments like deserts, forests, alpine zones, tundras, and polar regions due to their unique physiology. They can grow on natural surfaces such as tree bark, soil and rocks, as well as on man-made structures like monuments and buildings. Lichens are excellent indicators of air pollution in an area.
Throughout history, lichens have been utilized for various purposes, including perfumes, dyes, food additives, fodder, and cosmetics. Many cultures around the world use lichen species for medicinal purposes. In ancient Ayurvedic medicine, species from the Parmotrema genus are commonly used to treat diarrhea, heart conditions, leprosy, skin ailments, and urinary disorders. Everniastrum cirrhatum is widely used as a crude drug2 for the treatment for blood disorders, stomach ailments, leprosy, kidney stones, carminative, and aphrodisiac disorders.3 Usneas species produce usnic acid, renowned for its antibiotic properties.4
Lichens produce over 1050 secondary metabolites, with more than half demonstrating antibiotic properties. These substances are found as crystals or liquids in the upper cortex or among medullary hyphae.5 Extracts from lichens, whether crude or purified, display diverse biological activities, including antimicrobial, analgesic, antiviral, anti-inflammatory, antioxidant, hepatoprotective, antiulcer, anti-HIV, and antigen toxicity.6 The inefficiency of current antimicrobial drugs, along with drug resistance and emerging infectious diseases, is a major concern in medicine today.7 Staphylococcus aureus, for instance, has become resistant to multiple drugs, including methicillin, posing a significant global challenge. Many antibiotics developed in the mid-20th century are no longer effective due to the pathogens’ resistance.8 Lichens are gaining attention for their pharmacological properties, including potent bioactivities and antioxidant compounds like phenolics.9 India, known for its biodiversity, hosts numerous lichen species, but only 160 have been recognized for their medicinal potential based on folklore, ancient literature, and preliminary biological screening.
Lichen collection and identification
The lichen materials were collected from Thalkedar Hills in the Pithoragarh district of Uttarakhand (India) during May 2022 and June 2023. The lichens were identified at the Lichenology Laboratory, CSIR-NBRI, in Lucknow. A voucher specimen Pamotrema reticulatum (Taylor) M. Choisy, no. 24929 (CSIR-LWG); Everniastrum cirrhatum (Fr.) Hale no. 47849 (CSIR-LWG); Usnea orientalis Motyka, no-24984/B (CSIR-LWG) was deposited at the CSIR-National Botanical Research Institute, Lucknow, India (Figure 1).
Figure 1. Lichen utilized for antimicrobial and antioxidant study. (A). Everniastrum cirrhatum, (B). Parmotrema reticulatum, and (C). Usnea orientalis
Preparation of extracts
The dried lichen materials were finely ground using an electric grinder, and pulverized material (10 g) was extracted twice by soaking with 100 ml of chloroform, hexane, and methanol for 48 h at room temperature. Further, the separated extracts were filtered through Whatman No. 1 filter paper, and the extract filtrates were condensed to dryness using a rotary evaporator at 40 °C, yielding approximately 15-20% of the extracts.
GC-MS analysis
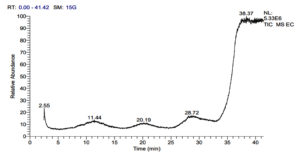
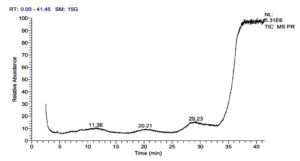
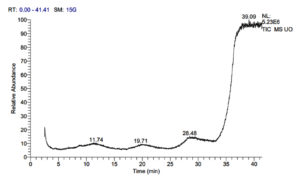
The GC-MS analysis was performed using a Thermo GC-Trace Ultra Ver: 5.0 and Thermo MS DSQ II, equipped with a DB 35-MS Capillary Standard Non-Polar Column (30 m length, 0.25 mm ID, 0.25 µm film thickness). The instrument was initially set to 110 °C, held for 2 minutes, then gradually increased to 260 °C at a rate of 6 °C/min and maintained for 9 minutes. The injection port temperature was set to 250 °C with a helium flow rate of 1 ml/min. A 1 µl sample was injected in split mode (10:1), and ionization was done at 70 eV. The mass spectral scan range was set between 45-450 MHz. Chemical constituents were identified by comparing fragmentation patterns with the National Institute of Standards and Technology Mass Spectral database (NIST-MS), which contains over 62,000 patterns. The percentage of each component was determined from the relative peak areas in the chromatogram, and the name, molecular weight, and structure of the compounds were confirmed through GC-MS analysis. Methanol extracts of all three lichen samples were prepared separately for GC-MS analysis.
The microorganism
Pathogenic bacteria and fungi were obtained from the National Centre for Cell Science (NCCS) in Pune, including Bacillus subtilis (B. subtilis, MTCC 4755), Staphylococcus aureus (S. aureus, MTCC 4734), Klebsiella pneumonia (K. pneumonia, MTCC 7028), Salmonella typhi (S. typhi, MTCC 734) and Candida albicans (C. albicans, MTCC 10231).
Evaluation of antimicrobial activity
Agar well diffusion assay
The agar well diffusion method was used to assess the antimicrobial activity of the extracts against selected bacterial strains, including Bacillus subtilis and Staphylococcus aureus being Gram-positive, Klebsiella pneumonia and Salmonella typhi being Gram-negative strains, and Candida albicans was a fungal strain. A subculture of each strain (200 µl, equivalent to 10v CFU/ml) was uniformly spread over the surface of nutrient agar or PDA plates, and five wells were created using a sterile gel borer. Dimethyl sulfoxide (DMSO) was used as the negative control, while ampicillin (0.1 mg/ml) for bacteria and fluconazole (0.1 mg/ml) for fungi served as positive controls. The remaining wells were filled with 100 µl of hexane, chloroform, and methanol extracts, each labeled accordingly. The plates were incubated at 37 °C for 20 hours for bacteria and at 22 °C for 2 days for fungi, after which the zones of inhibition (ZOI) around the wells were measured.10
Minimum inhibitory concentration (MIC) assay
The minimum inhibitory concentration (MIC) of the extracts was determined using the microdilution method with nutrient broth and PDA broth. The MIC was tested against bacterial strains (Bacillus subtilis, Klebsiella pneumonia, Salmonella typhi, Staphylococcus aureus, and the fungal strain Candida albicans). Five different concentrations of the extracts (1, 0.5, 0.250, 0.125, and 0.062 mg/ml) were prepared. Ampicillin and fluconazole were used as positive controls, while DMSO served as the negative control. A standardized suspension of the test bacteria and fungus was inoculated to create fresh cultures. Five milliliters of media were placed in each test tube, followed by the addition of 100 µl of the prepared extract concentrations and 20 µl of the fresh cultures. The test tubes were incubated for the appropriate time, and then turbidity was checked. The MIC value is defined as the lowest concentration of the extract at which there is complete inhibition of bacterial or fungal growth.10
Antioxidant activities
The samples were evaluated for their antioxidant potential using the following two assays:
Phosphomolybdenum method (Total antioxidant capacity-TAC)
An aliquot of 0.5 ml of the sample solution was mixed with 4.5 ml of reagent solution containing 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate. For the blank, 0.5 ml of DMSO was used instead of the sample. The extracts were added at a concentration of 1 mg/ml. The tubes were incubated in a boiling water bath at 95 °C for 90 minutes. After cooling to room temperature, the absorbance of each sample’s aqueous solution was measured at 695 nm against the blank using a UV-2450 spectrophotometer (Shimadzu, Japan). The total antioxidant capacity of the extracts was expressed as ascorbic acid equivalent (AAE), with the antioxidant capacity calculated using ascorbic acid as the standard for each extract.
Hydrogen peroxide radical scavenging test
The hydrogen peroxide (H2O2) scavenging activity of the extracts was determined by measuring the reduction of H2O2 in a system containing H2O2 and the scavenger, using the classical UV method at 330 nm. Phosphate buffer (pH 7.4) was added to all samples, each containing 0.6 ml of hydrogen peroxide. After incubating for 10 minutes, the absorbance was measured at 330 nm against a blank solution with phosphate buffer. The extracts were added at a concentration of 1 mg/mL.11 The percentage of H2O2 inhibition was calculated using the following formula:
Percentage (%) of hydrogen peroxide radical scavenging activity = [(A0-A1) / A0] x 100
where A0 is control, A1 is test sample.
Data analysis
All experiments were performed in triplicate, and the data are presented as the mean ± standard deviation (SD).
Gas chromatography-mass spectrometry (GC-MS)
The Gas chromatography-mass spectrometry (GC-MS) analysis of the methanol extracts of three lichens revealed a total of 57 pharmacological compounds (Figure 2-4). Further, comparison of obtained compounds in the NIST library and PubChem yielded their details and importance (Table 1-3). Everniastrum cirrhatum yielded 22 compounds such as Niacinamide (0.25%), Methotrexate (0.06%), Semicarbazide (0.09%), Carotene (0.15%), Lycoxanthin (0.41%), 2-Iodohiistidine (0.45%), Tetrazole (0.48%), Triprolidine (0.34%), Titanium (0.16%), Milbemycin B (0.21%), Chromone (0.22%), Carvacrol (0.12%), Androstane (0.15%), Trimethylsilyl (0.22%), Hexasiloxane (0.61%), Heptasiloxane (0.81%), Octasiloxane (0.29%), Tetradecanoic acid (0.23%), Colchicin (0.11%), Thymol (0.51%), Methadone (0.37%), W-18 (0.32%), which are of high pharmaceutical value.
Table (1):
List of compounds having pharmaceutical importance identified in Everniastrum cirrhatum
No. |
RT |
Compound Name |
Molecular Formula |
Mol. Weight |
Area % |
Biological activity |
|---|---|---|---|---|---|---|
1 |
5.18 |
Niacinamide |
C6H6N2O |
122 |
0.25 |
Antipruritic, antimicrobial |
2 |
5.56 |
Methotrexate |
C20H22N8O5 |
45 |
0.06 |
Anti-inflammatory actions |
3 |
11.69 |
Semicarbazide |
C19H23N3O2S |
357 |
0.09 |
Antiviral, antitumor |
4 |
11.81 |
Carotene |
C41H58O |
566 |
0.15 |
Antioxidant |
5 |
12.79 |
Lycoxanthin |
C40H56O |
552 |
0.41 |
Antimicrobial |
6 |
0.45 |
2-Iodohiistidine |
C6H8IN3O2 |
281 |
0.45 |
Antioxidant antiviral, antitumor |
7 |
14.39 |
Tetrazole |
C16H15N5 |
277 |
0.48 |
Antiviral, antitumor |
8 |
16.29 |
Triprolidine |
C19H22N2 |
278 |
0.34 |
Anticholinergic and sedative |
9 |
16.33 |
Titanium |
C16H16Br2Ti2 |
462 |
0.16 |
Antimicrobial |
10 |
16.66 |
Milbemycin B |
C33H47C1O7 |
590 |
0.21 |
Antiparasitic |
11 |
20.41 |
Chromone |
C14H16O6 |
280 |
0.22 |
Antiviral, antitumor |
12 |
21.52 |
Carvacrol |
C16H28OSi |
264 |
0.12 |
Antimutagenic, antigenotoxic |
13 |
24.98 |
Androstane |
C29H43NO3Si |
481 |
0.15 |
Antimicrobial |
14 |
27.31 |
Trimethylsilyl |
C14H24O3Si2 |
296 |
0.22 |
Antimicrobial |
15 |
27.40 |
Hexasiloxane |
C12H38O5Si6 |
430 |
0.61 |
Antimicrobial |
16 |
27.61 |
Heptasiloxane |
C14H44O6Si7 |
504 |
0.81 |
Antimicrobial |
17 |
27.80 |
Octasiloxane |
C16H50O7Si8 |
578 |
0.29 |
Antimicrobia |
18 |
29.38 |
Tetradecanoic acid |
C37H58O8 |
630 |
0.23 |
Antielastase, antioxidant |
19 |
29.86 |
Colchicine |
C31H33NO9 |
563 |
0.11 |
Anti-mitotic drug |
20 |
30.93 |
Thymol |
C16H28OSi |
264 |
0.51 |
Antibacterial |
21 |
32.43 |
Methadone |
C21H27NO |
309 |
0.37 |
Antagonist |
22 |
33.24 |
W-18 |
C19H20ClN3O4S |
421 |
0.32 |
Antitumor |
Table (2):
List of compounds having pharmaceutical importance identified in Parmotrema reticulatum
No. |
RT |
Compound Name |
Molecular Formula |
Mol. Weight |
Area % |
Biological activity |
|---|---|---|---|---|---|---|
1 |
3.76 |
Dialifor |
C14H17CINO4PS2 |
393 |
0.32 |
Organophosphate |
2 |
3.94 |
Tabersonine |
C21H24N2O2 |
336 |
0.73 |
Anticancerous |
3 |
4.02 |
Thiosemicarbazone |
C10H13N3S |
207 |
0.07 |
Antiparasitic |
4 |
4.55 |
Pentafluoropropionate |
C11H8F5NO4 |
313 |
0.79 |
Antimicrobial |
5 |
5.40 |
Hydrazinecarbothioamide |
C14H14N4O3S2 |
350 |
0.13 |
Antimicrobial |
6 |
5.78 |
Pentanal |
C11H14N4O4 |
266 |
0.14 |
Antifungal |
7 |
6.65 |
Normethadol |
C20H27NO |
297 |
0.29 |
Antimicrobial, anticancerous |
8 |
6.92 |
Propanedinitrile |
C18H22N2O |
282 |
0.63 |
Antibacterial |
9 |
7.01 |
Triprolidine |
C19H22N2 |
278 |
0.13 |
Anticholinergic and sedative |
10 |
7.34 |
Lansoprazole |
C16H14F3N3O2S |
369 |
0.13 |
Decreases gastricacid secretion |
11 |
7.89 |
Valsartan |
C24H29N5O3 |
435 |
0.25 |
Cardiac stimulation |
12 |
10.34 |
Papaveroline |
C19H21NO5 |
343 |
0.33 |
Antiviral, cardioprotective, anti-inflammatory |
13 |
10.34 |
Falcatine |
C16H13NO4 |
283 |
0.33 |
Antiviral |
14 |
11.20 |
Sarreroside |
C30H42O10 |
562 |
0.12 |
Wound healing, antioxidant |
15 |
11.20 |
Dehydrofelodipine |
C18H17CI2NO4 |
381 |
0.12 |
Antiviral |
16 |
11.20 |
Butanedial |
C20H18O6 |
354 |
0.12 |
Euphoric and sedative |
17 |
11.50 |
Colchicine |
C31H33NO9 |
563 |
0.40 |
Anti-mitotic |
18 |
14.03 |
Glafenin |
C19H17CIN2O4 |
372 |
0.10 |
Anti-inflammatory |
19 |
14.65 |
Buflomedil |
C17H25NO4 |
307 |
0.26 |
Antiplatelet |
20 |
14.93 |
Schisandrol B |
C23H28O7 |
416 |
0.21 |
Anti-inflammatory |
21 |
15.76 |
Pipemidic acid |
C14H17N5O3 |
303 |
0.10 |
Antibacterial |
22 |
16.01 |
Nitralin |
C13H19N3O6S |
345 |
0.08 |
Antimicrobial |
23 |
17.07 |
Apraclonidine |
C9H10CI2N4 |
244 |
0.68 |
Anti-inflammatory |
Table (3):
List of compounds having pharmaceutical importance identified in Usnea orientalis
No. |
RT |
Compound Name |
Molecular Formula |
Mol. Weight |
Area % |
Biological activity |
|---|---|---|---|---|---|---|
1 |
3.58 |
Tyramine |
C16H14N2O4 |
298 |
0.31 |
Increasing blood pressure |
2 |
3.58 |
Picolinamide |
C35H34N6O3 |
586 |
0.31 |
Antifungal, antibiotic |
3 |
3.58 |
Silane |
C14H24OS |
236 |
0.31 |
Antimicrobial |
4 |
3.65 |
Cholic acid |
C24H40O5 |
408 |
0.09 |
Mutagenic |
5 |
3.65 |
Carbonyl Oxy Camptothecin |
C28H30N4O6 |
518 |
0.09 |
Anticancer |
6 |
3.79 |
Piperidinethione |
C14H18N2O2S |
278 |
0.20 |
Antimicrobial, antioxidant |
7 |
3.79 |
Bitropenyl |
C14H14 |
182 |
0.20 |
Antioxidant, antimicrobial |
8 |
3.91 |
2H-Pyran |
C16H24O2 |
248 |
0.12 |
Antibacterial, antifungal, phytotoxic |
9 |
4.35 |
Hydrazine carbothioamide |
C14H14N4O3S2 |
350 |
0.20 |
Antibacterial and antifungal |
10 |
4.35 |
Benzodioxole |
C11H11NO6 |
253 |
0.20 |
Antitumor, antibacterial |
11 |
4.35 |
Hydrazone |
C12H16N4O4 |
280 |
0.20 |
Antibacterial, anticonvulsant |
12 |
4.53 |
Pyridazine |
C20H13N5O2 |
355 |
0.52 |
Analgesic, anti-tumor, antiviral |
13 |
4.53 |
Chlorendic anhydride |
C9H2CI6O3 |
368 |
0.52 |
Antibacterial |
14 |
4.92 |
Mitoxantrone |
C22H28N4O6 |
444 |
0.11 |
Antibiotics |
Parmotrema reticulatum yielded 23 pharmaceutically important compounds, such as Dialifor (0.32%), Tabersonine (0.73%), Thiosemicarbazone (0.07%), Pentafluoropropionate (0.79%), Hydra-zinecarbothioamide (0.13%), Pentanal (0.14%), Normethadol (0.29%), Propanedinitrile (0.63%), Triprolidine (0.13%), Lansoprazole (0.13%), Valsartan (0.25%), Papaveroline (0.33%), Falcatine (0.33%), Sarreroside (0.12%), Dehydrofelodipine (0.12%), Butanedial (0.12%), Colchicine (0.40%), Glafenin (0.10%), Buflomedil (0.26%), Schisandrol B (0.21%), Pipemidic acid (0.10%), Nitralin (0.08%), and Apraclonidine (0.68%).
Usnea orientalis yielded 14 compounds such as Tyramine (0.31%), Picolinamide (0.31%), Silane (0.31%), Cholic acid (0.09%), Carbonyloxycamptothecin (0.09%), Piperidinethione (0.20%), Bitropenyl (0.20%), 2H-Pyran (0.12%), Hydrazine carbothioamide (0.20%), Benzodioxole (0.20%), Hydrazone (0.20%), Pyridazine (0.52%), Chlorendic anhydride (0.52%) and Mitoxantrone (0.11%).
Antimicrobial activities
All the extracts demonstrated notable antibacterial activity, with zones of inhibition (ZOI) ranging from 8.3 ± 0.06 mm to 28.2 ± 0.23 mm. Among the extracts, methanol extracts from all three lichens were better than chloroform and hexane extracts. The methanol extract of P. reticulatum showed the greatest antibacterial effect against S. typhi (ZOI: 28.2 ± 0.23 mm). Methanol extracts also proved most effective for antifungal activity, with a ZOI range of 12.7 ± 0.36 mm. The methanol extract of E. cirrhatum showed the strongest activity against S. aureus (ZOI: 23.6 ± 0.08 mm). Similarly, Usnea orientalis hexane extract displayed the highest activity against S. aureus (ZOI: 24.3 ± 0.08 mm). Methanol extracts showed the highest antifungal activity, with a ZOI range of 16.3 ± 0.28 mm. Similar to antibacterial activity among the three extracts methanol, also showed better antifungal activity. It can be noted that although lichen extracts showed excellent antimicrobial activities, they are not better than the standards Ampicillin or Fluconazole. In most cases methanol extracts, of lichens showed minimum inhibitory concentrations of 0.125 mg against many microbes (Table 4).
Table (4):
Antimicrobial activity expressed as zone of inhibition for E. cirrhatum, P. reticulatum and U. orientalis extracts against S. aureus, B. subtilis, S. typhi, K. pneumonia and C. albicans (n = 3)
| No. | Extract | Organism | Zone of inhibition (in mm) evaluated at 1 mg/ml | Efficiency |
|---|---|---|---|---|
| 1 | E. cirrhatum – Hexane | S. aureus | 12.2 ± 0.03 | ++ |
| 2 | B. subtilis | 11.1 ± 0.05 | ++ | |
| 3 | S. typhi | 14.8 ± 0.21 | ++ | |
| 4 | K. pneumoniae | 12.4 ± 0.32 | ++ | |
| 5 | C. albicans | 10.9 ± 0.21 | + | |
| 6 | E. cirrhatum – Chloroform | S. aureus | 12.7 ± 0.04 | ++ |
| 7 | B. subtilis | 10.1 ± 0.16 | + | |
| 8 | S. typhi | 15.7 ± 0.13 | ++ | |
| 9 | K. pneumoniae | 10.4 ± 0.05 | + | |
| 10 | C. albicans | 11.3 ± 0.32 | ++ | |
| 11 | E. cirrhatum – Methanol | S. aureus | 23.6 ± 0.08 | +++ |
| 12 | B. subtilis | 18.2 ± 0.14 | ++ | |
| 13 | S. typhi | 23.5 ± 0.21 | +++ | |
| 14 | K. pneumoniae | 14.8 ± 0.32 | ++ | |
| 15 | C. albicans | 16.7 ± 0.27 | ++ | |
| 16 | P. reticulatum – Hexane | S. aureus | 10.9 ± 0.02 | + |
| 17 | B. subtilis | 08.3 ± 0.06 | + | |
| 18 | S. typhi | 13.1 ± 0.04 | ++ | |
| 19 | K. pneumoniae | 09.7 ± 0.24 | + | |
| 20 | C. albicans | 09.5 ± 0.31 | + | |
| 21 | P. reticulatum – Chloroform | S. aureus | 11.9 ± 0.13 | ++ |
| 22 | B. subtilis | 09.1 ± 0.14 | + | |
| 23 | S. typhi | 14.6 ± 0.08 | ++ | |
| 24 | K. pneumoniae | 13.9 ± 0.09 | ++ | |
| 25 | C. albicans | 09.1 ± 0.33 | + | |
| 26 | P. reticulatum – Methanol | S. aureus | 26.1 ± 0.15 | +++ |
| 27 | B. subtilis | 20.9 ± 0.32 | ++ | |
| 28 | S. typhi | 28.2 ± 0.23 | +++ | |
| 29 | K. pneumoniae | 13.8 ± 0.16 | ++ | |
| 30 | C. albicans | 12.7 ± 0.36 | ++ | |
| 31 | U. orientalis – Hexane | S. aureus | 24.3 ± 0.08 | +++ |
| 32 | B. subtilis | 16.7 ± 0.04 | ++ | |
| 33 | S. typhi | 20.4 ± 0.06 | +++ | |
| 34 | K. pneumoniae | 25.1 ± 0.15 | +++ | |
| 35 | C. albicans | 11.5 ± 0.27 | ++ | |
| 36 | U. orientalis – Chloroform | S. aureus | 15.7 ± 0.07 | ++ |
| 37 | B. subtilis | 12.9 ± 0.05 | ++ | |
| 38 | S. typhi | 24.1 ± 0.12 | +++ | |
| 39 | K. pneumoniae | 09.5 ± 0.31 | + | |
| 40 | C. albicans | 12.7 ± 0.34 | ++ | |
| 41 | U. orientalis – Methanol | S. aureus | 12.8 ± 0.05 | ++ |
| 42 | B. subtilis | 08.6 ± 0.09 | + | |
| 43 | S. typhi | 22.2 ± 0.08 | +++ | |
| 44 | K. pneumoniae | 13.8 ± 0.21 | ++ | |
| 45 | C. albicans | 16.3 ± 0.28 | ++ | |
| 46 | Amphicillin | S. aureus | 32.2 ± 0.04 | +++ |
| 47 | B. subtilis | 28.8 ± 0.23 | +++ | |
| 48 | S. typhi | 35.1 ± 0.26 | +++ | |
| 49 | K. pneumoniae | 33.9 ± 0.07 | +++ | |
| 50 | Flucanazole | C. albicans | 22.6 ± 0.30 | +++ |
+++ is excellent, ++ is good, + is moderate
MIC activities
The minimum inhibitory concentration (MIC) values for microorganisms sensitive to the methanol extracts ranged from 62.5 to 125 µg/ml. In this study, the lowest MIC values were observed in the methanol extracts of E. cirrhatum against S. aureus and S. typhi. Similarly, the methanol extract of P. reticulatum showed the lowest MIC values against S. aureus and S. typhi. For U. orientalis, the hexane extracts exhibited the lowest MIC values against S. aureus, S. typhi, and K. pneumoniae. No MIC values were recorded for the standard antibiotics used in the study (Table 5).
Table (5):
MIC values obtained against S. aureus, B. subtilis, S. typhi, K. pneumonia and C. albicans
| No. | Extract | Organism | Turbidity observed with drug concentration | MIC (mg) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 mg | 0.5 mg | 0.250 mg | 0.125 mg | 0.062 mg | ||||
| 1 | E. cirrhatum – Hexane |
S. aureus | clear | clear | clear | turbid | turbid | 0.250 |
| 2 | B. subtilis | clear | clear | clear | turbid | turbid | 0.250 | |
| 3 | S. typhi | clear | clear | clear | turbid | turbid | 0.250 | |
| 4 | K. pneumoniae | clear | clear | clear | turbid | turbid | 0.250 | |
| 5 | C. albicans | clear | turbid | turbid | turbid | turbid | 1 | |
| 6 | E. cirrhatum – Chloroform |
S. aureus | clear | clear | clear | turbid | turbid | 0.250 |
| 7 | B. subtilis | clear | clear | turbid | turbid | turbid | 0.5 | |
| 8 | S. typhi | clear | clear | clear | turbid | turbid | 0.250 | |
| 9 | K. pneumoniae | clear | clear | turbid | turbid | turbid | 0.5 | |
| 10 | C. albicans | clear | clear | turbid | turbid | turbid | 0.5 | |
| 11 | E. cirrhatum – Methanol |
S. aureus | clear | clear | clear | clear | turbid | 0.125 |
| 12 | B. subtilis | clear | clear | clear | turbid | turbid | 0.250 | |
| 13 | S. typhi | clear | clear | clear | clear | turbid | 0.125 | |
| 14 | K. pneumoniae | clear | clear | clear | turbid | turbid | 0.250 | |
| 15 | C. albicans | clear | clear | turbid | turbid | turbid | 0.5 | |
| 16 | P. reticulatum – Hexane |
S. aureus | clear | clear | turbid | turbid | turbid | 0.5 |
| 17 | B. subtilis | clear | clear | turbid | turbid | turbid | 0.5 | |
| 18 | S. typhi | clear | clear | clear | turbid | turbid | 0.250 | |
| 19 | K. pneumoniae | clear | clear | turbid | turbid | turbid | 0.5 | |
| 20 | C. albicans | clear | turbid | turbid | turbid | turbid | 1 | |
| 21 | P. reticulatum – Chloroform |
S. aureus | clear | clear | clear | turbid | turbid | 0.250 |
| 22 | B. subtilis | clear | clear | turbid | turbid | turbid | 0.5 | |
| 23 | S. typhi | clear | clear | clear | turbid | turbid | 0.250 | |
| 24 | K. pneumoniae | clear | clear | clear | turbid | turbid | 0.250 | |
| 25 | C. albicans | clear | turbid | turbid | turbid | turbid | 1 | |
| 26 | P. reticulatum – Methanol |
S. aureus | clear | clear | clear | clear | turbid | 0.125 |
| 27 | B. subtilis | clear | clear | clear | turbid | turbid | 0.250 | |
| 28 | S. typhi | clear | clear | clear | clear | turbid | 0.125 | |
| 29 | K. pneumoniae | clear | clear | clear | turbid | turbid | 0.250 | |
| 30 | C. albicans | clear | clear | turbid | turbid | turbid | 0.5 | |
| 31 | U. orientalis – Hexane |
S. aureus | clear | clear | clear | clear | turbid | 0.125 |
| 32 | B. subtilis | clear | clear | clear | turbid | turbid | 0.250 | |
| 33 | S. typhi | clear | clear | clear | clear | turbid | 0.125 | |
| 34 | K. pneumoniae | clear | clear | clear | clear | turbid | 0.125 | |
| 35 | C. albicans | clear | clear | turbid | turbid | turbid | 0.5 | |
| 36 | U. orientalis – Chloroform |
S. aureus | clear | clear | clear | turbid | turbid | 0.250 |
| 37 | B. subtilis | clear | clear | clear | turbid | turbid | 0.250 | |
| 38 | S. typhi | clear | clear | clear | clear | turbid | 0.125 | |
| 39 | K. pneumoniae | clear | clear | turbid | turbid | turbid | 0.5 | |
| 40 | C. albicans | clear | clear | turbid | turbid | turbid | 0.5 | |
| 41 | U. orientalis – Methanol |
S. aureus | clear | clear | clear | turbid | turbid | 0.250 |
| 42 | B. subtilis | clear | clear | turbid | turbid | turbid | 0.5 | |
| 43 | S. typhi | clear | clear | clear | clear | turbid | 0.125 | |
| 44 | K. pneumoniae | clear | clear | clear | turbid | turbid | 0.250 | |
| 45 | C. albicans | clear | clear | turbid | turbid | turbid | 0.5 | |
| 46 | Ampicillin | S. aureus | clear | clear | clear | clear | clear | NA |
| 47 | B. subtilis | clear | clear | clear | clear | clear | NA | |
| 48 | S. typhi | clear | clear | clear | clear | clear | NA | |
| 49 | K. pneumoniae | clear | clear | clear | clear | clear | NA | |
| 50 | Fluconazole | C. albicans | clear | clear | clear | clear | clear | NA |
Antioxidant activities
The lichen extracts exhibited high total antioxidant capacities, with the methanol extract of E. cirrhatum having the highest antioxidant capacity at 1.991 AAE. The methanol extract of U. orientalis had an AAE of 1.504, and the methanol extract of P. reticulatum had an AAE of 1.256. However, the percentage of hydrogen peroxide radical scavenging varied for different extracts with hexane extract, of E. cirrhatum showed maximum activity of 91.13%. The hexane extracts of P. reticulatum showed maximum activity of 90.52%. The hexane extracts of U. orientalis showing maximum activity of 65.05. It was observed that among all the lichen extracts, the hexane extracts exhibited the highest radical scavenging activity, followed by the chloroform extracts, and then the methanol extracts (Table 6).
Table (6):
Total antioxidant activity of lichen extracts through Phosphomolybdenum method and Hydrogen peroxide radical scavenging capacity (n = 3)
| No. | Extracts at 1 mg/ml | Phosphomolybdenum method | Hydrogen peroxide radical scavenging capacity | ||
|---|---|---|---|---|---|
| OD (695 nm) | Total Antioxidant Capacity (mg/gm AAE) | OD (330 nm) | Percentage (%) | ||
| 1 | E. cirrhatum – Hexane | 0.176 ± 0.05 | 0.053 | 0.454 ± 0.04 | 91.13 |
| 2 | E. cirrhatum – Chloroform | 0.354 ± 0.08 | 0.125 | 1.208 ± 0.06 | 76.40 |
| 3 | E. cirrhatum – Methanol | 1.380 ± 0.14 | 1.991 | 2.989 ± 0.09 | 41.62 |
| 4 | P. reticulatum – Hexane | 0.331 ± 0.09 | 0.196 | 0.485 ± 0.12 | 90.52 |
| 5 | P. reticulatum – Chloroform | 0.402 ± 0.10 | 0.251 | 0.862 ± 0.10 | 83.16 |
| 6 | P. reticulatum – Methanol | 0.998 ± 0.16 | 1.256 | 2.983 ± 0.05 | 41.73 |
| 7 | U. orientalis – Hexane | 0.429 ± 0.19 | 0.258 | 1.789 ± 0.08 | 65.05 |
| 8 | U. orientalis – Chloroform | 0.235 ± 0.08 | 0.094 | 2.864 ± 0.16 | 44.06 |
| 9 | U. orientalis – Methanol | 1.198 ± 0.09 | 1.504 | 2.992 ± 0.12 | 41.56 |
The crude extracts or isolated compounds from lichens have shown a range of biological activities that are available in the public domain. These lichen constituents are being utilized in the development of ointments for treating fungal skin conditions, including tinea, ringworm, and athlete’s foot.12 Usnea species are said to be used in many medicinal applications as a potent antibiotic and antifungal agent.13 In the study, the methanol extract of E. cirrhatum exhibited relatively low antibacterial activity against P. aeruginosa, E. coli, and S. aureus, with inhibition zones measuring 2.40 ± 0.10 mm, 2.97 ± 0.15 mm, and 2.83 ± 0.05 mm, respectively.14 In another study, the crude extract of the lichens E. cirrhatum showed significant antifungal activities against Aspergillus flavus.15 Several studies have investigated the antioxidant activities of lichen extracts using a similar set of organisms as those in the present study. The highest value of radical scavenging activity for U. orientalis and correlated it with phenolic content.16 In the recent studied, antimicrobial, antioxidant activities and phytochemical analysis for 12 lichen species which also included E. cirrhatum and U. orientalis. The antibacterial activity of E. cirrhatum ranged from zero to 12.7 ± 0.8 mm of ZOI. For U. orientalis the maximum ZOI for antibacterial activity was 13.5 ± 0.5 mm. In both cases methanol extract showed better antibacterial activity. However, in the case of antifungal both lichens showed poor activities. In case of the antioxidant study E. cirrhatum (48%) had poorer free radical scavenging activity in comparison to U. orientalis (62.87%).17
The current study demonstrates that lichen-specific substances exhibit a wide range of biological activities. This highlights the potential for these species in phytochemistry and medicine, suggesting they could be further explored through in vivo studies.18 The choice of extraction methods and solvents significantly impacts the nature and concentration of the extracts, while maintaining their physical and chemical integrity.19,20 The methanolic extracts of lichens, in particular, show strong antioxidant activity, likely due to the high polarity of methanol, which effectively extracts these bioactive compounds.21 GC-MS analysis confirms the presence of antimicrobial and antioxidant compounds in the extracts, indicating their potential for pharmacological applications. Overall, these lichens serve as valuable benchmarks for their high biotechnological potential, demonstrating excellent antimicrobial and antioxidant properties.
Lichens have been utilized since ancient times for various purposes, including medicine, colorants, perfumes, food additives, and other medicinal uses, due to their diverse range of secondary metabolites. Lichen metabolites like niacinamide, methotrexate, tetrazole, chromone, carvacrol, tabersonine, pentanal, normethadol, lansoprazole, falcatine, colchicine, apraclonidine, silane, picolinamide, mitoxantrone belonging to several chemical classes and exhibit a broad spectrum of biological activities, including antibiotic, antiviral, antimycobacterial, anti-inflammatory, antipyretic, analgesic, cytotoxic, antiproliferative, and antioxidant properties. In the present study, lichen extracts have shown potent antimicrobial and antioxidant activities which may be attributed to the presence of lichen compounds. Among the three lichens E. cirrhatum has shown excellent antimicrobial and antioxidant study. Further, methanol extracts were found to contain strong antimicrobial and antioxidant effects. Therefore, more insights into in vivo studies and further research on isolation and characterization of active compounds are much needed in future research.
ACKNOWLEDGMENTS
The authors are thankful to the Head, Department of Chemistry, D.S.B Campus, Kumaun University, Nainital, Uttarakhand and Director, CSIR – NBRI, Lucknow, for providing the laboratory facilities during the work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PJ and DKU designed the study. SN performed experimental analysis. KP performed the analytical calculations and numerical simulations. PJ and JD wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Nayaka S, Haridas B. Lichen forming and lichenicolous fungi of the Western Ghats, India. In Pullaiah, T. (ed.). Biodiversity Hotspots of the Western Ghats and Sri Lanka. Apple Academic Press Inc., Canada. Co-published with CRC Press (Taylor & Francis), U.K. 2024:89-107.
Crossref - Nayaka S, Upreti DK, Khare R. Medicinal lichens of India. In Drugs from plants. (Ed. PC Trivedi) Avishkar Publishers and Distributors, India. 2010:1-54
- Upreti DK, Bajpai R, Nayaka S, Singh BN. Ethnolichenological studies in India: Future prosects. In: Jain AK (ed.) Emerging dimentions in Indian Ethnobotany, Scientific publishiers, Jaipur, 2015;199-237.
- Cansaran D, Kahya D, Yurdakulol E, Atakol O. Identification and quantitation of usnic acid from the lichen Usnea species of Anatolia and antimicrobial activity. Zeitschriftf r Naturforschung C. 2006;61:773-776.
Crossref - Huneck S. The significance of lichens and their metabolites. Naturwissenschaften. 1999;86:559-70.
Crossref - Solarova Z, Liskova A, Samec M, Kubatka P, Busselberg D, Solar P. Anticancer potential of lichens secondary metabolites. Biomolecules. 2020;10(1):87.
Crossref - Walsh C. Where will new antibiotics come from? Nat Rev Microbiol. 2003;1:65-70.
Crossref - Kosanic M, Rankovic B. Lichen Secondary Metabolites as Potential Antibiotic Agents. In: Rankovic, B. (eds) Lichen Secondary Metabolites. Springer Cham. 2019:99-127.
Crossref - Pradhan S, Dash S, Parida S, Sahoo B, Rath B. Antioxidant and antimicrobial activities and GC/MS-based phytochemical analysis of two traditional Lichen species Trypethellium virens and Phaeographis dendritica. J Genet Eng Biotechnol. 2023;21(1):41.
Crossref - Srivastava P, Upreti DK, Dhole TN, Srivastava AK, Nayak MT. Antimicrobial property of extracts of Indian lichen against human pathogenic bacteria. Interdiscip Perspect Infect Dis. 2013;2013(1):709348.
Crossref - Hussen EM, Endalew SA. In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina. BMC Complement Med Ther. 2023;23(1):146.
Crossref - Wilson CO, Gisvold O, Delgado JN, Remers WA. Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry. Lippincott Williams & Wilkins. 2004;991
- Shukla P, Upreti DK, Tewari LM. Secondary metabolite variability in lichen genus Usnea in India: A potential source for bioprospection. G-Journal of Environmental Science and Technology. 2015;2(3):44-55.
- Kumar SVP, Kekuda TRP, Vinayaka KS, Swathi D, Mallikarjun N, Nishanth BC. Studies on proximate composition, antifungal and anthelmintic activity of a macro-lichen Ramalina hossei H. Magn & G. Awasthi. Int J Biotechnol Biochem. 2010;6(2):191-201.
- Furmanek L, Czarnota P, Seaward MRD. The effect of lichen secondary metabolites on Aspergillus fungi. Arch Microbiol. 2022;204:100.
Crossref - Rankovic B, Rankovic D, Kosanic M, Maric D. Antioxidant and antimicrobial properties of the lichens Anaptychya ciliaris, Nephroma parile, Ochrolechia tartarea and Parmelia centrifuga. Cent Eur J Biol. 2010;5(5):649-655.
Crossref - Kumar P, Nayaka S, Verma T, Niranjan A, Upreti DK. Comparative analysis of antimicrobial, antioxidant activities and phytochemicals of Himalayan lichens. Biomass Conv Bioref. 2024;1:1-21.
Crossref - Adesalu TA, Agadagba T. Isolation of symbionts and GC-MS analysis of lichens collected from Obudu mountain resort, South-South, Nigeria. Ife Journal of Science. 2016;18(2):427-434.
- Dirar AI, Alsaadi DHM, Wada M, Mohamed MA, Watanabe T, Devkota HP. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S Afr J Bot. 2019;120:261-267.
Crossref - Keneni YG, Bahiru LA, Marchetti JM. Effects of different extraction solvents on oil extracted from jatropha seeds and the potential of seed residues as a heat provider. Bioenerg Res. 2021;14:1207-1222.
Crossref - Azmir J, Zaidul ISM, Rahman MM, et al. Techniques for extraction of bioactive compounds from plant materials: A review. J Food Eng. 2013;117(4):426-436.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.